Abstract
Background. Although human immunodeficiency virus (HIV)-positive persons on antiretroviral therapy (ART) frequently have chronic liver enzyme elevation (cLEE), the underlying cause is often unclear.
Methods. Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study participants without chronic viral hepatitis were observed to the earliest of cLEE (elevated aminotransferase ≥6 months), death, last follow-up, or January 2, 2014. Antiretroviral treatment exposure was categorized as follows: no exposure and ongoing short- and long-term exposure (<2 or ≥2 years) after initiation. Association between development of cLEE and ART exposure was investigated using Poisson regression.
Results. Among 21 485 participants observed for 105 413 person-years (PY), 6368 developed cLEE (incidence 6.04/100 PY; 95% confidence interval [CI], 5.89–6.19). Chronic liver enzyme elevation was associated with short-and long-term exposure to didanosine (<2 years rate ratio [RR] = 1.29, 95% CI, 1.11–1.49; >2 years RR = 1.26, 95% CI, 1.13–1.41); stavudine (<2 years RR = 1.51, 95% CI, 1.26–1.81; >2 years RR = 1.17, 95% CI, 1.03–1.32), and tenofovir disoproxil fumarate (<2 years RR = 1.55, 95% CI, 1.40–1.72; >2 years RR = 1.18, 95% CI, 1.05–1.32), but only short-term exposure to nevirapine (<2 years RR = 1.44, 95% CI, 1.29–1.61), efavirenz (<2 years RR = 1.14, 95% CI, 1.03–1.26), emtricitabine (<2 years RR = 1.18, 95% CI, 1.04–1.33), and atazanavir (<2 years RR = 1.20, 95% CI, 1.04–1.38). Chronic liver enzyme elevation was not associated with use of lamivudine, abacavir, and other protease inhibitors. Mortality did not differ between participants with and without cLEE.
Conclusions. Although didanosine, stavudine, nevirapine, and efavirenz have been described to be hepatotoxic, we additionally observed a consistent association between tenofovir and cLEE emerging within the first 2 years after drug initiation. This novel tenofovir-cLEE signal should be further investigated.
Keywords: alanine aminotransferase, antiretroviral therapy, hepatotoxicity, HIV, liver disease
Human immunodeficiency virus (HIV)-infection has evolved into a chronic disease that requires lifelong antiretroviral therapy (ART). Therefore, drug-related toxicities have emerged as a key issue, including long-term ART-related liver injury [1].
Chronic liver enzyme (alanine aminotransferase [ALT]) elevation, a marker of persistent hepatocyte injury, is frequent among HIV-positive persons, even in the absence of hepatitis C (HCV) and hepatitis B (HBV) coinfection [2–4]. The cause of these elevations, its clinical significance, the need for evaluation, a reasonable diagnostic approach, and its management are often unclear, particularly when the elevation is modest. Most studies on liver disease focused on HCV- or HBV-coinfected persons or on the incidence and prevalence of severely elevated liver enzymes, defined as 3–5 times the upper limit of normal (ULN) or more [5]. Only limited data are available on HIV-monoinfected individuals with ALT values just above normal limits.
In previous studies, metabolic factors, including obesity, dyslipidemia and diabetes mellitus, severe alcohol use, high HIV viral load, and ART were predictive of persistently elevated ALT levels [2–4, 6]. Some antiretrovirals, such as the older stavudine (d4T) and didanosine (ddI), have been linked to liver injury [3, 6–11]. Data on the association of newer antiretrovirals and chronic liver disease are limited.
Study results on the outcome of liver enzyme elevations are controversial. A previous analysis of the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study suggested that in HIV/HCV-coinfected persons, ALT levels above the normal limit are associated with a higher mortality [12], whereas in another investigation of HIV-monoinfected persons ALT elevation was not predictive of an increased death rate, although the observation period in this study was relatively short [11]. Two liver biopsy series demonstrated a high prevalence of liver fibrosis (FIB) and steatosis among HIV-monoinfected adults with chronic elevated liver enzymes [13, 14]. Such results emphasize that ALT is an important surrogate marker for relevant liver pathology.
Our study aims were (1) to identify risk factors associated with chronic ALT elevation (cLEE), focusing on the individual antiretroviral drugs and (2) to evaluate the outcome of liver enzyme elevation with regard to liver-related and all-cause mortality, within the frame of the D:A:D Study with its large size and long-term prospective observation.
METHODS
Study Design
The D:A:D Study, founded in 1999, is a prospective observational study of 11 previously established cohorts as described in detail [15]. Currently, more than 49 000 HIV-positive participants are followed in Europe, the United States, and Australia. The primary study aim was to investigate the associations between exposure to antiretroviral drugs and risk of myocardial infarction, and, subsequently, other major clinical events. Data, including sociodemographic characteristics, acquired immune deficiency syndrome (AIDS), viral hepatitis, deaths, known risk factors for cardiovascular disease, laboratory markers, and ART, are collected prospectively during routine clinical care. Information on causes of death is captured using the Coding of Causes of Death in HIV (CoDe) form [16]. Clinical events are regularly monitored and centrally adjudicated. The present analyses were limited to the participating cohorts that provided data on ALT levels.
Definitions
Chronic ALT elevation was defined as ALT levels greater than the ULN (males/females >50/>35 U/L) at ≥2 visits spanning at least 6 months within 2 years. We used the date of the first elevated ALT as the event date. A single normal ALT measurement between 2 elevated values was permitted and therefore did not signal the end of a period of cLEE. Hepatitis C virus infection was defined by HCV seropositivity or detectable HCV RNA. Hepatitis B virus infection was defined by a positive HBV surface antigen, HBV e antigen, HBV core antibodies, or detectable HBV DNA. Participants with unknown HBV or HCV status were excluded. Fibrosis-4 and aspartate aminotransferase (AST)-to-platelet index (APRI), 2 noninvasive biomarkers of liver FIB, were calculated as follows: FIB-4: (age × AST)/[platelet count (109 cells/L) × sqrt(ALT)], and APRI: [(AST/ULN)/platelet count] × 100. A FIB-4 value >3.25 or an APRI score >1.5 is considered indicative of cirrhosis, whereas with a FIB-4 ≤ 1.45 and an APRI score ≤0.5, respectively, significant FIB is unlikely [17, 18].
