Abstract
Background
A national survey in 2006 identified that UK referral practice for pediatric CNS tumors ranked poorly in international comparisons, which led to new National Health Service (NHS) Evidence accredited referral guidelines published in 2008 by the Royal College of Paediatrics and Child Health and a campaign to raise awareness of early features of CNS tumors and the need for timely imaging.
Methods
The “HeadSmart: Be Brain Tumour Aware” campaign was launched in June 2011 across the UK as a quality improvement strategy directed at reducing the total diagnostic interval (TDI) from a pre-campaign (2006) median of 14 (mean, 35.4) weeks to a target of 5 weeks in order to equal the best reported internationally. Professional and public awareness was measured by questionnaire surveys. TDI was collected by clinical champions in 18 regional children's cancer centers and the public campaign was coordinated by a national charity, working with a network of community champions.
Results
The guidelines and campaign raised awareness among pediatricians and were associated with reduction in TDI to a median of 6.7 (mean, 21.3) weeks by May 2013. This change in referral practice was most pronounced in the time from first medical contact to CNS imaging, for which the median was reduced from 3.3 to 1.4 weeks between January 2011 and May 2013 (P = .009).
Conclusion
This strategy to accelerate brain tumor diagnosis by the NHS using a public and professional awareness campaign is a “world first” in pediatric cancer and is being emulated internationally and acknowledged by a series of NHS and charity awards for excellence.
Keywords: awareness campaign, diagnostic delay, pediatric brain tumour, total diagnostic interval
Delayed diagnosis of brain tumors in children has been reported by parents and young people in the media, in Parliament,1,2 and in the courts. These reports have identified a disturbing lack of public confidence in UK health systems.3,4
Brain tumors account for a quarter of all childhood cancers, affecting 1 in 2400 children under the age of 16 annually in the UK, putting ∼32 000 life-years at risk.5 More children die of brain tumors than any other cancer, accounting for the loss of ∼10 000 life-years annually in the UK.5 Death occurs either as a result of catastrophic presentations with raised intracranial pressure or as a result of tumor recurrence and resistance to further treatment.6 The development of a specialist network of UK childhood brain tumor centers, working with centers across Europe, has improved survival rates by introducing new treatments through clinical trial programs over the past 3 decades.7 Five-year survival rates have risen from 50% to over 70%,8 and the majority of these patients go on to be long-term survivors. Despite this, 60% of long-term survivors of childhood brain tumors are moderately or severely neurologically disabled,9,10 accounting for an estimated gain of ∼20 000 disabled life-years annually.5
Against this backdrop, public and professional concerns about diagnostic delays precipitated an initiative to investigate the severity of the problem and develop an intervention to promote a measurable improvement in quality of care. In 2004 the Brain Pathways project was initiated. This project entailed a systematic literature review and meta-analysis of symptomatology and referral practice for childhood brain tumors6 and a UK 4-center cohort study, recording referral paths, symptomatology, and total diagnostic interval (TDI, time between first symptom onset and diagnosis) for newly diagnosed cases of brain tumor in children and young people under the age of 18.6 This work informed a Delphi consensus process aimed at producing revised guidelines11 for selection of patients for reassurance, timely review, or fast track referral to CNS imaging. The new clinical guidelines were developed according to the criteria of AGREE II (Appraisal of Guidelines for Research and Evaluation)12 and were endorsed and published by the Royal College of Paediatrics and Child Health (RCPCH) in 200813; they received NHS Evidence accreditation in 2011.
The Problem
Data from 4 pediatric neuro-oncology centers in 2004–2006 showed that TDIs ranged widely from a day to 6.9 years, with a median of 3.3 months (14 wk).14 UK studies were ranked in the lower half of international comparisons for TDI, with no discernible trend of improvement over time (1993–2006). A population-based study using cancer registrations linked to routine records from primary care (1989–2006, n = 181) and secondary care (1997–2006, n = 2959) in England also showed that primary care consultation rates rose 40-fold, from 3.1 per 100 person-months one year before diagnosis to 148.9 at diagnosis; and hospital admission rates rose 100-fold, from 1.3 per 100 person-months at one year before diagnosis to 134.0 at diagnosis.15,16 These data implied that neither the patient, family, nor the doctors were sufficiently aware of the risk or symptoms to confidently request CNS imaging during early symptom development.
A quality improvement program17 based on the RCPCH-endorsed guidelines was therefore designed to raise public and professional awareness of the symptomatology of brain tumors, using the TDI as the “driver for change” across the UK.
