Abstract
Background
One of the most consistent features of the autism spectrum disorders (ASDs) is the predominance among males, with approximately four males to every female. We sought to examine sex differences among children who met case definition for ASD in a large, population-based cohort with respect to age at first developmental evaluation, age of diagnosis, influence of cognitive impairment on these outcomes, and sex-specific behavioral characteristics.
Methods
We conducted a secondary analysis of data collected for a population-based study of the prevalence of ASD. The sample comprised 2,568 children born in 1994 who met the case definition of ASD as established by the Autism and Developmental Disabilities Monitoring (ADDM) Network for ASD surveillance. Children who had a history of developmental disability and behavioral features consistent with the DSM-IV-TR criteria for autistic disorder, Asperger's disorder, and Pervasive Developmental Disorder–Not Otherwise Specified in existing evaluation records were classified as ASD cases via two paths: streamlined and nonstreamlined. Streamlined reviews were conducted if there was an ASD diagnosis documented in the records. Data were collected in 13 sites across the United States through the ADDM Network, funded by the Centers for Disease Control and Prevention.
Results
Males constituted 81% of the sample. There were no differences by sex in average age at first evaluation or average age of diagnosis among those with an existing documented chart diagnosis of an ASD. Girls were less likely than boys to have a documented diagnosis (odds ratio [OR] = 0.76, p = .004). This analysis was adjusted for cognitive impairment status. In the logistic model, with the interaction term for sex and cognitive impairment, girls with IQ of 70 or less were less likely than boys with IQ of 70 or less to have a documented diagnosis (OR = 0.70, 95% confidence interval [CI] = 0.50–0.97, p = .035). Boys with IQ greater than 70 were less likely than boys with IQ of 70 or less to have a documented diagnosis (OR = 0.60, 95% CI = 0.49–0.74, p < .001). This finding (less likely to have a documented diagnosis) was also true for girls with IQ greater than 70 (OR = 0.45, 95% CI = 0.32–0.66, p < .001). Girls were more likely to have notations of seizure-like behavior (p < .001). Boys were more likely to have notations of hyperactivity or a short attention span and aggressive behavior (p < .01).
Conclusions
Girls, especially those without cognitive impairment, may be formally identified at a later age than boys. This may delay referral for early intervention. Community education efforts should alert clinicians and parents to the potential of ASDs in boys and girls.
Keywords: Autism spectrum disorder, Children, Sex differences
Autism spectrum disorders (ASDs) are a group of developmental disorders characterized by deficits in socialization, communication, and restricted interests and repetitive or stereotyped behaviors. ASDs include autistic disorder, Asperger's disorder, and Pervasive Developmental Disorder–Not Otherwise Specified (PDD-NOS). Recent epidemiologic studies have confirmed that ASDs are more common than previously thought, with a rate of approximately 6 to 7 per 1,000 children reported in the literature [1–3]. The most recent estimates in the United States are as high as 10.6 per 1,000 overall and range between 5.0 to 16.8 per 1,000 males and 1.4 to 4.0 per 1,000 females [4]. Early identification of children with ASDs leads to earlier treatment, which is associated with improved developmental outcomes [5]; however, the median age of diagnosis in multiple areas of the United States was found to be between 4.5 and 5.5 years of age [4,6].
A sex difference in the prevalence of ASDs has been well documented in epidemiologic studies since the 1960s, and males with an ASD outnumber females with an ASD by a ratio of about 4–5:1 [7–12]. All subtypes of ASDs are more commonly observed in males with the exception of Rett syndrome, an X-linked dominant neuro-developmental disorder that affects predominantly females. However, there are limited data that describe patterns of identification and behavioral features relative to the sex of the child in population-based, rather than clinically based, cohorts.
The purpose of this paper is to describe patterns of identification and diagnosis and behavioral features relative to the sex of the child who is identified as having ASD. In the context of this paper, cases of ASD refer only to those individuals who meet the case status criteria as applied by the Autism and Developmental Disabilities Monitoring (ADDM) Network of the Centers for Disease Control and Prevention (CDC) [4] for 8-year-old children in the surveillance year 2002. These criteria are described in detail in Methods and were used to identify a surveillance (not clinical) case of ASD [4,6]. In addition, we discuss ways in which this information can be used to inform clinical judgments.
Background
Diagnosing autism spectrum disorders—state of the science
Since autism was first described by Kanner [13], there have been multiple adaptations of diagnostic criteria and significant changes to the classification system. Each adaptation has expanded the criteria and qualified the differentiation of autism from other disorders, such as intellectual disability (ID) and schizophrenia. The DSM-IV [14] expanded the concept of pervasive developmental disorders (PDDs) to include autistic disorder, Asperger's disorder, Rett syndrome, childhood disintegrative disorder, and PDD-NOS. For the DSM-IV [14], 12 criteria are provided in three areas: social interaction, communication, and repetitive or restricted behaviors or interests. For a diagnosis of autistic disorder, there must be 6 of the 12 criteria, with a least 2 indicating social interaction deficits, and 1 criterion in each of the communication and stereotyped patterns of behavior. Most recently, the DSM-IV-TR [15] included minor changes to clarify the criteria for PDD-NOS. The International Classification of Diseases (ICD) criteria for the autism spectrum of disorders are comparable to DSM-IV. In addition, there have been practice parameters that state the diagnosis of an ASD should involve detailed birth, medical, and developmental histories; physical and neurologic examinations; parent interviews; child observations; and clinical judgment. These parameters, along with DSM and ICD criteria, continue to be the gold standard in ASD assessment and diagnosis.