Statistical Analyses
Cohort-specific baseline dates were chosen according to the introduction of routine ALT monitoring in the individual cohorts. All D:A:D participants without HBV and HCV infection, with ≥3 ALT measurements, ≥6 months of follow-up, and normal ALT at baseline, were followed from baseline to the earliest of cLEE, death, 6 months prior to a date of a first positive HCV/HBV test, 6 months after last visit, or February 1, 2014. The incidence of cLEE was defined as the number of first events divided by the total person years of follow-up (PYFU).
We used Poisson regression models to assess the incidence of cLEE and its association with ART and other risk factors. Models were manually built using a standard structured approach including variables into a multivariable model in groups. At each stage, factors that were not significantly associated with cLEE were removed before moving to the next set of variables. Fixed covariables were sex, ethnicity, and participating cohort. Time-updated covariables were age, calendar year, hypercholesterolemia, hypertriglyceridemia, use of lipid-lowering drugs, lipodystrophy, body mass index (BMI), arterial hypertension, smoking status, and exposure to the individual antiretrovirals. Antiretroviral therapy exposure was categorized as follows for each individual drug: no exposure, ongoing short- and long-term exposure (<2 or ≥2 years) after initiation; and discontinuation for <2 or ≥2 years. We do not present risk estimates for drugs after discontinuation because interpretation is difficult without considering the subsequent drugs persons were switched to. Use of ritonavir (RTV) includes both full and boosting doses.
We also investigated the association between the total number and duration of cLEE episodes with all-cause mortality using Poisson regression models. For these analyses, individuals were followed to the earliest of death, 6 months prior to a date of a first positive HCV/HBV test, 6 months after last visit, or February 1, 2014, and all periods of cLEE, including any subsequent episodes, were included. Analyses were adjusted for age, sex, ethnicity, mode of HIV acquisition, calendar year, cohort, smoking status, BMI, and in a subsequent model additionally for time-updated CD4 cell counts and HIV viral loads. Note that these models did not include adjustment for the ART drugs received, because it was anticipated that any impact of ART on mortality would be largely driven by changes in CD4 counts and HIV viral loads. Multivariable analyses were not feasible for liver-related deaths because of small numbers. All analyses were performed using SAS version 9.3.
RESULTS
Patient Characteristics
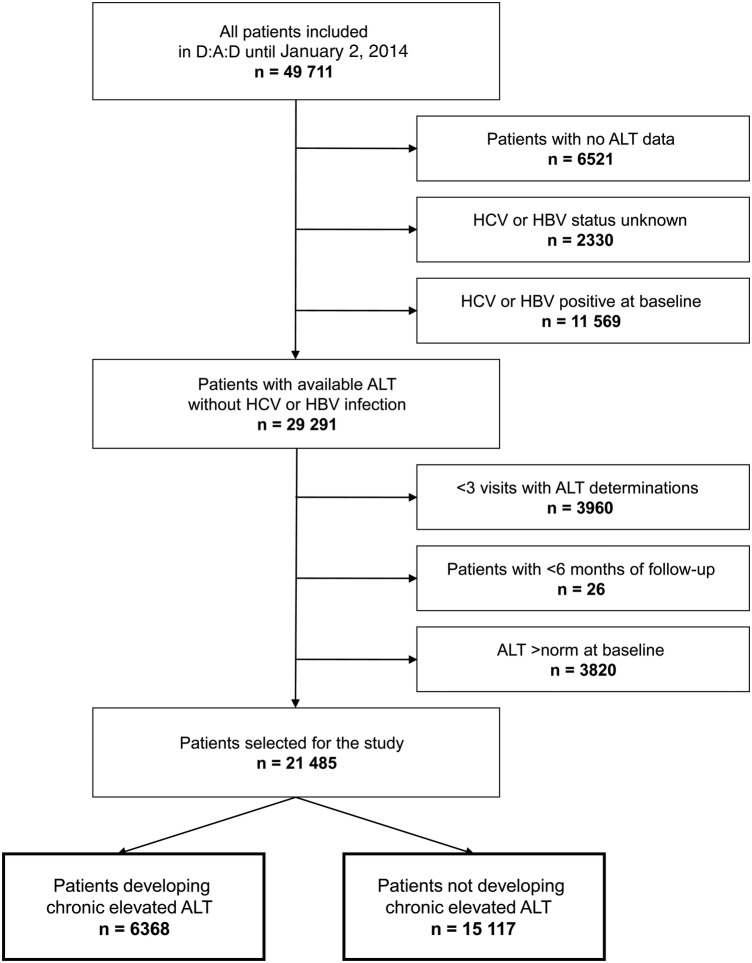
Of 49 711 participants included in D:A:D until the cutoff date for the study of February 1, 2014, 29 291 (58.9%) were HCV and HBV negative and had available ALT results. We further excluded 3986 (8%) with <3 visits with ALT determinations or <6 months of follow-up and 3820 participants (7.7%) because of preexisting elevated ALT levels at time of baseline visit. Excluded participants were similarly compared with those who were included in terms of sex, age, ethnicity, mode of HIV acquisition, previous AIDS, and date of D:A:D enrollment, except that the group with incomplete follow-up contained more intravenous drug users (IDU) and persons of unknown ethnicity, and the group with increased ALT levels at baseline were more often IDUs and participants with previous AIDS. The present study is thus based on 21 485 HIV-positive individuals with normal ALT values at baseline (Figure 1).