Materials and Methods
The “HeadSmart: Be Brain Tumour Aware” campaign, launched in June 2011, was a UK-wide intervention. The aim of the campaign was to raise awareness among the public and profession by distributing the symptom checklist, signposting to the website so that both the public and profession could access guidance to initiate medical assessment, and select patients for reassurance, review, or referral for CNS imaging. The driver for change that was selected for the quality improvement method17 was TDI, aiming for less than 5 weeks, equal to the best published result.6
Planning the Intervention
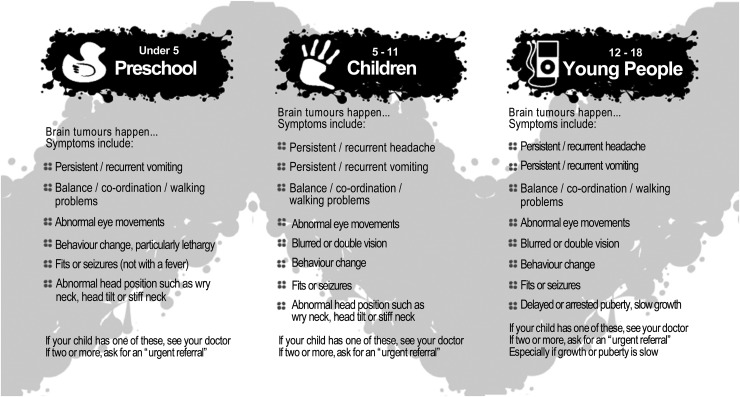
The prior systematic review and meta-analysis had identified age-related differences in symptoms, which, together with the Delphi consensus statements, were used to create age-stratified symptom lists for children under 5 years, aged 5–11 years, and aged 12–18 years (Fig. 1).6 The quality improvement strategy was therefore to spread the information to individuals, groups, and community networks (broader populations, the health care service system, political systems, and other stakeholders, such as relevant professional colleges and other brain tumor charities, etc) using different products and dissemination approaches.18
Fig. 1.
HeadSmart age-stratified symptom list.
HeadSmart Campaign Materials
We designed awareness materials, usable during a consultation and consisting of a handy age-stratified symptom checklist, with instructions that 1 symptom required medical assessment and 2 required an urgent referral (http://www.headsmart.org.uk/headsmart-materials/). HeadSmart materials were distributed to health care professionals via conferences and seminars and to general practitioner (GP) surgeries, health organizations, and professional bodies by direct mail. Materials were also distributed to the public through our community champions (directly to local schools, nurseries, GP surgeries, hospital waiting rooms, etc), as well as via local authorities and other charities and commercial networks (eg, a national chain of toy shops).
HeadSmart Website
We designed an open access decision support website (www.headsmart.org.uk) with complementary guidance and links to existing NHS and related health sites providing advice on relevant signs and symptoms, emphasizing where to reassure patients, which symptoms required timely review with recommendations for review intervals, and finally, which patients needed immediate imaging. The language of the website was carefully designed to be accessible and interpretable for a wide reading capability.
Community Champions, Network, and Social Media
Community champions are individuals and families around the country who have volunteered to be the “voice of the campaign” to help raise awareness in their local areas. They are recruited by our charity partner, The Brain Tumour Charity, through its website and social media to distribute symptom checklists in communities, schools, general practices, and local government and by using their personal contacts and experiences in print, radio, TV, and social media (http://www.thebraintumourcharity.org/awareness/community-champions).
As new community champions were recruited, The Brain Tumour Charity provided them with one-to-one telephone support to explain the purpose and history of the campaign. They were also provided with a toolkit made up of all the materials to support their role in explaining the HeadSmart message. This included a comprehensive guide to spreading the word (ie, advice on how to use social media effectively, lobby a Member of Parliament, and contact their local authority and give a good presentation), as well as HeadSmart materials. Our charity partner supplied printed resources as needed and provided backup if they had any difficulties.
Clinical Champions
A network of clinical champions was established through 18 UK Children's Cancer and Leukaemia Group (CCLG) treatment centers (www.cclg.org.uk). The clinical champion at each center was asked to collect contemporaneous TDI data for patients with new diagnoses and was provided with information and materials to distribute to regional primary and secondary care colleagues. Seventeen of the 18 centers participated. Clinical champions also received campaign newsletters reporting TDI data as they were collated.
Conferences and Education Outreach Events
Regular presentations about the campaign's progress were made at national professional meetings.
The acceptability and context of the RCPCH guidelines and associated awareness campaign among health professionals and the public were optimized by:
developing the guidelines using AGREE criteria12 and seeking National Institute for Health and Clinical Excellence accreditation;
timing the launch of the awareness campaign with the support of the National Awareness and Early Diagnosis Initiative19 as part of Improving Outcomes: A Strategy for Cancer20; and
linking the guidance to policies for childhood and cancer practice in England, Wales, and Scotland.2,19,21–27
Methods of Evaluation
Total diagnostic interval
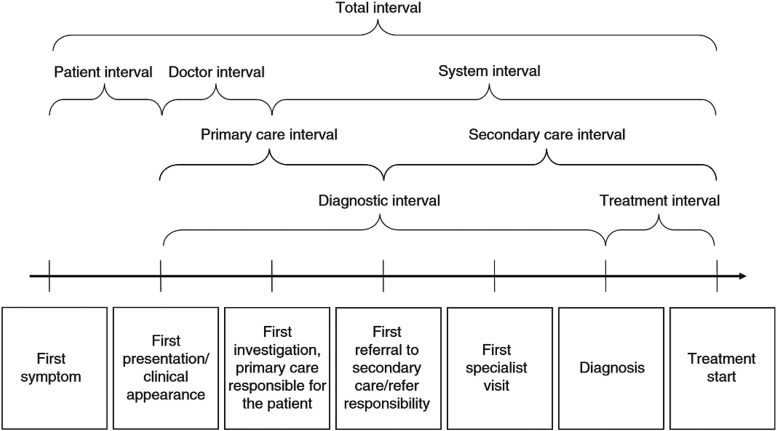
Clinical champions were asked to provide data on (i) date of symptom onset, (ii) date of initial presentation to health care, and (iii) date of diagnosis for each incident case diagnosed from January 2011. TDI was defined as the interval between symptom onset and diagnosis. “Patient interval” and “diagnostic interval” were monitored throughout the project period (see Fig. 2 for definitions).