To meet diagnostic criteria for autism, symptoms must be present before the age of 3 years, although symptoms are often apparent before the age of 2 years [16]. Furthermore, diagnosis of an ASD by a qualified professional remains relatively stable from 2 years to middle childhood [17,18]. Early identification and diagnosis of children with ASDs are important because early identification leads to earlier treatment, which is associated with improved developmental outcomes [5]. Furthermore, screening for ASD has been sporadic and generally not implemented in clinical practice until most recently, when screening instruments have been tested and validated. Screening for other than developmental delays was not a priority until recently with the advent of the Children's Health Act [19], which mandates establishing the ADDM Network and the publication of reliable and valid instruments to assess risk [20,21].
Sex differences in evaluation and classification
Although a sex difference in the prevalence of ASDs is consistently reported in the literature, little is known about the relationship between sex and the evaluation and classification of children with ASDs. For instance, diagnostic criteria do not include consideration of sex and few studies to date have explored the relationship among sex, age of first evaluation, presence of an ASD classification, and age of first ASD classification [8,11,22,23]. One study by Wiggins and colleagues found that despite a heightened awareness of the higher rate of ASDs among boys, there was a tendency for girls to be evaluated and diagnosed with an ASD at earlier ages than boys, and there was a longer delay between first evaluation and diagnosis for girls than for boys [23]. Even though findings were not statistically significant in this small sample, the earlier evaluation and diagnosis of girls suggest a possible sex bias in diagnostic patterns of autism spectrum conditions.
Differences and behavioral patterns
In contrast to the strong evidence of a sex difference in prevalence, there is little information about sex differences in behavioral features and developmental characteristics. Studies of these relationships have used small clinical samples and diagnoses were based on different sets of criteria. Carter and colleagues' study of toddlers reported that sex differences emerged in developmental profiles using the Autism Diagnostic Observation Schedule [17], Autism Diagnostics Interview–Revised [24], Vineland Adaptive Behavior Scales [25], and Mullen Scales of Early Learning [26]. In this clinically derived sample, girls achieved higher visual reception scores and boys attained higher language, motor scores, and social-competence ratings. In a 2007 study, Carter and colleagues [27] reported that significant sex differences in children's behaviors were reported by parents, but no significant sex differences emerged on direct assessment of social and language functioning [27]. A separate 2007 study reported no sex difference in symptoms of core dysfunction but a significant difference in coexisting psychopathology [7]. While these studies add to the literature on potential sex differences in ASD and associated symptoms, findings have not been consistent and the use of small, clinically recruited samples makes it difficult to generalize findings to the larger population of children with ASDs.
Cognitive ability
Epidemiologic studies of ASD commonly include estimates of intellectual functioning. Some studies report that the male-to-female ratio of ASD decreases with greater levels of impairment in intellectual functioning [3,28]. Two older studies reported that females with autism manifested lower IQs than males and ID occurred in higher percentages of girls with autism than in boys [8,29]. These studies did not evaluate the spectrum of autism. A higher male prevalence is pronounced in clinical samples of children with an ASD (full spectrum). For instance, a linear relationship between sex ratio and IQ in children with ASDs was reported in an early study Wing [12] with higher male-to-female ratios associated with increased IQs. This difference was also found among individuals with typical cognitive functioning [30,31].
The suggestion of a sex bias in diagnostic patterns of ASDs is potentially salient when considered in the context of past research on other childhood conditions with a higher male prevalence. For instance, Berry and colleagues [32] found that girls diagnosed with attention-deficit/hyperactivity disorder (ADHD) were younger than boys at the time of referral for medical attention. This was possibly due to the increased rate of cognitive impairment in girls or to the fact that girls were displaying disruptive and uncontrolled behaviors on referral. The authors concluded that girls with ADHD must show greater impairment or display externalizing symptoms perceived to be related to male child development and behavior before they are referred for evaluation and treatment.
In summary, ASD is diagnosed more often in boys than in girls. However, there is limited understanding of the sex differences in the patterns of evaluation and diagnosis, and there are no data from large, population-bases studies of behavioral features by sex. An age-delineated, population-based sample could provide evidence that is more broadly generalizable. To further explore the relationship among sex and patterns of evaluation and diagnosis, we evaluated the following questions:
How do (a) age of first developmental evaluation, (b) the likelihood of having a documented ASD diagnosis, and (c) the age of the diagnosis differ by the sex of the child?
Does cognitive impairment affect (a) mean age of first evaluation, (b) likelihood of a documented diagnosis and (c) mean age of diagnosis differentially by sex?
Is there a sex difference in the report of behavioral features and other diagnoses among children who met surveillance criteria for ASD but did not have a documented diagnosis?
The use of surveillance data to explore this topic offered a unique opportunity to analyze data from a large, well-defined cohort, from multiple areas of the United States. The population-based data helped us overcome power and sampling limitations of previous studies.
Methods
This study was a secondary analysis of data collected between 2001 and 2006 by a large, multisite, surveillance program conducted in 13 different U.S. states by the ADDM Network. The ADDM Network collects data by study year, and the 2002 data were used for this report as they represent the largest and most complete dataset compiled in the United States using the same and a standardized methodology [33]. The participating states were Alabama, Arkansas, Arizona, California, Colorado, Florida, Georgia (CDC), Maryland/Delaware, Missouri, New Jersey, North Carolina, Pennsylvania, South Carolina, and Wisconsin. ADDM Network sites estimated the prevalence of ASD and described behavioral characteristics with respect to sex, race/ethnicity, and cognitive functioning of ASDs among children 8 years old in 2002 [33]. Prevalence estimates did not involve contact with the child but were based on detailed data abstracted from health and education records followed by clinician review using DSM-IV-TR criteria.
Population
A child was eligible if he or she was 8 years old in 2002 (born between January 1 and December 31, 1994) and had at least one parent who resided in the respective study areas, representing a base population of over 400,000 children aged 8 years (10% of the U.S. population of children this age). The sample included in these analyses comprised children (n = 2,568) identified by the ADDM Network as meeting the surveillance case definition for ASD.
