Decades of research have greatly increased our understanding of genetic alterations in cancer.1 Tumors of the CNS are no exception, and we now have fairly sophisticated understanding of the major genetic alterations.2,3 Testing for certain genetic abnormalities, such as 1p/19q co-deletion, has been used in the clinical setting for many years, whereas others, such as isocitrate dehydrogenase (IDH)1/2 mutations, have been in clinical use for shorter periods of time.4 Recent advances in sequencing technologies have led to a plethora of studies that have greatly facilitated the understanding of genetic alterations in CNS neoplasms, further refining their classification.3,5–8 These advances include the discovery of BRAF-KIAA1549 and BRAFV600E alterations in the majority of pilocytic astrocytomas, IDH mutations and 1p/19q co-deletions in oligodendrogliomas, IDH mutations with TP53 and/or ATRX mutations in astrocytic diffuse gliomas, TERT promoter mutations in meningiomas, and WNT and SHH pathway alterations in medulloblastomas.8–11 Despite the progress made in recent years, translating these advances in sequencing technologies into meaningful clinical information for patients with CNS malignancies remains challenging. One can anticipate that we will continue to see further improvements in tumor classification and better definition of prognostic tumor subclasses(to help tailor clinical management) as clinical molecular diagnostics continue to evolve in the field of neuro-oncology.12
Molecular subclassification of brain tumors has identified important subgroups of gliomas and medulloblastomas with better prognoses (Fig. 1).3,5,10 The excellent prognosis of a subgroup of patients with medulloblastoma has allowed neuro-oncologists to consider reduced-intensity treatment for specific, molecularly defined medulloblastoma patients (NCT01878617). BRAF inhibitors have been considered as treatment for ganglioglioma and pleomorphic xanthoastrocytoma carrying the BRAFV600E mutation.13,14 There is no doubt that the benefits of molecular characterization of brain tumors are slowly making their way into the clinical arena and influencing patient management. The validation of a clinical test, tailored to detect genetic alterations in CNS neoplasms, is an extremely important step prior to its use in the clinic and its incorporation into defining personalized treatment strategies for patients with brain tumors.
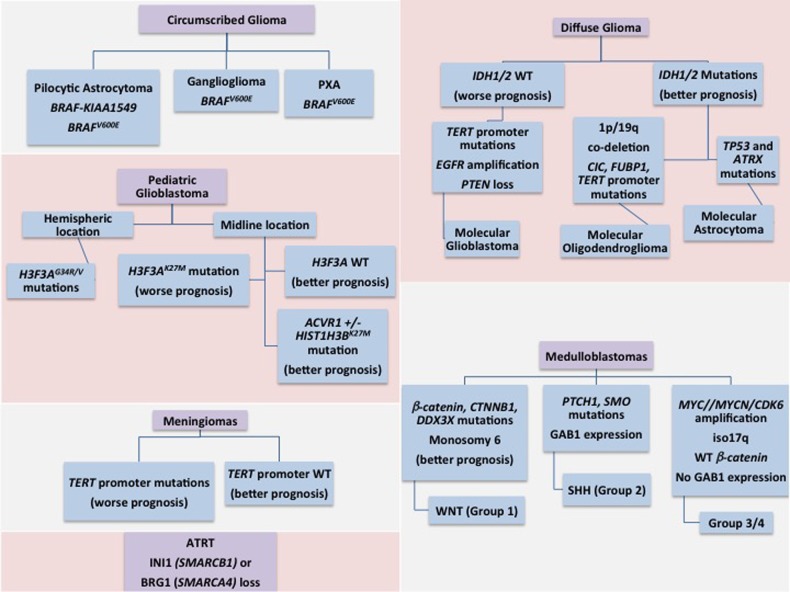
Fig. 1.
Simplified view of current key molecular alterations in CNS neoplasms with diagnostic, prognostic, or predictive utility. The current landscape of important biomarkers in CNS neoplasms is rapidly changing as the importance of additional markers is established. Comprehensive molecular profiling with next-generation sequencing technologies will likely soon lead to more refined molecular classification of CNS tumors. Abbreviations: ATRT, atypical teratoid rhabdoid tumor; PXA, pleomorphic xanthoastrocytoma; SHH, sonic hedgehog pathway; WNT, wnt signaling pathway.