Fig. 2.
Key milestones and time intervals in the pathways from first symptom until start of treatment (Weller et al 2012)28.
Public awareness
Two face-to-face National Opinion Poll omnibus surveys, using computer-assisted personal interviews, were conducted, in February 2011 and October 2011, to assess the levels of awareness of symptoms and signs of childhood brain tumors in the public before and after the HeadSmart media campaign launch.
Professional awareness
Two web-based surveys were distributed (in spring 2011 and autumn 2012) via pediatric, emergency, and GP professional email networks to assess practitioners' awareness of symptoms and signs of childhood brain tumors, as well as their self-rated confidence in diagnosing brain tumors.
Analysis
A monthly run chart of TDI was produced to track improvement. Kruskal–Wallis or Mann-Whitney U-tests were used to compare the differences of TDIs among different cohorts. Descriptive analysis was used to analyze data from public and professional awareness surveys. SPSS 22 was used for all statistical analyses of data.
Approval
HeadSmart: Be Brain Tumour Aware is a quality improvement project and awareness campaign that poses no risk to patients. The public awareness survey was approved by the Nottingham University Medical School Research Ethics Committee (reference no: A/12/2010).
Results
Total Diagnostic Interval
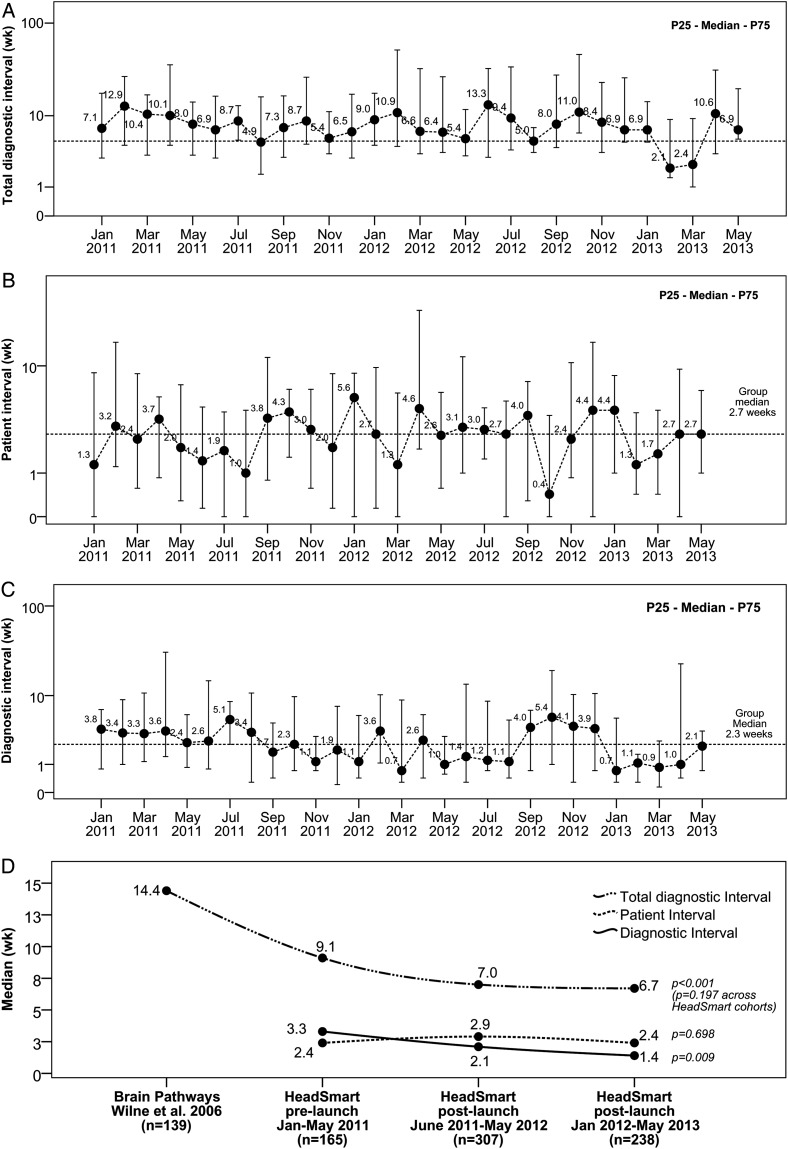
Clinical champions at 18 CCLG treatment centers submitted data on 710 patients diagnosed between January 2011 and May 2013. Monthly run charts of TDI, patient interval, and diagnostic interval are shown in Fig. 3.
Fig. 3.