Case ascertainment
Definition of a Surveillance Case of ASD
The methodology of the ADDM Network was based on the CDC's Metropolitan Atlanta Developmental Disabilities Surveillance Program [34], which uses a common case definition and standardized procedures for data abstraction, clinician review, and quality assurance. A child was assigned a status of “surveillance case of ASD” if he or she either (1) had a documented previous classification of an ASD and no conflicting diagnostic information by a qualified professional or (2) did not have a documented ASD classification but had at least one evaluation record from a school or clinical sources indicating the developmental history and behaviors consistent with the DSM-IV-TR [15] for autistic disorder, Aspeger's disorder, or PDD-NOS. Children with Rett syndrome and childhood disintegrative disorder were not included in this study in order to focus on the most common ASDs. Details of the methodology are described elsewhere [35].
Origin of the dataset
Surveillance sites identified agencies that evaluated, educated, treated, and/or maintained records for children with developmental delays and requested a list of all children served at that agency who were evaluated under a range of ICD-9 codes and/or educational exceptionalities, including ASD diagnoses or common co-morbid conditions (such as ID or obsessive-compulsive disorder). The clinical health agencies and schools were the sources of data. Data from schools included information used to determine eligibility for special education services including developmental evaluations and diagnostic test scores. Data from health sources included evaluations performed by psychologists, developmental pediatricians, neurologists, and psychiatrists, and scores were from behavioral, adaptive, and cognitive evaluations and tests. Information from multiple sources was combined into a single anonymous record for each child and was systematically reviewed by the clinician reviewers. The quality of data abstraction was assured through procedures used to maintain the interrater reliability among abstractors. When clinician review was completed, the child was classified as having ASD or not having ASD. For a complete description of this process see Rice and colleagues [33] and Van Naarden Braun and colleagues [35].
Determining autism spectrum disorder surveillance case status
Case determination was determined by a team of clinician reviewers applying DSM-IV-TR criteria. The team reviewed all descriptions in evaluations, test scores, and diagnostic summaries compiled for each child through a systematic review. Children met ASD case criteria through two paths (Table 1). In Path 1, children were classified as an ASD case if a classification (a diagnosis or special education eligibility) of an ASD was documented in an educational or clinical record and no information existed that contradicted an ASD classification. This path was referred to as streamlined. An ASD diagnosis included a clear statement made by the professional who evaluated the child, that the child met the diagnostic criteria for Autistic disorder, PDD-NOS (or ASD without further specification), or Asperger's disorder; or that the child met eligibility for special education services under an autism exceptionality. A child was not automatically classified as a case based on a previous classification; if there was information that called the ASD classification into question, such as contradictory diagnoses or behaviors incompatible with an ASD, a more detailed abstraction and review process was undertaken as described later.
Table 1.
Criteria for Potential Autism Spectrum Disorder (ASD) Case Identification and Confirmation
| Inclusion Criteria for “Potential ASD Case” |
| 1. Child born between January 1, 1994 and December 31, 1994, and |
| 2. Child resides in the study area (specified by each surveillance site), and |
| 3. The parent or guardian of the target child must have been a resident in the catchment area sometime during the 2002 abstraction year, and |
| 4. Child has been evaluated at a health or educational source for an eligible disability. |
| Inclusion Criteria for “Case of ASD” |
| PATH 1 (Streamlined) |
| 1. Meets all abstraction inclusion criteria above, and |
| 2. Child has been identified as having ASD by a qualified professional who has documented the diagnosis or educational classification in the child's health or education records, and |
| 3. The ADDM clinician reviewer reviews the diagnostic summaries from all records and confirms that no exclusions are present to preclude an ASD classification. |
| PATH 2 (Nonstreamlined) |
| 1. Meets all abstraction inclusion criteria above, and |
| 2. Child DOES NOT HAVE a diagnosis or classification of ASD recorded in the chart, but |
| 3. Child may have a diagnosis of another educational exceptionality or developmental disability, or has been evaluated for developmental concerns, and |
| 4. Has demonstrated behaviors that conform to DSM-IV-TR criteria for either autistic disorder, PDD-NOS, or Asperger's syndrome. |
In the absence of a documented ASD classification in the child's existing evaluation records, case status was determined by a second path. In Path 2, a child was considered a case if an ADDM clinician reviewer found that recorded behaviors were consistent with the DSM-IV-TR criteria for autistic disorder, PDD-NOS, or Asperger's disorder based on a systematic coding and data review process. The information reviewed included verbatim descriptors of behaviors associated with the core features of ASDs, diagnostic test scores, and associated features. Some associated features are eating, drinking, or sleeping difficulties; mood difficulties; aggressive behaviors; delayed motor milestones; odd responses to sensory stimuli; self-injurious behaviors; and seizures or seizure-like behaviors. The scheme for coding descriptors also included noting the presence of developmental delays before 36 months of age, developmental regression or developmental plateau, each of the DSM-IV criteria for ASDs, and diagnoses noted in developmental evaluations.
Information abstracted from the records was reviewed by clinician reviewers to determine case status. All clinician reviewers used common procedures and met initial and ongoing reliability standards. Clinician reviewers rigorously and systematically applied a standardized coding scheme based on DSM-IV-TR [15] criteria for autistic disorder, PDD-NOS (including atypical autism), and Asperger's disorder. For example, in the case definition, the DSM-IV-TR social criterion of “limited social or emotional reciprocity” was operationalized to require evidence of specific symptoms such as “rarely responds verbally or nonverbally to social approaches from others in a familiar setting” [4]. After evaluating the presence of each of the DSM-IV-TR criteria and associated features, a clinician reviewer could initiate an independent second review of any case to confirm case status if they had concerns about the applicability of the final case definition. Final review status was based on either a high degree of certainty that the child met the number and pattern of criteria for an ASD or by a consensus review following by two independent reviews of all information for that child.