The authors of the study by Nikiforova et al. have elegantly designed and validated a customized, amplification-based, targeted next-generation sequencing (NGS) assay for detecting a wide range of genetic alterations in CNS neoplasms. The customized multigene NGS panel targets hotspots in 30 genes of known relevance for CNS tumors to detect mutations (ie, point mutations and small insertion/deletions) as well as gene fusions and copy number changes through a single assay. By using stringent criteria of at least 50% tumor cells in the tissue samples, the NGS assay was able to detect all of the known genetic alterations present in the validation sample set (100% sensitivity). Although it remains to be seen how well NGS assays will perform when testing an array of clinical specimens with lower tumor content, the current results are certainly encouraging. Importantly, the authors showed reliable results for both frozen and formalin-fixed, paraffin-embedded (FFPE) tissues. This is of particular relevance in analysis of clinical samples since the vast majority of specimens analyzed are FFPE tissue.
In the current era of rapidly evolving sequencing technology, the financial and technical limitations of simultaneously testing for multiple genes have been drastically reduced. However, in the absence of updated patient management algorithms that utilize the vast amounts of genomic data generated by NGS assays, the clinical utility of routine NGS testing for brain tumors remains limited. Currently, mutations in IDH, TP53, ATRX, and 1p/19q co-deletion are sufficient for the accurate molecular classification of diffuse gliomas. Testing for the BRAF-KIAA1549 fusion could serve as an adjuvant diagnostic marker for a pilocytic astrocytoma with ambiguous histologic features,15 and the presence of a BRAFV600E mutation in an optic pathway glioma could open the possibility of using a targeted BRAF inhibitor for an otherwise nonresectable tumor.
Today we have an unprecedented ability to perform genetic profiling of brain tumors in a fast, accurate, and cost-effective manner, as demonstrated in the current article by Nikiforova et al. The questions of when to test, which tumor to test, and what alterations should be tested for have a set of complex and rapidly evolving answers. For patients with brain tumors, clinical testing for predictive markers is currently limited, and most of the molecular testing relates to defining subgroups with prognostic significance.5,8
Several ongoing studies including early phase clinical trials using IDH inhibitors in gliomas, however, are currently underway and may help unmask the clinical significance of some of these markers (clinicaltrials.gov). In addition, it is expected that multigene NGS panels will soon be incorporated into clinical trials to facilitate patient stratification into groups with prognostic or predictive relevance based on the molecular characteristics of the tumors. Nonetheless, more studies integrating patient outcome data and clinical trials with targeted agents are needed to advance the field. As the use of comprehensive NGS analysis becomes an integral part of the personalized clinical management of patients with CNS malignancies, we should also familiarize ourselves with the strengths and weaknesses of NGS methodologies to successfully take advantage of its full potential in everyday clinical practice.
References
- 1.Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frattini V, Trifonov V, Chan JM, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan CW, Verhaak RGW, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan K-M, Fang D, Gan H, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur K, Kakkar A, Kumar A, et al. Integrating Molecular Subclassification of Medulloblastomas into Routine Clinical Practice: A Simplified Approach. Brain Pathol. 2015 doi:10.1111/bpa.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones DTW, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skowron P, Ramaswamy V, Taylor MD. Genetic and molecular alterations across medulloblastoma subgroups. J Mol Med. 2015;93(10):1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2015;108(5). doi:10.1093/jnci/djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis DN, Perry A, Burger P, et al. International Society of Neuropathology-Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Bufalo F, Carai A, Figà-Talamanca L, et al. Response of recurrent BRAFV600E mutated ganglioglioma to Vemurafenib as single agent. J Transl Med. 2014;12:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neurooncol. 2013;114(2):237–240. [DOI] [PubMed] [Google Scholar]
- 15.Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401–405. [DOI] [PubMed] [Google Scholar]