Monthly run charts of all patients diagnosed from 6 months before HeadSmart campaign launch (January 2011) to 2 years post-launch (May 2013) and a comparison across 3 time periods. (A) Total diagnostic interval, time from symptom onset to diagnosis; (B) patient interval, time from symptom onset to first presentation to health care professionals; (C) diagnostic interval, time from first presentation to health care to diagnosis; (D) comparison across 3 time periods.
The median TDI was reduced from 9.1 (mean, 25.2) weeks (Jan–June 2011) pre-launch to 6.7 (mean, 21.3) weeks in the second year post-launch (2012–13) (Kruskal–Wallis test, P = .197; Fig. 3D). The change in referral practice was most pronounced in the median interval from first medical contact to the CNS imaging “diagnostic interval,” reduced from 3.3 (mean, 17.3) weeks to 1.4 (mean, 11.9) weeks (Kruskal–Wallis test, P = .009). No significant change in patient intervals was observed.
We also compared the HeadSmart second year data with our Brain Pathways multicenter study in 2006, which predated the publication of the RCPCH clinical guidelines in 2008. There was a significant improvement in median TDI (Mann–Whitney U-test, P < .001).
The range and frequency of presenting symptoms in the cohort of patients with diagnoses of brain tumor between January 2011 and May 2013 mirrored previous reports. Use of the “two-week wait” urgent referral pathway29 remained low, at about 2%–3% (data not shown).
Public Awareness
The omnibus survey sample is designed to be representative of adults over the age of 16 in the UK. Response rates were 59% (647 of 1105) and 56% (648 of 1154) in baseline and follow-up surveys, respectively. About 88% of respondents had regular contact with children or young people, and 15% were between 16 and 24 years old (Table 1).
Table 1.
Key results of the pre-launch public awareness baseline surveys (February 2011) and post-launch follow-up survey (September/October 2011)
| Baseline Survey (n = 647) | Follow-up Survey (n = 648) | |
|---|---|---|
| Sources of medical information if worried that a child might have serious condition |
|
|
|
Length of time before public would make an appointment to discuss high-risk symptoms with a doctor (a list of high-risk symptoms based on HeadSmart symptom card was provided) |
|
|
|
Percentage of public that are aware of the number of brain tumor incident cases (informed that it is 500 cases a year) |
|
NA |
| Percentage of public that were aware of warning signs of brain tumors |
High awareness (identified by >80% of respondents) Vomiting, headaches, seizures, and vision problems |
High awareness (identified by >80% of respondents) Vomiting, headaches, seizures, and vision problems |
|
50%–79% Deterioration in balance and behavior change |
50%–79% Deterioration in balance and behavior change |
|
|
Low awareness (identified by <50% of respondents) Abnormal head position, 46% Lethargy, 45% Delayed puberty or slow growth,14% Excessive thirst, 12% Passing a lot of urine, 11% |
Low awareness (identified by <50% of respondents) Abnormal head position, 44% Lethargy, 46% Passing a lot of urine, 9% Excessive thirst, 13% Early/delayed puberty or slow growth, 13% |
When shown a list of high-risk symptoms based on the HeadSmart symptom card (Fig. 2), 70% of respondents would seek medical advice on all symptoms within 2 weeks, except delayed puberty and/or slow growth. Over 50% of respondents thought that deterioration in balance or behavior change was a warning sign.
HeadSmart's launch day in June 2011 saw a series of broadcast highlights that reached an estimated 14 million people. Results from our public awareness survey showed that 89% (range, 74%–94% depending on respondent's age group, data not shown) of the general population aged 16 years and above had regular contact with children or young people, either a child of their own, in the family, someone they knew from work, or a child of a friend or close family contact. We therefore estimate that at least 74% of the 14 million reached subjects were our target population. The HeadSmart post-launch surveys in 2011 and the 2014 Charity Awareness Monitor Survey revealed that 11% and 15%, respectively, of the population were aware of HeadSmart. From the 2014 survey, awareness among adults with children under 18 was 20%.
Professional Awareness
Over 300 health care professionals took part in the 2 surveys (pre-launch in 2011 and 3 mo post-launch in 2011), of which two thirds were pediatricians (Table 2). Prior to the launch overall, 63% thought that for current practice, the average time to diagnosis was 3 months or longer. The symptom with the lowest awareness level was abnormal head position or head tilt.
Table 2.
Key results of the pre-launch professional baseline surveys (March/April 2011) and post-launch follow-up survey (November 2011–February 2012)
| Baseline Survey (n = 323) | Follow-up Survey (n = 340) | |
| Area of medicine practiced |
|
|
| Confidence in ability to recognize when a child might have a brain tumor |
|
|
| View on the average symptom interval of children in the UK |
|
|
|
Respondents' opinion on the statement: “A prolonged symptom interval in childhood brain tumors is associated with worse outcome” |
|
|
|
Symptoms that could be a sign of a childhood brain tumor (Identify from a list of 15 symptoms; may or may not be specifically related to brain tumor) |
|
|
| Respondents’ opinion on: “children with brain tumors have multiple signs and symptoms” |
|
|
After launch, self-rated diagnostic confidence rose from 32% to 54% for pediatricians but remained low for GPs (11% to 12%). Awareness of HeadSmart materials was higher among pediatricians (73%) compared with GPs (26%). Most respondents (>60%) had heard of the campaign through their professional body. Up to 37% had seen or used the HeadSmart materials in practice; over 66% thought the materials to be useful.