Data quality and control
Interrater reliability was established among clinician reviewers. ADDM clinician reviewers had an advanced degree and specialized training and/or certification in the assessment and diagnosis of children with developmental disabilities (e.g., clinical/developmental psychologists, developmental pediatricians, child psychiatrists, pediatric neurologists, and speech pathologists). The clinician reviewers met initial and ongoing reliability standards in applying a standardized coding scheme based on the DSM-IV criteria. Only those who met reliability standards were permitted to assign case status. Continuing interrater reliability checks were conducted periodically on a random sample of 15% of fully abstracted records. For all 14 sites, the percent agreement for final case definition was acceptable (79%–100%; kappa = 0.55–1.00; overall average = 89%, kappa = .77).
Ethical considerations
Each of the ADDM surveillance sites received approval from the institutional review boards (IRB) as required by their institutions to conduct the original public health surveillance program. Approval and any additional IRB requirements were obtained with all cooperating agencies and at each site that was a source of data. Authorization to review records was obtained via agency authorization to access existing data for surveillance as public health. Direct contact was not made with the children. Data were de-identified before being pooled and analyzed.
Data analysis
We analyzed sex difference in age at first developmental evaluation, the presence of an identified ASD classification, age at first documented ASD diagnosis, and behavioral features associated with ASDs. Cognitive impairment was defined as an IQ score of 70 points or less on the most recent intelligence test documented in the child's evaluation records. Descriptive data were obtained as percentages or means and standard deviations as a function of the sex of the child. ANOVA analyses were used to estimate sex differences in age at first identified evaluation and age at first ASD classification. Chi-square tests were used to examine sex differences in the likelihood of a documented ASD classification and differences in the presence of behavioral features associated with ASDs for children without a documented ASD classification (identified as ASD cases via Path 2). Logistic regression was used to estimate the association of each independent variable with the presence of an ASD classification in any record after adjusting for cognitive impairment status. Finally, we used the maximum likelihood chi-square test for difference of proportions (G-statistic) [36] to describe non-ASD diagnoses among children without an ASD classification. This test provided a more precise estimate of the standard error that traditional chi-square or t-test statistics.
Results
There were 2,568 children who met the surveillance case definition of ASD. This sample was composed of 1,497 (58%) children who had a clearly documented ASD classification (Path 1–Streamlined) and 1,071 children who did not have a documented ASD diagnosis but were determined to meet ASD surveillance criteria by the clinician reviewer (Path 2). The racial composition of the entire sample was 63% white; 23% black; 10% Hispanic, Asian, or American Native; and 4% other or not stated. The racial composition for Path 1 was 65% white; 22% black; 9% Hispanic, Asian, or American Native; and 4% other or not stated. The racial composition for Path 2 was 60% white; 24% black; 12% Hispanic, Asian, or American Native; and 4% other or not stated.
Boys constituted 81% of the sample. The male-to-female ratio was 4:1 for all 2,568 ASD cases. One thousand nine hundred nineteen (75%) children had IQ data documented in their records. Of these children, 55% had no cognitive impairment (IQ > 70) and 45% had cognitive impairment (IQ ⩽ 70).
Specifically, 82% of the children with a documented ASD classification (Path 1) were boys compared with 80% of the children determined to meet ASD surveillance criteria by the clinician reviewer (Path 2). The presence of cognitive impairment also did not differ by the path of case ascertainment. Of the 1,086 children with IQ data in Path 1, 51% had no cognitive impairment and 49% had cognitive impairment. Of the 833 children with IQ data in Path 2, 61% had no cognitive impairment and 39% had cognitive impairment.
Age at first developmental evaluation and likelihood of a documented classification
For the entire sample (n = 2,567), the average age at first developmental evaluation was not different for boys compared with girls (46 months and 47 months, respectively). More than half (58%) of the entire sample (n = 1,497), had a documented ASD classification. Boys were more likely than girls to have a classification of ASD [, p = .014]—specifically, 59% of all boys (n = 2,077) and 54% of all girls (n = 491). Among those children with a documented ASD1 classification, the age at first ASD classification was not significantly different by sex (average age was 61 months for boys and 59 months for girls).
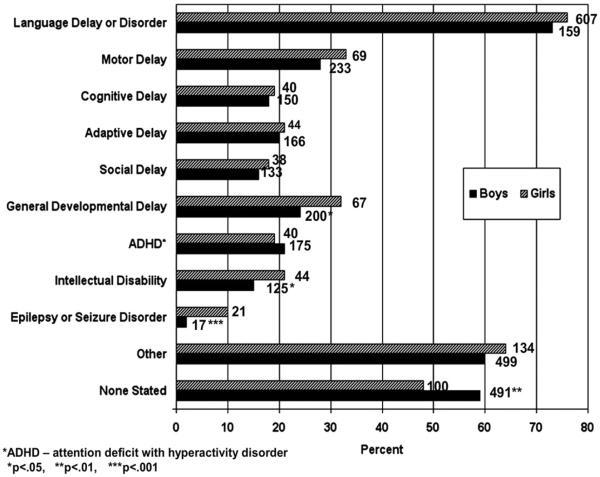
When an ASD classification was not documented in the child's existing records (Path 2, n = 1,071), the most common non-ASD diagnoses were “other,” “not stated,” “language delay,” “language disorder,” “motor delay,” and “general developmental delay” (Fig. 1). The diagnoses of “general developmental delay,” “epilepsy or seizure disorder,” and “intellectual disability” were more common in girls than in boys from Path 2. Sex differences were statistically significant for “none stated” (p = .0038), “epilepsy” (p < .001), “intellectual disability” (p = .0401), and “general delay” (p = .0200) (see Fig. 1).