Figure 1.
Patient flowchart. ALT, alanine aminotransferase; HBV, hepatitis B virus; HCV, hepatitis C virus.
The study population consisted of 72.9% men with a median age of 40 years. Of the participants, 51.9% were white, and 51.2% had acquired HIV through sex between men. At study entry, the median CD4 lymphocyte count was 470 cells/µL and 72.1% of participants were on ART. In total, 77.2% had ever been exposed to ART for a median duration of 3.9 years. Aspartate aminotransferase-to-platelet score was >1.5 in 523 (2.4%) and FIB-4 score was >3.25 in 647 (3.0%) of 12 857 (60%) persons with available scores (Table 1). Participants contributed a median of 13 (interquartile range [IQR], 6–23) ALT measurements to the analyses. Overall, the median baseline ALT level was 22 (IQR, 16–30) U/L.
Table 1.
Baseline Characteristics of 21 485 D:A:D Study Participants Without HCV or HBV Coinfection
| Total of participants, no. (%) | 21 485 (100) | |
| Sex, no (%) | Male | 15 661 (72.9) |
| Age, years | Median (IQR) | 40 (33, 49) |
| Ethnicity, no. (%) | White | 11 159 (51.9) |
| Black | 2110 (9.8) | |
| Other | 580 (2.7) | |
| Unknown | 7636 (35.5) | |
| Mode of HIV acquisition, no. (%) | Heterosexual | 8916 (41.5) |
| Homosexual | 10 990 (51.2) | |
| Injection drug use | 243 (1.1) | |
| Other/unknown | 1336 (6.2) | |
| Duration of D:A:D cohort follow-up | Median (IQR) | 6.6 (2.1, 10.8) |
| Previous clinical AIDS | No. (%) | 5090 (23.7) |
| CD4 cells/µL | Median (IQR) | 470 (318, 656) |
| Ever received antiretroviral therapy | No. (%) | 16 578 (77.2) |
| Cumulative ART exposure (years) | Median (IQR) | 3.9 (1.8, 7.0) |
| Ever received NRTIs | No. (%) | 16 360 (76.2) |
| Cumulative ART exposure (years) | Median (IQR) | 3.7 (1.7, 6.7) |
| Ever received PIs | No. (%) | 11 922 (55.9) |
| Cumulative ART exposure (years) | Median (IQR) | 2.5 (1.1, 4.2) |
| Ever received NNRTIs | No. (%) | 10 094 (47.0) |
| Cumulative ART exposure (years) | Median (IQR) | 1.9 (0.7, 4.4) |
| Body mass index, kg/m2, no. (%) | <18 | 545 (2.5) |
| ≥18, ≤26 | 14 232 (66.2) | |
| >26, ≤30 | 3129 (14.6) | |
| >30 | 1154 (5.4) | |
| Unknown | 2425 (11.3) | |
| Diabetes mellitus | No. (%) | 704 (3.3) |
| Total cholesterol, mmol/L | Median (IQR) | 5.0 (4.2–5.8) |
| HDL cholesterol, mmol/L | Median (IQR) | 1.2 (1.0–1.5) |
| Triglycerides, mmol/L | Median (IQR) | 1.5 (1.0–2.4) |
| Use of lipid-lowering drugs | No. (%) | 1855 (8.6) |
| Lipodystrophy | No. (%) | 4260 (19.8) |
| Smoking status, n (%) | Current | 7144 (33.3) |
| Former | 4735 (22.0) | |
| Never | 7459 (34.7) | |
| Unknown | 2147 (10.0) | |
| FIB-4 score | ≤1.45 | 10 352 (48.2) |
| >1.45, ≤3.25 | 1858 (8.7) | |
| >3.25 | 647 (3.0) | |
| Unknown | 8628 (40.2) | |
| APRI score | ≤0.5 | 11 385 (53.0) |
| >0.5, ≤1.5 | 949 (4.4) | |
| >1.5 | 523 (2.4) |
Abbreviations: AIDS, acquired immune deficiency syndrome; ALT, alanine aminotransferase; APRI, aspartate aminotransferase-to-platelet ratio; ART, antiretroviral therapy; D:A:D, Data Collection on Adverse Events of Anti-HIV Drugs; FIB, fibrosis; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Predictors for Chronic Alanine Aminotransferase Elevation
During a median follow-up of 6.6 years (IQR, 2.1–10.8) and 105 413 PYFU, 6368 (29.6%) participants experienced episodes of cLEE, resulting in an incidence of 6.04 per 100 PYFU (95% CI, 5.89–6.19). Increased BMI (>26 kg/m2), dyslipidemia, use of lipid-lowering drugs, arterial hypertension, high HIV levels (≥5 log10 copies/mL), and earlier calendar years were associated with cLEE. Older age (≥50 years), black ethnicity, male gender, and current smoking were inversely correlated (Supplementary Figure 1).
Among the nucleoside reverse-transcriptase inhibitors, cLEE was associated with ongoing exposure to regimens containing ddI, d4T, tenofovir disoproxil fumarate (TDF), and with exposure to emtricitabine (FTC) during the first 2 years. Exposure to lamivudine (3TC) was inversely correlated with cLEE. Among the nonnucleoside reverse-transcriptase inhibitors, the first 2 years of exposure to nevirapine (NVP) and efavirenz (EFV) were only associated with cLEE. The only protease inhibitor (PI) to demonstrate an association with cLEE was atazanavir (ATV) during the first 2 years of exposure. There was no evidence for an association with increased risk for the other PIs. Exposure to darunavir, RTV, and ATV for periods in excess of ≥2 years appeared to be protective for cLEE (Figure 2, Supplementary Table 1).
Figure 2.