Other Outcomes
The HeadSmart webpage currently has a total of 34 999 likes on Facebook and 2253 followers on Twitter. The website has nearly 12 000 visits per month, and mobile symptom cards have now been accessed 2767 times. In 2014/15 we encountered a rising number of patients reporting that they had used the HeadSmart checklist during clinical consultations leading to diagnostic referrals for scanning in primary and secondary care.
Meetings have been held in the UK and EU Parliaments, where political representatives have pledged support for the campaign. There was also a Westminster Hall Debate in Parliament in September 2013.1 The campaign materials and messages are being prepared for dissemination in at least 6 countries beyond the UK.30
The campaign has also been supported by Glenis Willmott, Member of the European Parliament, in Brussels, and was discussed by that body as part of Childhood Cancer Awareness Week in 2013.31
Discussion
We are unaware of previous population-based attempts to accelerate diagnosis in childhood brain tumors worldwide. We selected a quality improvement approach, using an awareness intervention, as this offered the opportunity to change practice. Alternative research strategies identifying red flag symptoms have been explored. However, they do not yield significant positive predictive values for individual symptoms that can be used for stratifying childhood patients in primary care.32 We rejected a randomized trial due to concerns about denying children the benefits of enhanced awareness in this life-threatening and disabling disease and the risk of contamination between geographical regions of awareness strategies. We placed the risk of excessive public alarm and the potential for swamping imaging facilities at the top of our risk assessment and designed our materials to prioritize reassurance. We are unaware of any evidence of public alarm or excess imaging referrals and received appreciative feedback from pediatricians, who valued the reassuring advice for those children who did not require a brain scan.
This report describes the methodology and impact of the RCPCH guidance document and the HeadSmart campaign, designed to amplify the impact of the RCPCH clinical guidelines.13 At the end of the project in 2013 we observed a significant change in TDI from 14.4 weeks in 2008, prior to the publication of the RCPCH guidelines, to 6.7 weeks.
We have been promoting early diagnosis of pediatric brain tumors for over 10 years. The TDI data prior to the publication of the RCPCH guidelines was stable in the published UK studies. The changes in TDI after 2006, prior to the HeadSmart launch, suggested that practice was changing, perhaps related to enhanced awareness surrounding the development and publication of the RCPCH clinical guidelines in 2008. At this time, high-profile cases were announced attracting media attention as well as government initiatives prioritizing early cancer diagnosis (the National Awareness and Early Diagnosis Initiative) and perhaps easier access to imaging.33
The differential impact of the campaign on awareness between GPs and pediatricians in our surveys at 3 to 6 months after HeadSmart launch is worthy of note. The number of respondents varied between the 2 groups, and the dissemination campaign had actively targeted pediatricians through RCPCH-directed communications to all pediatricians across the UK, while the GPs were targeted in some regions but not in others and the professional email network of the Royal College of General Practitioners was not used, for policy reasons. The follow-up surveys, however, did provide useful information regarding the impact of the media launch by identifying the most useful campaign material(s) for health care professionals and as an indication of professional awareness improvement among pediatricians. While there have been measurable improvements in the “diagnostic interval” suggesting prioritized referrals, there has not, as yet, been a significant improvement in the “patient interval” by the end of year 2, although there are a rising number of reports where the HeadSmart checklist is being used during clinical consultations, which we believe is evidence of campaign materials having reached the targeted population and influenced awareness during the consultation as originally intended.
To measure the net effect of the HeadSmart campaign is methodologically challenging. The nationwide approach we took means that there was no control or comparison community. The assessment requires additional information on all relevant confounding factors, such as introduction of new policies or guidelines; MRI service availability; changes in health care service; education and public awareness of the importance of early diagnosis overall; etc. There were a few campaigns promoting early diagnosis during the project period, but none of them were for adult or pediatric brain tumors; and there was no change in policies affecting the referral pathway. Inequalities in health care access may potentially have a negative impact on time to diagnosis; unfortunately, we were not able to measure or quantify such impact with our current dataset. Our dataset represents an estimated 75% of the predicted incident cases, suggesting that it is representative of the UK pediatric brain tumor population.
In the absence of control or comparator, continuous measurement of the desired outcome is the only way to provide the evidence of the change. In order to sustain momentum, we have negotiated that “time to diagnosis” be a compulsory cancer registry data item; recent reviews have identified that this is now available in 30% of childhood brain tumor cases registered by the National Cancer Intelligence Network or brain tumor registry check. We are currently working on using linked population-based data from the National Cancer Registry, the Clinical Practice Research Datalink, and Hospital Episode Statistics, which provide objective descriptions of the pathways of referral.15
The TDI data showed a large difference between median and mean values, indicating right-skewed distribution of the curve due to very prolonged intervals in some. Those falling into the top quartile (TDI ≥ 20.1 wk) or ranked above the 90th percentile (54 wk to maximum 435 wk) are of particular interest and are the focus of a separate study. The reduction in TDI was associated with some closing of the mean to median differences, indicating a possible preferential effect on very long delays.