Figure 1.
Sex differences in frequencies of non–autism spectrum disorder (ASD) diagnoses among surveillance cases of ASD.
Cognitive impairment and earliest developmental evaluation
When available, IQ data were collected. The IQ score noted was the most recently available (closest to the chronologic age of 8 years in 2002). As previously noted, 42% of children who met the surveillance case definition, by either path, and who had IQ data documented in their records, had no cognitive impairment (IQ > 70) and 33% had cognitive impairment (IQ ⩽ 70). Girls were more likely than boys to have documented cognitive impairment [, p < 0.00].
Logistic regression was used to predict the likelihood of having a documented classification of ASD based on sex and cognitive impairment. Results are presented (Table 2) in terms of the main effects (i.e., sex and cognitive impairment examined separately) and interactions (i.e., sex and cognitive impairment examined together). Odds ratios show the increased likelihood of receiving a documented ASD classification for each main effect and interaction compared to a specific reference category. As Table 2 reveals, without the interaction term between sex and cognitive impairment, girls had 0.76 the odds of having a documented classification of ASD compared with boys. Surveillance cases without cognitive impairment were less likely to have a documented ASD classification than surveillance cases with cognitive impairment (OR = 0.66, 95% CI = 0.55–0.80). With the interaction term for sex and cognitive impairment, girls with IQ of 70 or less were less likely than boys with IQ of 70 or less to have a documented classification of ASD (OR = 0.70, 95% CI = 0.50–0.97). Girls with IQ greater than 70 also were less likely than boys with IQ greater than 70 to have a documented diagnosis (OR = 0.60, 95% CI = 0.49–0.74). This finding was more pronounced for girls with IQ greater than 70 (OR = 0.45, 95% CI = 0.32–0.66). Findings did not differ when the level of cognitive impairment was divided into mild, moderate, and severe level of impairment. These results suggest that having cognitive impairment increased the likelihood of having a documented ASD classificiation for boys, but not for girls.
Table 2.
Adjusted Logistic Regression Predicting the Presence of a Classification of ASD Recorded in Developmental Evaluations of Children Aged 8 Years in 2002*
| Adjusted Odds Ratio | 95% Confidence Interval | p | |
|---|---|---|---|
| Main effects | |||
| Girlsa | 0.76 | 0.65–0.97 | .024** |
| No cognitive impairmentb | 0.66 | 0.55–0.80 | <.001** |
| Interactions | |||
| Girl, IQ ⩽70c | 0.70 | 0.50–0.97 | .035** |
| Girl, IQ >70c | 0.45 | 0.32–0.66 | <.001** |
| Girl, IQ unknownc | 0.78 | 0.55–1.12 | .183 |
| Boy, IQ >70c | 0.60 | 0.49–0.74 | <.001** |
| Boy, IQ unknownc | 1.00 | 0.79–0.28 | .966 |
The n changes for each regression analysis. For instance, the n for the sex main effect is 2,568 while the n for the cognitive impairment main effect is limited to children with cognitive data.
Significant.
Compared with boys.
Compared with Autism Spectrum Disorder surveillance cases with cognitive impairment.
Compared with boys with IQ ⩽70.
Sex differences and behavioral features
For the subset of ASD cases that did not have a clearly documented ASD classification in their existing records (n = 1,071 in Path 2), behavioral features were abstracted from the children's medical and education records. Boys who did not have a documented ASD classification in their existing records had more externalizing behaviors than girls, such as aggression [, p = .004] and hyperactivity or attention deficits [, p = .003]. Girls without an ASD classification were more likely than boys to have staring spells and seizure-like activity [, p = .001]. There were no other significant differences by sex in other documented behavioral characteristics (Table 3).
Table 3.
Sex Differences in Behavioral Features Associated with a Classification of Autism Spectrum Disorder (n = 1,071) (Cases of Autism Spectrum Disorder via Path 2)
| Presence of Abnormality (%) |
p Value | ||
|---|---|---|---|
| Associated Feature | Boys | Girls | |
| Eating/drinking/sleeping difficulties | 52 | 52 | 1.00 |
| Mood difficulties | 62 | 61 | .961 |
| Scatter in cognitive skills | 48 | 49 | .914 |
| Aggressive behaviors | 50 | 41 | .008* |
| Argumentative or oppositional behaviors | 58 | 54 | .436 |
| Delayed motor milestones | 62 | 64 | .728 |
| Hyperactivity or short attention span | 85 | 78 | <.001* |
| Lack of fear or excessive fearfulness | 36 | 35 | .846 |
| Odd response to sensory stimuli | 56 | 51 | .355 |
| Self-injurious behaviors | 28 | 26 | .745 |
| Seizures or seizure-like behaviors | 24 | 34 | <.001* |
| Temper tantrums | 47 | 42 | .234 |
Significant.
Discussion
In this study, we examined differences between girls and boys with respect to age of first developmental evaluation, age at diagnosis, the presence of any indication in educational or clinical records that the child was identified as having ASD, and the differential role of cognitive impairment in whether girls and boys with ASD were identified as such in their records. We used a pooled dataset collected from a large population-based surveillance program to decrease the risk of selection bias from a clinically referred sample and to overcome the limitation of a small sample size found in previous studies.