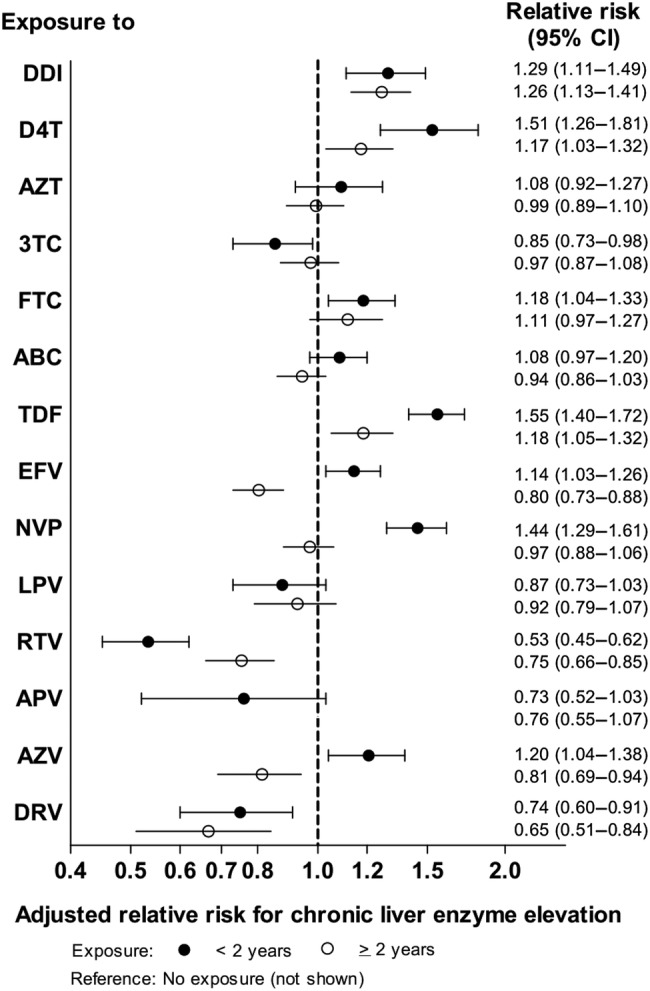
Incidence rate ratios and 95% confidence intervals (CIs) for the development of chronic liver enzyme elevation as a function of duration of antiretroviral drug exposure. Results are from Poisson regression based on 6368 events among 21 485 participants with 105 413 person years of follow-up. Models were adjusted for exposure to the other antiretrovirals, sex, age, ethnicity, body mass index, lipids, use of lipid-lowering drugs, lipodystrophy, arterial hypertension, smoking status, calendar year, and participating cohort, and for each drug discontinued for <2 years and ≥2 years. Abbreviations: ABC, abacavir; APV, amprenavir; AZT, zidovudine; AZV, atazanavir; D4T, stavudine; DDI, didanosine; DRV, darunavir; EFV, efavirenz; FTC, emtricitabine; LPV, lopinavir; NVP, nevirapine; RTV, ritonavir; TDF, tenofovir disoproxil fumarate; 3TC, lamivudine.
Because the association of TDF with cLEE was unexpected, we further analyzed commonly used TDF-containing regimens. The association was found to be more pronounced when TDF was used in combination with FTC and/or EFV (Figure 3).
Figure 3.
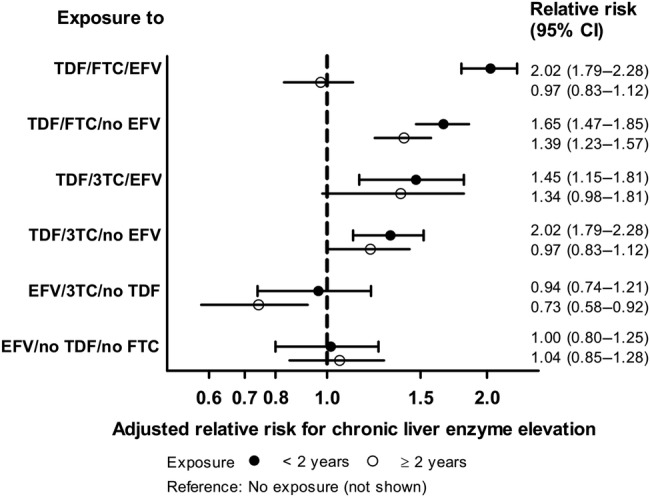
Incidence rate ratios and 95% confidence intervals (CIs) for the development of chronic liver enzyme elevation as a function of duration of antiretroviral drug exposure. Analyses are based on 6368 events among 21 485 participants with 105 413 person years of follow-up. Poisson regression models were adjusted for exposure to all other antiretrovirals (excluding lamivudine [3TC], emtricitabine [FTC], efavirenz [EFV], and tenofovir disoproxil fumarate [TDF]), sex, age, ethnicity, body mass index, lipids, use of lipid lowering drugs, lipodystrophy, arterial hypertension, smoking status, calendar year, and participating cohort.
Sensitivity Analyses
To substantiate the positive association between TDF and cLEE, we performed several sensitivity analyses. First, we excluded all participants who acquired HBV/HCV during follow-up, or who ultimately developed end-stage liver disease (ESLD), who might have received TDF for an unreported HBV infection or because of its perceived good liver safety profile. Second, we limited the analyses to ART-naive persons initiating TDF to rule out hepatotoxicity caused by a previous drug exposure. Third, we used a modified LEE definition of a single ALT value of ≥100 U/L. Results of all sensitivity analyses were consistent with our main analyses.
Clinical Outcome
Of the 6368 participants with ≥1 episode of cLEE, 1758 (27.6%) had at least 1 subsequent additional episode of cLEE. In total, the participants experienced a median of 1 (IQR, 1–6) episodes of cLEE with a median total duration of 1.5 (IQR, 0.5–13.7) years.