It is not feasible to categorize patients into GP referred, pediatrician referred, or accident and emergency (A&E) cases, as the range of referral routes to CNS imaging is so diverse, and diagnostic pathways of childhood brain tumor are complex—they are rarely linear, in most cases involving consultation with and referral between multiple health care professionals, including GPs, general and specialty pediatricians, opticians, optometrists, ophthalmologists, paramedics, A&E specialists, etc. HeadSmart chose to monitor “place of care at the time of imaging.” We observed that more patients with TDI ≥ 20.1 weeks were outpatients when they had their CNS scan (104/171, 60.8%), while patients in the lowest TDI quartile were mainly under the care of A&E (58/173, 33.5%) or were inpatients (71/173, 41.0%).
We were greatly assisted by funding for HeadSmart branding and the media launch by The Health Foundation and The Brain Tumour Charity. Brain Pathways was renamed HeadSmart: Be Brain Tumour Aware as part of that marketing process. The networks of clinical and community champions exhibited widespread enthusiasm, creating a groundswell of support for the campaign. While we have been able to demonstrate clear evidence of enhanced awareness among pediatricians, this was not demonstrable in general practice. The 2015 National Institute for Health and Clinical Excellence Cancer Referral Guidance has recently been published.34 Although HeadSmart had been accepted as a stakeholder, the experience of the campaign was not included in the evidence to drive adjustments to the campaign referral guidelines for childhood brain tumors. The model adopted for the NICE guidelines relied upon positive predictive values for individual symptoms, which did not meet the significance threshold to act as a justification for change of the established guidance launched with a consensus method in 2005. It still does not identify scanning as the endpoint for referral. The National Cancer Intelligence Network (NCIN) has agreed to record TDI for all new childhood tumor registrations. The Royal College of General Practitioners launched a web-based training package on childhood brain tumor diagnosis in 2013 (“Brain Tumours in Children”. This online course is available through the Cancer Education Hub at the following link: http://elearning.rcgp.org.uk/course/), which has attracted 1938 users in 18 months, receives positive reviews, and is associated with measurable increase in knowledge scores by those completing the training.
Dixon Woods in her work on quality improvement in clinical communities35 identifies the importance of considering quality improvement methodology not so much as a science, but more as an intervention within a defined community using a clearly articulated program, including theory of change and harnessing the right resources and training. However, its effectiveness is dependent upon motivating the community and dealing successfully with conflict. The selection and collection of data as a driver for change is central to any change in practice acceptability.
In this project, the community that was the focus of change was the UK childhood population, their families, and health practitioners, aided by the advocacy of community and clinical champions. Community champions were supported by the high profile of the campaign, their personal awareness of the problem, and the clear theory of change that was promoted, that is, enhancement of public and professional awareness within the clinical consultation using campaign materials and providing a focus on TDI to accelerate diagnosis at a time when health policy was supporting change in both pediatric and cancer care.21–27
The opportunity to identify the groups for whom it was most and least effective supports the original theories of change developed for the intervention. The lack of change in the “patient interval” highlights the need to reconsider the approach to the public, although the recent rising reports of materials being used in consultations indicates that there is a reservoir of materials now in the population acting as timely reminders, justifying ongoing prioritization for community champions.
Conclusions
This quality improvement program has delivered change in NHS practice, using a public and professional awareness campaign about a rare childhood condition as the intervention. It was necessary to strike a balance between raising awareness and avoiding public alarm. We have evidence of enhanced public and professional awareness and a measurable change in TDI compatible with our theory of change and predictions, based upon our knowledge of the range of conditions that constitute childhood brain tumor. The program is ongoing; the adolescent population and GPs are priorities for the campaign relaunch. We are in the process of reviewing the evidence base for the guideline and refocusing the campaign to tackle areas of greatest impact. We are working with linked population databases16 to validate our TDI data and investigate evidence for impact upon survival and disability.
Awards
NHS Innovation Challenge Prize 2013
-
Charity Times Award for Best Cross-Sector Partnership of the Year 2013
The judges praised HeadSmart as “an outstanding partnership making a real impact through crucial early diagnosis of brain tumours: it was a thoroughly researched, well-planned and innovative project to engage healthcare professionals as well as raise public awareness.”
-
Third Sector Excellence Award 2013
This award was given in the Charity Partnership category.
-
Quality in Care Cancer Charity Initiative of the Year 2013
This award recognizes the best in oncology health care practice and is supported by partners in industry, NHS, and charities.
-
Association of Medical Research Charities (AMRC) Science Communication Award
This award celebrates the way the AMRC'S leading research charities communicate and engage with patients and the public; given every 2 years.
-
Finalist, the National Lottery Award 2014 Health Category
We competed against 6 other projects for public votes in the Health category.
HeadSmart: Be Brain Tumour Aware—Group Authorship
David Walker: Principal Investigator (PI); co-director CBTRC; co-designer of HeadSmart website; content editing of clinical awareness materials, web-based training packages; interpretation of analysis; drafting of manuscript.