In this cohort of children, the timing of the first documented developmental evaluation was roughly the same for boys and girls and occurred at slightly under 4 years of age. The absence of a sex difference in the age of the child's first evaluation suggested that both boys and girls were manifesting behavioral indicators of ASD, and these behaviors were called to the attention of clinicians at approximately the same age. Furthermore, for children who went on to be classified as having an ASD by a diagnosis or education eligibility for special education, boys and girls received the classification at approximately the same age (around 5 years). However, the age of the first evaluation and age of the first ASD classification are well above the age at which screening can identify many children who are at risk for ASDs during the toddler years [5,20,37,38]. Both boys and girls in our sample experienced a delay of approximately 12 to 15 months from the time they were first evaluated for developmental concerns until the ASD classification was documented. This represents a 2- to 3-year delay from the manifestation of symptoms of ASD to the time of first documented ASD classification for children who were 8 years old in 2002 (born in 1994). It is important to note that since the birth of this cohort, the DSM-IV criteria have undergone a minor update [15] to further clarify the criteria for PDD-NOS. These data provide important baseline information on the status of early evaluation and subsequent classification of girls and boys with ASDs. These baseline data indicate that improved early identification and evaluation of ASD symptoms were needed several years ago and point to the value of training programs for parents and professionals that instruct on the early symptoms of ASDs and the need to refer to a diagnostic specialist as soon as deficits or delays are suspected. Ongoing comparison of early identification and classification patterns will be important to evaluate the effect of the recent emphasis on public and professional education that focuses on the early identification of ASD and other developmental disabilities [6].
While the timings of the first developmental evaluation and first ASD classification were equivalent in boys and girls, boys were more likely than girls to have a documented ASD classification. This suggested to us that the clinicians (in the population cohort) were more likely to classify boys with ASD than girls, even when both sexes had symptoms associated with the disorders documented in educational and clinical records. The implications of this sex difference are important in that a formal ASD classification may provide the advantage of earlier access to a range of diagnosis-specific treatments and services associated with improved outcomes. The advantage may be available to male more than female children.
Clinicians should be aware that there may be a gap between first evaluation and first ASD classification and should consider factors that might forestall ASD classification in girls. For instance, the sex difference in the likelihood of a documented classification may arise from the social context in which evaluation and classification takes place, and the profile of associated features (e.g., cognitive impairment) and co-occurring conditions (e.g., multiple medical concerns and seizure activity).
Since males are more likely to have ASDs, and comorbid cognitive impairment is common in children with ASDs, clinicians may be cued to suspect an ASD in a boy who presents with a developmental concern such as an ID. Conversely, when a girl presents with an ID, an ASD diagnosis may not be considered the primary problem and ID may be diagnosed, instead. This hypothesis is supported by the fact that, among children without a documented ASD classification in our sample, it was more common for girls than boys to be diagnosed with ID. In addition, among children without a documented ASD classification, it was more common for girls than boys to be diagnosed with general developmental delay and epilepsy or seizure disorder. Staring spells and seizure-like activity were 5 times more commonly reported for girls than for boys. Thus, clinicians evaluating girls with a complex developmental profile may erroneously exclude a classification of ASD based on the presence of other intellectual, developmental, and medical conditions.
Another possible explanation for the sex difference in the presence of an ASD classification is “interpreting bias,” which is the difference between observed and expected behaviors. A significant and essential source of information on a child's behaviors is provided by parents to clinicians, who then use these and other data during screening and evaluation and to make a diagnosis. If a clinician relied solely on a parent's report, he or she may presume the existence of behaviors that may not occur on direct assessment. Carter and colleagues [27] report that significant sex differences in children's behaviors were reported by parents, but no significant sex differences emerged on direct assessment of social and language functioning. Specific to the study of ASD, Holtmann and colleagues [7] reported the possibility of “interpreting bias” among parents of children with ASDs, who may expect more socially desirable behaviors from daughters than sons. This theory is further supported by Crick and Zahn-Waxler [39], who reported that girls with ASDs were perceived as having a greater level of impairment, despite a comparability of symptoms reported and observed in the Autism Diagnostic Inventory—Revised and Autism Diagnostic Observation Schedule [39].
Hirt [40] proposed an expectancy-guided retrieval model in which preconceived ideas necessarily influenced perception and judgment. Factors such as sex of the clinician and sex of the child, or even sex of the parent observing and reporting behaviors, may have an influence on symptom documentation and interpretation. The effect of “interpreting bias” might also explain some of the differences in reporting more externalizing behaviors, such as aggression and hyperactivity, in boys compared to girls. In our study, boys were much more likely than girls to have externalizing symptoms documented in educational and clinical records. We must ultimately consider how both biological etiology and social expectation influence the evaluation and diagnosis of ASDs in boys and girls.
Strengths and limitations
Our study was strengthened by our use of data that were collected using consistently defined and implemented case identification and classification procedures for a large population of children with ASD from multiple communities in the United States. During data collection, careful attention was paid to initial and ongoing quality control. The use of the large, systematically collected data set enabled us to examine developmental information from multiple education and clinical sources [34]. The records from these sources contained elaborate information on behaviors associated with ASDs. Examining of the occurrence of comprehensive ASD symptoms across a widely diverse cohort of children is not possible using clinically referred sample but is more informative when population-based samples are used.
Our study also has some limitations. First, it is possible that some children with symptoms of ASDs were not evaluated at the educational and clinical sources. Some children may have had milder forms of ASDs or complex symptoms of ASDs that were not captured in the child's evaluation records. These children would not have been recognized by our surveillance program, and therefore were either missed as a surveillance case or clinician review was inconclusive and the label of “suspected case” was applied. Second, case status relied on the review of information contained in educational and clinical records rather than direct clinical reassessment of the child. One might presume that this method is less reliable and less accurate than the “gold standard” of one-on-one evaluation of the child by an expert clinician. Ascertaining cases of ASD by direct observation is idea, but is impractical and costly use with large population-based samples and may have response-bias problems. This limitation is offset by the absolute rigor applied to the development and quality assessment and control of the surveillance methodology used to collect the original data. Indeed, the data collected from the child's educational and clinical records were extensive and the clinicians who reviewed the data had specific expertise in the evaluation of children with ASD and other developmental disabilities. They meticulously applied a coding schema that was derived explicitly from the DSM-IV-TR criteria and had a systematic process for incorporating clinical judgment on final case status. Preliminary results from a validation study to compare surveillance and clinical methods for identification of cases suggest very good positive and negative predictive values and specificity, but sensitivity estimates indicate some missed ASD cases based on record review (Avchen et al., unpublished data). Therefore, the prevalence estimates presented here may be slight underestimates.