Overall, 924 persons died over a total of 151 191 PYFU (rate 0.61/100 PYFU; IQR, 0.57–0.65). All-cause mortality was slightly higher in those who ever had an episode of cLEE compared with participants without cLEE (0.66/100 PYFU versus 0.60/100 PYFU), but this was not significant, either when unadjusted (rate ratio [RR] = 1.10; IQR, 0.95–1.28; P = .19), adjusted for basic demographic variables (RR = 1.13; IQR, 0.97–1.32; P = .11), or adjusted additionally for the latest CD4 cell count and HIV viral load (RR = 1.15; IQR, 0.99–1.34; P = .08). Neither number of episodes of cLEE nor total duration of cLEE was associated with an increased risk of mortality in unadjusted or adjusted analyses (Table 2). The total number of liver-related deaths was 15 with 5 events among persons with cLEE and 10 events among those without any cLEE.
Table 2.
Associations between cLEE, number of Episodes of cLEE, Total Duration of cLEE, and All-Cause Mortalitya
| Events | All-Cause Mortality |
Unadjusted |
Adjusted (1) |
Adjusted (2) |
||||
|---|---|---|---|---|---|---|---|---|
| Deaths/PY | Rate (95% CI)/100 PY | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| Overall | 924/151191 | 0.61 (.57, .65) | ||||||
| Any cLEE | ||||||||
| No | 682/114389 | 0.60 (.55, .64) | Ref. | Ref. | Ref. | |||
| Yes | 242/36802 | 0.66 (.57, .74) | 1.10 (.95, 1.28) | .19 | 1.13 (.97, 1.32) | .11 | 1.15 (.99, 1.34) | .08 |
| No. of episodes of cLEE | ||||||||
| None | 682/114389 | 0.60 (.55, .64) | ||||||
| 1 | 192/28835 | 0.67 (.57, .76) | ||||||
| 2 | 41/6539 | 0.62 (.43, .82) | ||||||
| 3 | 9/1168 | 0.77 (.35, 1.46) | ||||||
| ≥4 | 0/230 | 0 (, 1.60) | ||||||
| Per cLEE | 1.05 (.95, 1.17) | .35 | 1.06 (.96, 1.18) | .25 | 1.07 (.96, 1.19) | .20 | ||
| Total duration of cLEE, years | ||||||||
| ≤0.5 | 685/117583 | 0.58 (.54, .63) | ||||||
| >0.5–1 | 90/13227 | 0.68 (.54, .82) | ||||||
| >1–1.5 | 40/5745 | 0.70 (.48, .91) | ||||||
| >1.5–2 | 30/3935 | 0.76 (.49, 1.04) | ||||||
| >2–3 | 51/4566 | 1.12 (.81, 1.42) | ||||||
| >3–4 | 18/2527 | 0.71 (.42, 1.13) | ||||||
| >4 | 10/3609 | 0.28 (.13, .51) | ||||||
| Per year | 1.02 (.96, 1.07) | .61 | 1.01 (.95, 1.07) | .76 | 1.01 (.96, 1.07) | .67 | ||
Abbreviations: CI, confidence interval; cLEE, chronic liver enzyme elevation; HIV, human immunodeficiency virus; PY, patient years; Ref., reference; RR, rate ratio.
a Adjusted for (1) sex, age, ethnicity, mode of HIV acquisition, body mass index, smoking status, calendar year, and participating cohort, and (2) additionally for the latest CD4 cell count and HIV RNA value.
DISCUSSION
In this large longitudinal cohort analysis, the incidence of cLEE among HIV-positive individuals without viral hepatitis was high. In adjusted analyses, cLEE was associated with ongoing exposure to regimens containing ddI, d4T, and TDF and short-term exposure to NVP, EFV, FTC, and ATV. However, mortality did not appear to be increased in HIV-monoinfected participants with cLEE.
The potential for drug-induced liver toxicity of d4T, ddI, NVP, and EFV has previously been described [19]. In HIV-monoinfected and HCV-coinfected individuals, d4T and ddI have been associated with transaminase elevation, FIB, steatosis, and ESLD, most likely related to mitochondrial toxicity [3, 6–10]. In our study, NVP and EFV, known to cause early acute hepatitis driven by an immune-mediated hypersensitivity reaction, were associated with elevated ALT in the first 2 years of exposure [19, 20].
We were surprised to find a strong relationship between TDF and the development of cLEE emerging within the first 2 years after drug initiation. We confirmed this association using different sensitivity analyses. In another recent D:A:D Study of HIV-monoinfected and HCV/HBV-coinfected participants, TDF was also found to be associated with ESLD/hepatocellular carcinoma, supporting our observation [10]. In addition, our results are consistent with small case studies [21–24]. There are a few reports of liver injury and hepatic failure, including 1 person requiring liver transplantation, developing a few months after starting treatment with TDF/FTC/EFV in persons without preexisting liver disease [22–24]. Lattuada et al [21] reported on 3 individuals with liver enzyme elevation after the addition of TDF to an EFV-containing regimen. The drug registration trials did not identify a link between TDF and hepatotoxicity [25, 26]. The STEAL study, a randomized trial comparing abacavir/3TC versus TDF/FTC, showed a small but statistically significant increase in mean ALT levels on TDF/FTC [27], whereas the AIDS Clinical Trials Group A5202, a similar study, showed inconsistent results regarding elevated ALT values [28]. Randomized trials usually enroll participants who are healthier and younger and may therefore not be representative of the general population. Moreover, these trials with viral primary outcomes often lack statistical power to detect rare adverse drug effects due to their limited sample size.
The mechanisms of a possible TDF-related liver injury are unclear. In vitro data showed that TDF is not associated with mitochondrial toxicity [29]. In an animal study, it was found that TDF induced marked oxidative stress in renal and hepatic tissues of Wistar rats [30]. Our finding of the enhanced TDF signal when used in combination with EFV could reflect drug-drug interactions of TDF, which have been described with other antiretrovirals including ddI and ATV [31, 32]. It is interesting to note that in slow EFV metabolizers, such as those with CYP2B6 loss/diminished-function alleles, EFV plasma area-under-the-curve values were highest among patients receiving TDF [33].