Sophie Wilne: co-PI; Research Fellow Brain Pathways project; first author of linked publications including CBTRC clinical guideline; co-designer of HeadSmart website; content editing of clinical awareness materials and web-based training packages; interpretation of analysis; coordination of clinical champions; drafting of manuscript.
Richard Grundy: member of Brain Pathways and HeadSmart Project Boards; co-designer of clinical awareness materials; interpretation of analysis; drafting of manuscript.
Colin Kennedy: member of Brain Pathways and HeadSmart Project Boards; co-designer of clinical awareness materials; interpretation of analysis; drafting of manuscript.
Neil and Angela Dickson: founders of Samantha Dickson Brain Tumour Trust (SDBTT), now The Brain Tumour Charity; applicant to Community Fund for Brain Pathways project; members of Brain Pathways and HeadSmart project boards; lead community champions.
Sarah Lindsell (2011–now): CEO of The Brain Tumour Charity (formerly SDBTT); Member HeadSmart Project Board.
Julia Trusler (2009–12), Alison Evans (2012–now): Head of Research and Policy of The Brain Tumour Charity (formerly SDBTT); member of HeadSmart Project Board; content editing of HeadSmart website.
Jan Dudley: Chair of HeadSmart Project Board; representative of RCPCH Academic Board.
Alistair Thomson: Chair RCPCH Academic Board.
Monica Lakhanpaul: representative Health Services Committee RCPCH.
Lucy Clough: Project Manager HeadSmart.
Maureen Baker: Royal College of General Practice Steering Group Representative on HeadSmart Project Board, now President RCGP; drafting of manuscript.
Thomas Chu: PhD student at the London School of Hygiene & Tropical Medicine; external epidemiology advisor to HeadSmart board (2010–13); CBTRC HeadSmart Research Fellowship (2013–now); member of HeadSmart Project Board.
Jo-Fen Liu: CBTRC coordinator; member of HeadSmart Project Board responsible for professional and public surveys; data management and analysis.
Emma Pearson: CBTRC fundraiser.
Emma Rayner and Emma Thorne: Public Relations and Communications advisors, University of Nottingham.
Sue Franklin: CBTRC Secretary; project management support.
Clinical Champions (by CCLG center)
Clinical champion, treatment center: Veronica Neefjes, Royal Aberdeen Children's Hospital; Anthony McCarthy, Royal Belfast Hospital for Sick Children; Martin English, Birmingham Children's Hospital; Steve Lowis, Rachel Perrow, Bristol Royal Hospital for Children; Matthew Garnett, Addenbrooke's Hospital; Cathy Morley Jacob, Heidi Traunecker, Children's Hospital for Wales; Jane Pears, Our Lady's Children's Hospital; Alf Nicholson, Temple Street Children's University Hospital; Hamish Wallace, Mark Brougham, Royal Hospital for Sick Children, Edinburgh; Jairam Sastry, Royal Hospital for Sick Children, Glasgow; Antony Michalski, Great Ormond Street Hospital; Simone Wilkins, Leeds Children's Hospital; Barry Pizer, James Hayden, Alder Hey Children's Hospital; Eddy Estlin, Royal Manchester Children's Hospital; Juliet Hale, The Great North Children's hospital; Sophie Wilne, Nottingham Children's Hospital; Denise Tritton, John Radcliffe Hospital; Darren Hargrave, The Royal Marsden Hospital; Vicki Lee, Sheffield Children's Hospital.
Funding
The project is funded by The Health Foundation Closing the Gaps Award, The Brain Tumour Charity, and Children′s Brain Tumour Research Centre, University of Nottingham.
Acknowledgments
The authors would like to thank The Health Foundation, the professional colleges that endorsed the RCPCH guidelines and the HeadSmart campaign, the National Cancer Registration Service, Children's Cancer and Leukaemia Group, and all clinical and community champions for their support to the HeadSmart project.
Conflict of interest statement. None declared.
Contributor Information
Collaborators: HeadSmart: Be Brain Tumour Aware, David Walker, Sophie Wilne, Richard Grundy, Colin Kennedy, Neil, Angela Dickson, Sarah Lindsell, Julia Trusler, Alison Evans, Jan Dudley, Alistair Thomson, Monica Lakhanpaul, Lucy Clough, Maureen Baker, Thomas Chu, Jo-Fen Liu, Emma Pearson, Emma Rayner, Emma Thorne, and Sue Franklin
References
- 1.House of Commons Hansard. Brain tumours in children; 2013. http://www.publications.parliament.uk/pa/cm201314/cmhansrd/cm130903/halltext/130903h0002.htm#13090344000003. Accessed September 11, 2015.
- 2.Brain Tumour Action. A manifesto for everyone affected by a brain tumour; 2010.
- 3.Jimmyteens.tv. All they need is a scan; 2012. http://jimmyteens.tv/2012/04/20/all-they-need-is-a-scan/. Accessed September 11, 2015.
- 4.HeadSmart. Stories of families affected by a brain tumour; 2011. http://www.headsmart.org.uk/personal-stories/. Accessed September 11, 2015.