Another limitation is missing data on specific subsets of children. For instance, not all children had cognitive testing and the age at which this testing was done may have been as young as age 4 and as old as age 8. This absence of data, however, reflects common clinical practice in that every child who is evaluated for ASD does not necessarily receive a standardized test of cognitive function. Furthermore, we cannot say for certain that what we reported fully captures the range and distribution of behavioral features associated with ASDs, which were available on only 42% of the sample. Describing behavioral differences among all children would offer a comprehensive profile of male and female cases of ASD. Although limitations are present, and further and updated analyses are needed, this methodology allowed us to examine population characteristics of ASDs and to examine sex differences in the presence and absence of an ASD classification. Ongoing data collection using the same methods by the ADDM Network will enable future evaluation of changes in the age of ASD evaluation and classification for boys and girls. The findings of the present study report a sex difference in the classification of ASDs among the largest sample to date.
Appendix 1
Autism and Developmental Disabilities Monitoring (ADDM) Network Surveillance Year 2002
Network Principal Investigators
Catherine Rice, Ph.D., Jon Baio, Ed.S., Kim Van Naarden Braun, Ph.D., and Nancy Doernberg, B.A. (National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention); Russell S. Kirby, Ph.D. (University of South Florida, Tampa); Carole Canino and Mark Swanson, M.D., M.P.H. (University of Arkansas); Sydney Pettygrove, Ph.D., Chris Cunniff, M.D., and F. John Meaney, Ph.D. (University of Arizona); Lisa Miller, M.D., M.S.P.H. (Colorado Department of Public Health and Environment); Cordelia Robinson, Ph.D., R.N. (University of Colorado at Denver and Health Sciences Center); Craig Newschaffer, Ph.D. (Johns Hopkins University); Rebecca Landa, Ph.D. (Kennedy Krieger Institute); Edwin Trevathan, M.D., M.P.H., and John Constantino, M.D. (Washington University in St. Louis); Julie Daniels, Ph.D. (University of North Carolina at Chapel Hill); Walter Zahorodny, Ph.D., and Franklin Desposito, M.D. (University of Medicine and Dentistry of New Jersey); Jennifer Pinto-Martin, Ph.D., M.P.H., and Ellen Giarelli, Ed.D., R.N. (University of Pennsylvania); Susan Levy, M.D. (Children's Hospital of Philadelphia); Jane Charles, M.D. (Medical University of South Carolina); Judith Zimmerman, Ph.D., and William McMahon, M.D. (University of Utah); Barbara Becker-Cottrill, Ed.D. (Marshall University); and Maureen Durkin, Ph.D., Dr.P.H. (University of Wisconsin-Madison).
Other ADDM Principal Investigators
Judith Grether, Ph.D., and Gayle Windham, Ph.D. (California Department of Health Services); Lisa Croen, Ph.D. (Northern California Kaiser Permanente Division of Research); and Keith Scott, Ph.D., and Marygrace Yale Kaiser, Ph.D. (University of Miami).
Footnotes
See Appendix 1 for a list of principal investigators.
Financial disclosure: The authors of this manuscript were principal investigators or co-investigators participating in the ADDM Network Surveillance Project. The principal investigators and co-investigators received salary support over several years during the collection of data that was provided by the Centers for Disease Control and Prevention (CDC). There are no conflicts of interest, financial or otherwise, relevant to the subject matter of the manuscript that have occurred over the last 2 years or that are expected in the foreseeable future, beyond participation in the ADDM network. These projects were funded by the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC. For additional information, see www.cdc.gov/autism.
References
- [1].Charman T. The prevalence of autism spectrum disorders. Recent evidence and future challenges. Eur Child Adolesc Psychiatry. 2002;11:249–256. doi: 10.1007/s00787-002-0297-8. [DOI] [PubMed] [Google Scholar]
- [2].Charman T. Epidemiology and early identification of autism: research challenges and opportunities. Novartis Found Symp. 2003;251:10–19. discussion 19–25, 109–111, 281–297. [PubMed] [Google Scholar]
- [3].Yeargin-Allsopp MC, Rice C, Karapurkar T, et al. Prevalence of autism in a US metropolitan area. JAMA. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention Prevalence of autism spectrum disorders: Autism and Developmental Monitoring Network, 14 sites, United States, 2002. MMWR Morb Mortal Wkly Rep. 2007;58(SS-1):12–28. [PubMed] [Google Scholar]
- [5].Filipek PA, Accardo PJ, Baranek GT, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29:439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention Learn the signs. Act early. 2007 Available at http://www.cdc.gov/ncbddd/autism/actearly.