In line with other recent reports, most PIs were not associated with liver toxicity [34]. We found that ATV during the first 2 years of exposure was predictive, but with ≥2 years of exposure ATV was protective for cLEE. In a previous study, ATV was associated with grade 3 LEE in HIV/HCV-seropositive individuals [34], whereas other investigations suggested that liver tolerability of ATV was good [35, 36]. Continuing surveillance for possible ATV-related hepatotoxicity is warranted.
Our data confirm that metabolic features, including dyslipidemia and a high BMI, are one of the main causes of liver disease in HIV-positive persons without chronic hepatitis B and C coinfection, similar to the general population. Our finding of black ethnicity as a negative predictor for ALT elevation is in agreement with other investigations, which found an inverse association of black ethnicity and hepatic steatosis in the general population and among HIV-infected patients [37, 38]. A high HIV viral load was associated with an increased risk of hepatopathy compared with individuals on ART with suppressed viral load, supporting the evidence that HIV itself is contributing to liver injury [39].
We found a low number of liver-related deaths among HIV-monoinfected individuals, in accordance with a previous D:A:D Study [40]. All-cause mortality was not significantly increased among persons with cLEE. Because patients are regularly monitored for their ALT levels, this allows the clinician an early warning and the possibility to switch drugs or introduce risk modification (eg, alcohol reduction, diet), reducing the potential impact from ART-related cLEE on mortality. Another possibility for why we did not see a link between cLEE and mortality may be a relatively short follow-up period. In the large population-based US National Health and Nutrition Examination Survey III study, elevated ALT levels in HCV/HBV-negative persons were associated with a higher risk of liver-related death but not with all-cause death [41]. In contrast, studies including HCV/HBV-positive persons suggested both increased liver-related and all-cause mortality among persons with liver enzyme elevation in the general and HIV-positive population [12, 42]. Recent data indicate that cLEE is a marker of serious liver diseases. A large liver biopsy series of HIV-monoinfected individuals with cLEE revealed in two thirds of patients significant histological abnormalities with nonalcoholic steatohepatitis (NASH) in 55% and bridging FIB in 17% [14]. Given the long lag time between asymptomatic liver enzyme elevation and liver-related death, a study with a longer observation period may find an association between the two.
The strength of this study is its large size and the long-term prospective observation of a multinational population-based cohort collaboration. Such large observational studies are crucial to detect infrequent drug-related toxicities on the population level. To assess an individual drug effect might be challenging because antiretrovirals are always used in combination regimens. The large D:A:D Study, however, with extensive follow-up of patients drawn from heterogeneous settings should allow to disentangle individual drug effects because there is generally enough variability in the way an individual drug is used in combination with other drugs. Our study has several limitations. Information on alcohol use was not collected systematically. However, it is unlikely that the observed associations between antiretrovirals and cLEE are confounded by alcohol because alcohol consumption does not modify a physician's choice of ART. Moreover, by excluding HCV/HBV-coinfected persons and therefore most injection drug users with risk for multiple substance-dependence syndromes, including alcohol, many at-risk individuals were excluded. Furthermore, we did not have information on potentially hepatotoxic non-antiretroviral drugs. Finally, as a significant number of antiretrovirals were included in the analysis, we cannot rule out that findings may be a result of multiple testing.
CONCLUSIONS
In summary, the long-term observation of HIV-monoinfected individuals in this large cohort revealed a high incidence of cLEE. Besides the antiretrovirals ddI, d4T, NVP, and EFV, previously described to be hepatotoxic, FTC, and ATV restricted to the first 2 years of treatment, we observed an additional strong association between TDF exposure and cLEE emerging within the first 2 years after drug initiation. Although mortality was not increased in our study, other studies found relevant histological abnormalities in HIV-monoinfected persons, with elevated ALT underscoring its link to significant liver morbidity [13, 14]. In this context, our results emphasize (1) that ddI and d4T should be avoided if alternative treatment is available and (2) that close monitoring of liver enzymes in persons on NVP and EFV is essential. Finally, the observed novel association between TDF and cLEE calls for further investigations to understand the pathophysiological mechanisms and its clinical implications. Ongoing surveillance of drug-related liver injury in large cohort collaborations is of paramount importance.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
D:A:D Steering Committee: Cohort representatives: F. Dabis (Aquitaine), O. Kirk (EuroSIDA), M. Law (AHOD), A. d'Arminio Monforte (ICONA), C. Pradier (Nice), P. Reiss (ATHENA), R. Weber (SHCS), S. De Wit (Brussels), W. El-Sadr (CPCRA). Central coordination: L. Ryom, J. D Lundgren (chair). Statisticians: C. A Sabin, A. N Phillips. Oversight Committee representatives: B. Powderly, N. Shortman, C. Moecklinghoff, G. Reilly, X. Franquet. Members of the D:A:D Steering Committee From the Oversight Committee: B. Powderly, N. Shortman, C. Moecklinghoff, G. Reilly, and X. Franquet. D:A:D Central Coordination: C. I. Hatleberg, L. Ryom, C. A. Sabin, D. Kamara, C. Smith, A. Phillips, A. Mocroft, A. Bojesen, J. Nielsen, C. Matthews, D. Raben, and J. D. Lundgren (chair). D:A:D Data Managers: R. Salbøl Brandt (coordinator), M. Rickenbach, I. Fanti, E. Krum, M. Hillebregt, S. Geffard, Jaohar Mourabi, A. Sundström, M. Delforge, E. Fontas, F. Torres, H. McManus, S. Wright, J. Kjær, and Dennis Kristensen.