- 5.Stiller C. Childhood Cancer in Britain: Incidence, Survival and Mortality. Oxford: Oxford University Press; 2007. [Google Scholar]
- 6.Wilne S, Collier J, Kennedy C, et al. Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncol. 2007;8(8):685–695. [DOI] [PubMed] [Google Scholar]
- 7.Doz F, Picton S, Rutkowski S, et al. SIOP brain tumour trials. Eur J Cancer Suppl. 2009;7(2):26. [Google Scholar]
- 8.Cancer Research UK. Childhood cancer incidence statistics; 2012. http://www.cancerresearchuk.org/cancer-info/cancerstats/childhoodcancer/incidence/. Accessed September 11, 2015.
- 9.Boman K, Hovén E, Anclair M, et al. Health and persistent functional late effects in adult survivors of childhood CNS tumours: a population-based cohort study. Eur J Cancer. 2009;45(14):2552–2561. [DOI] [PubMed] [Google Scholar]
- 10.Packer R, Gurney J, Punyko J, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(17):3255–3261. [DOI] [PubMed] [Google Scholar]
- 11.Wilne S, Koller K, Collier J, et al. The diagnosis of brain tumours in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Arch Dis Child. 2010;95(7):534–539. [DOI] [PubMed] [Google Scholar]
- 12.Brouwers M, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 2010;182(18):E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royal College of Paediatric and Child Health. The Brain Pathways Guideline: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour; 2008.
- 14.Wilne S, Collier J, Kennedy C, et al. Progression from first symptom to diagnosis in childhood brain tumours. Eur J Pediatr. 2012;171(1):87–93. [DOI] [PubMed] [Google Scholar]
- 15.Chu TP, Shah A, Walker D, Coleman MP. Pattern of symptoms and signs of primary intracranial tumours in children and young adults: a record linkage study. Arch Dis Child. 2015. DOI:10.1136/archdischild-2014-307578. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Chu T. Pattern of Presentation in Hospital and Primary Care in Children and Young Adults with an Intracranial Tumour. London: London School of Hygiene & Tropical Medicine, University of London; 2012. [Google Scholar]
- 17.NHS Institute for Innovation and Improvement. Quality and service improvement tools for the NHS; 2010. [Google Scholar]
- 18.de Silva D. Spreading Improvement Ideas – Tips from Empirical Research. London: The Health Foundation; 2014. [Google Scholar]
- 19.Richards M. The National Awareness & Early Diagnosis Initiative in England: assembling the evidence. Br J Cancer. 2009;101(suppl.):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health & Clinical Excellence. Service Guidance for Improving Outcomes for People with Brain and Other Central Nervous System Tumours. London: National Institute for Health & Clinical Excellence; 2006. [Google Scholar]
- 21.Department of Health. Equity and Excellence: Liberating the NHS. London: Department of Health; 2010. [Google Scholar]
- 22.Department of Health. Achieving Equity and Excellence for Children; 2010. [Google Scholar]
- 23.Department of Health. Improving Outcomes: A Strategy for Cancer; 2011. [Google Scholar]
- 24.Kennedy I. Getting It Right for Children and Young People: Overcoming Cultural Barriers in the NHS so As to Meet Their Needs. Department of Health; 2010. [Google Scholar]
- 25.National Patient Safety Agency. Delayed Diagnosis of Cancer: Thematic Review; 2010.
- 26.The Scottish Government. Detect cancer early; 2012. http://www.scotland.gov.uk/Topics/Health/Services/Cancer/Detect-Cancer-Early. Accessed September 11, 2015.
- 27.Welsh Government. Together for health, cancer delivery plan for the NHS to 2016; 2012. http://wales.gov.uk/topics/health/publications/health/strategies/cancer/?lang=en. Accessed September 11, 2015.
- 28.Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106(7):1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Department of Health. The NHS Cancer Plan: A Plan for Investment, A Plan for Reform; 2000.
- 30.eCancer. HeadSmart child brain tumour awareness initiative launched in Europe; 2012. http://ecancer.org/news/2519-headsmart-child-brain-tumour-awareness-initiative-launched-in-europe.php. Accessed September 11, 2015.
- 31.Glenis Willmott MEP. http://www.europarl.europa.eu/meps/en/35743/GLENIS_WILLMOTT_home.html. Accessed September 11, 2015.
- 32.Dommett RM, Redaniel T, Stevens MC, et al. Risk of childhood cancer with symptoms in primary care: a population-based case-control study. Br J Gen Pract. 2013;63(606):e22–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Awareness and Early Diagnosis Initiative (NAEDI). http://www.cancerresearchuk.org/health-professional/early-diagnosis-activities/national-awareness-and-early-diagnosis-initiative-naedi. Accessed September 11, 2015. [DOI] [PMC free article] [PubMed]
- 34.National Institute for Health & Care Excellence. Suspected Cancer: Recognition and Referral; 2015. [PubMed]
- 35.Aveling EL, Martin G, Armstrong N, et al. Quality improvement through clinical communities: eight lessons for practice. J Health Organ Manag. 2012;26(2):158–174. [DOI] [PubMed] [Google Scholar]