- [7].Holtmann M, Bolte S, Poustka F. Autism spectrum disorders: sex differences in autistic behavior domains and coexisting psychopathology. Dev Med Child Neurol. 2007;49:361–366. doi: 10.1111/j.1469-8749.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- [8].Lord C, Schopler E, Revicki D. Sex differences in autism. J Autism Dev Disord. 1982;12:317–330. doi: 10.1007/BF01538320. [DOI] [PubMed] [Google Scholar]
- [9].McLennan JD, Lord C, Schopler E. Sex differences in higher functioning people with autism. J Autism Dev Disord. 1993;23:217–227. doi: 10.1007/BF01046216. [DOI] [PubMed] [Google Scholar]
- [10].Rutter M, Lockyer L. A five to fifteen year follow-up study of infantile psychosis: I. Description of sample. Br J Psychiatry. 1967;113:1169–1182. doi: 10.1192/bjp.113.504.1169. [DOI] [PubMed] [Google Scholar]
- [11].Tsia L, Beilser JM. The development of sex differences in infantile autism. Br J Psychiatry. 1983;142:373–378. doi: 10.1192/bjp.142.4.373. [DOI] [PubMed] [Google Scholar]
- [12].Wing L. Sex ratios in early childhood autism and related conditions. Psychiatric Res. 1981;5:129–137. doi: 10.1016/0165-1781(81)90043-3. [DOI] [PubMed] [Google Scholar]
- [13].Kanner L. Autistic disturbances in affective contact. Nerv Child. 1943;2:217–250. [Google Scholar]
- [14].American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- [15].American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition Text Revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- [16].Stone WL, Coonrod EE. Brief report: screening tool for autism in two-year olds: developmental and preliminary data. J Autism Dev Disord. 2000;30:607–612. doi: 10.1023/a:1005647629002. [DOI] [PubMed] [Google Scholar]
- [17].Lord C, Risi S, Di Lavore PS, et al. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- [18].Stone W, Lee E, Ashford L. Can autism be diagnosed accurately in children under 3 years? J Child Psychol Psychiatry. 1999;40:219–226. [PubMed] [Google Scholar]
- [19].U.S. Congress . Children's Health Act of 2000. [Google Scholar]
- [20].Baron-Cohen S, Wheelwright S, Cox A, et al. Early identification of autism by the CHecklist for Autism in Toddlers (CHAT) J R Soc Med. 2000;93:521–525. doi: 10.1177/014107680009301007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].American Academy of Pediatrics, Committee a Children with Disabilities Developmental surveillance and screening of infants and young children. Pediatrics. 2001;108:192–196. [Google Scholar]
- [22].Pilowsky T, Yirmiya N, Shulman C, et al. The Autism Diagnostic Interview-Revised and the Childhood Autism Rating Scale: differences between diagnostic systems and comparison between genders. J Autism Dev Disord. 1998;28:143–151. doi: 10.1023/a:1026092632466. [DOI] [PubMed] [Google Scholar]
- [23].Wiggins L, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Behav Pediatr. 2006;27(2 suppl):S79–S87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- [24].Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- [25].Sparrow SB, Balla DA, Cichetti DV, et al. Vineland Adaptive Behavior Scales: Interview Edition, Survey Form Manual. American Guidance Service; Circle Pines, MN: 1984. [Google Scholar]
- [26].Mullen EM. Mullen Scales of Early Learning, AGS Edition. Pearson Assessments; Bloomington, MN: 1995. [Google Scholar]
- [27].Carter AS, Black DO, Tewani S, et al. Sex difference in toddlers with autism spectrum disorders. J Autism Dev Disord. 2007;37:86–97. doi: 10.1007/s10803-006-0331-7. [DOI] [PubMed] [Google Scholar]
- [28].Baird G, Simonoff E, Pickles A, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- [29].Volkmar FR, Szatmari P, Sparrow SS. Sex differences in pervasive developmental disorders. J Autism Dev Disord. 1993;23:579–591. doi: 10.1007/BF01046103. [DOI] [PubMed] [Google Scholar]
- [30].Dyck MJ, Pick JP, Hay D, et al. Are abilities abnormally interdependent in children with autism? J Clin Child Adolesc Psychol. 2006;35:20–33. doi: 10.1207/s15374424jccp3501_3. [DOI] [PubMed] [Google Scholar]
- [31].Fombonne E. Epidemiologic surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. 2003;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- [32].Berry CA, Shaywitz SE, Shaywitz BA. Girls with attention deficit disorder: a silent minority? A report on behavioral and cognitive characteristics. Pediatrics. 1985;76:801–809. [PubMed] [Google Scholar]
- [33].Rice CE, Baio J, Van Nearden-Braun K, et al. A public health collaboration for the surveillance of autism spectrum disorders. Paediatr Perinatal Epidemiol. 2007;21:179–190. doi: 10.1111/j.1365-3016.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- [34].Yeargin-Allsopp M, Murphy CC, Oakley GP, et al. A multiple-source method for studying the prevalence of developmental disabilities in children: the Metropolitan Atlanta Developmental Disabilities Study. Pediatrics. 1992;89:624–630. [PubMed] [Google Scholar]
- [35].Van Naarden Braun K, Pettygrove S, Daniels J, et al. Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorder: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2002. MMWR Morb Mortal Wkly Rep. 2007;56(SS-1):29–40. [PubMed] [Google Scholar]
- [36].Sokal RR, Rolff FJ. Biometry. 2nd Ed W.H. Freeman and Company; New York: 1981. [Google Scholar]
- [37].Chakrabarti S, Fombonne EE. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- [38].Eaves LC, Ho HH. The very early identification of autism: outcome to age 4 1/2-5. J Autism Dev Disord. 2004;34:367–378. doi: 10.1023/b:jadd.0000037414.33270.a8. [DOI] [PubMed] [Google Scholar]
- [39].Crick NR, Zahn-Waxler C. The development of psychopathology in females and males: current progress and future challenges. Dev Psychopathol. 2005;75:719–742. [PubMed] [Google Scholar]
- [40].Hirt ER. Do I see only what I expect? Evidence for an expectancy guided retrieval model. J Person Soc Psychol. 1990;58:937–951. [Google Scholar]