Verification of Endpoints: A. Sjøl (cardiovascular disease primary endpoint), P. Meidahl (oncology, new endpoint), J. Helweg-Larsen (hematology, new endpoint), and J. Schmidt Iversen (nephrology, new endpoint). Kidney Working Group: L. Ryom, A. Mocroft, O. Kirk, P. Reiss, M. Ross, C. A. Fux, P. Morlat, O. Moranne, A. M. Kesselring, D. A. Kamara, C. Smith, and J. D. Lundgren (chair). Mortality Working Group: C. Smith, L. Ryom, A. Phillips, R. Weber , P. Morlat, C. Pradier, P. Reiss, N. Friis-Møller, J. Kowalska, and J. D. Lundgren (chair). Cancer Working Group: C. Sabin, M. Law, A. d'Arminio Monforte, F. Dabis, M. Bruyand, P. Reiss, C. Smith, D. A. Kamara, M. Bower, G. Fätkenheuer, A. Grulich, L. Ryom, and J. D. Lundgren (chair).
The members of the 11 cohorts are as follows.
AIDS Therapy Evaluation Project Netherlands (ATHENA). Central Coordination: P. Reiss, S. Zaheri, M. Hillebregt, and L. Gras.
Participating physicians (site coordinating physicians): Academisch Medisch Centrum bij de Universiteit van Amsterdam, Amsterdam: Prof. Dr. J. M. Prins, Prof. Dr. T. W. Kuijpers, Dr. H. J. Scherpbier, Dr. J. T. M. van der Meer, Dr. F. W. M. N. Wit, Dr. M. H. Godfried, Prof. Dr. P. Reiss, Prof. Dr. T. van der Poll, Dr. F. J. B. Nellen, Prof. Dr. J. M. A. Lange, Dr. S. E. Geerlings, Dr. M. van Vugt, Dr. D. Pajkrt, Dr. J. C. Bos, Dr. M. van der Valk, Dr. M. L. Grijsen, Dr. W. J. Wiersinga, Dr. A. Goorhuis, and Dr. J. W. R. Hovius. Academisch Ziekenhuis Maastricht, Maastricht: Dr. S. Lowe, Dr. A. Oude Lashof, and Dr. D. Posthouwer. Catharina-Ziekenhuis, Eindhoven: Dr. M. J. H. Pronk and Dr. H. S. M. Ammerlaan. Erasmus Medisch Centrum, Rotterdam: Dr. M. E. van der Ende, Dr. T. E. M. S. de Vries-Sluijs, Dr. C. A. M. Schurink, Dr. J. L. Nouwen, Dr. A. Verbon, Dr. B. J. A. Rijnders, Dr. E. C. M. van Gorp, and Dr. M. van der Feltz. Erasmus Medisch Centrum–Sophia, Rotterdam: Dr. G. J. A. Driessen, and Dr. A. M. C. van Rossum. Flevoziekenhuis, Almere: Dr. J. Branger. HagaZiekenhuis, Den Haag: Dr. E. F. Schippers, Dr. C. van Nieuwkoop, and Dr. E. P. van Elzakker. Isala Klinieken, Zwolle: Dr. P. H. P. Groeneveld and Dr. J. W. Bouwhuis. Kennemer Gasthuis: Dr. R. Soetekouw and Prof. Dr. R. W. ten Kate. Leids Universitair Medisch Centrum, Leiden: Dr. F. P. Kroon, Prof. Dr. J. T. van Dissel, Dr. S. M. Arend, Dr. M. G. J. de Boer, Dr. H. Jolink, Dr. H. J. M. ter Vollaard, and Dr. M. P. Bauer. Maasstadziekenhuis, Rotterdam: Dr. J. G. den Hollander and Dr. K. Pogany. Medisch Centrum Alkmaar, Alkmaar: Dr. G. van Twillert, Dr. W. Kortmann, Dr. J. W. T. Cohen Stuart, and Dr. B. M. W. Diederen. Medisch Centrum Haaglanden, Den Haag: Dr. E. M. S. Leyten and Dr. L. B. S. Gelinck. Medisch Spectrum Twente, Enschede: Dr. G. J. Kootstra and Dr. C. E. Delsing. Onze Lieve Vrouwe Gasthuis, Amsterdam: Prof. Dr. K. Brinkman, Dr. W. L. Blok, Dr. P. H. J. Frissen, Dr. W. E. M. Schouten, and Dr. G. E. L. van den Berk. Sint Elisabeth Ziekenhuis, Tilburg: Dr. M. E. E. van Kasteren and Dr. A. E. Brouwer. Sint Lucas Andreas Ziekenhuis, Amsterdam: Dr. J. Veenstra and Dr. K. D. Lettinga. Slotervaartziekenhuis, Amsterdam: Dr. J. W. Mulder, Dr. S. M. E. Vrouenraets, and Dr. F. N. Lauw. Stichting Medisch Centrum Jan van Goyen, Amsterdam: Dr. A. van Eeden and Dr. D. W. M. Verhagen. Universitair Medisch Centrum Groningen, Groningen: Dr. H. G. Sprenger, Dr. R. Doedens, Dr. E. H. Scholvinck, Dr. S. van Assen, and Dr. W. F. W. Bierman. Universitair Medisch Centrum Sint Radboud, Nijmegen: Dr. P. P. Koopmans, Dr. M. Keuter, Dr. A. J. A. M. van der Ven, Dr. H. J. M. ter Hofstede, Dr. A. S. M. Dofferhoff, Dr. A Warris, and Dr. R. van Crevel. Universitair Medisch Centrum Utrecht, Utrecht: Prof. Dr A. I. M. Hoepelman, Dr. T. Mudrikova, Dr. M. M. E. Schneider, Dr. P. M. Ellerbroek, Dr. J. J. Oosterheert, Dr. J. E. Arends, Dr. M. W. M. Wassenberg, and Dr. R. E. Barth. Vrije Universiteit Amsterdam, Amsterdam: Dr. M. A. van Agtmael, Dr. R. M. Perenboom, Dr. F. A. P. Claessen, Dr. M. Bomers, and Dr. E. J. G. Peters. Wilhelmina Kinderziekenhuis, Utrecht: Dr. S. P. M. Geelen, Dr. T. F. W. Wolfs, and Dr. L. J. Bont. Ziekenhuis Rijnstate, Arnhem: Dr. C. Richter, Dr. J. P. van der Berg, and Dr. E. H. Gisolf. Admiraal De Ruyter Ziekenhuis, Vlissingen: Dr. M. van den Berge and Dr. A. Stegeman. Medisch Centrum Leeuwarden, Leeuwarden: Dr. M. G. A. van Vonderen and Dr. D. P. F. van Houte. Medisch Centrum Zuiderzee, Lelystad: Dr. S. Weijer and Dr. R. el Moussaoui. Sint Elisabeth Hospitaal, Willemstad - Curaçao: Dr. C. Winkel, Dr. F. Muskiet, Dr. Durand, and Dr. R. Voigt.
The following is a list of the Aquitaine Cohort (France).
Coordination: F. Bonnet, F. Dabis. Scientific Committee: F. Bonnet, S. Bouchet, D. Breilh, G. Chêne, F. Dabis, M. Dupon, H. Fleury, V. Gaborieau, D. Lacoste, D. Malvy, P. Mercié, P. Morlat, D. Neau, I. Pellegrin, J. L. Pellegrin, S. Reigadas, S. Tchamgoué. Epidemiology and Methodology: M. Bruyand, G. Chêne, F. Dabis, C. Fagard, S. Lawson-Ayayi, L. Richert, R. Thiébaut, L. Wittkop. Infectious Diseases and Internal Medicine: K. André, F. Bonnet, N. Bernard, L. Caunègre, C. Cazanave, J. Ceccaldi, I. Chossat, C. Courtault, F. A. Dauchy, S. De Witte, D. Dondia, M. Dupon, A. Dupont, P. Duffau, H. Dutronc, S. Farbos, I. Faure, V. Gaborieau, Y. Gerard, C. Greib, M. Hessamfar-Joseph, Y. Imbert, D. Lacoste, P. Lataste, E. Lazaro, D. Malvy, J. Marie, M. Mechain, J. P. Meraud, P. Mercié, E. Monlun, P. Morlat, D. Neau, A. Ochoa, J. L. Pellegrin, M. Pillot-Debelleix, T. Pistone, I. Raymond, M. C. Receveur, P. Rispal, L. Sorin, S. Tchamgoué, C. Valette, M. A. Vandenhende, M. O. Vareil, J. F. Viallard, H. Wille, G. Wirth. Immunology: J. F. Moreau, I. Pellegrin. Virology: H. Fleury, M. E. Lafon, S. Reigadas, P. Trimoulet. Pharmacology: S. Bouchet, D. Breilh, F. Haramburu, G. Miremont-Salamé. Data Collection, Project Management and Statistical Analyses: M. J. Blaizeau, I. Crespel, M. Decoin, S. Delveaux, F. Diarra, C. D′Ivernois, C. Hanappier, D. Lacoste, S. Lawson-Ayayi, O. Leleux, F. Le Marec, E. Lenaud, J. Mourali, E. Pernot, A. Pougetoux, B. Uwamaliya-Nziyumvira, A. Tsaranazy, A. Valdes. IT Department and eCRF Development: V. Conte, I. Louis, G. Palmer, V. Sapparrart, D. Touchard.
Australian HIV Observational Database, Australia (AHOD):
Central coordination: M. Law, K. Petoumenos, H. McManus, S. Wright, C. Bendall (Sydney, New South Wales);
Participating physicians: R. Moore, S. Edwards, J. Hoy, K. Watson, N. Roth, J. Nicholson (Melbourne, Victoria); M. Bloch, T. Franic, D. Baker, R. Vale, A. Carr, D. Cooper (Sydney, New South Wales); J. Chuah, M. Ngieng (Gold Coast, Queensland), D. Nolan, J. Skett (Perth, Western Australia).
BASS (Spain):
Central coordination: G. Calvo, F. Torres, S. Mateu (Barcelona). Participating physicians: P. Domingo, M. A. Sambeat, J. Gatell, E. Del Cacho, J. Cadafalch, M. Fuster (Barcelona); C. Codina, G. Sirera, A. Vaqué (Badalona).
The Brussels St. Pierre Cohort (Belgium):
Coordination: S. De Wit, N. Clumeck, M. Delforge, C. Necsoi. Participating physicians: N. Clumeck, S. De Wit, A. F. Gennotte, M. Gerard, K. Kabeya, D. Konopnicki, A. Libois, C. Martin, M. C. Payen, P. Semaille, Y. Van Laethem.
Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) (United States):
Central coordination: J. Neaton, G. Bartsch, W. M. El-Sadr, E. Krum, G. Thompson, D. Wentworth. Participating physicians: R. Luskin-Hawk (Chicago, IL); E. Telzak (Bronx, NY); W. M. El-Sadr (Harlem, NY); D. I. Abrams (San Francisco, CA); D. Cohn (Denver, CO); N. Markowitz (Detroit, MI); R. Arduino (Houston, TX); D. Mushatt (New Orleans, LA); G. Friedland (New Haven, CT); G. Perez (Newark, NJ); E. Tedaldi (Philadelphia, PA); E. Fisher (Richmond, VA); F. Gordin (Washington, DC); L. R. Crane (Detroit, MI); J. Sampson (Portland, OR); J. Baxter (Camden, NJ).
EuroSIDA (multinational) Coordinating Centre: J. Lundgren (chair), O. Kirk, A. Mocroft, A. Cozzi-Lepri, D. Grint, D. Podlekareva, J. Kjær, L. Peters, J. Reekie, J. Kowalska, J. Tverland, A. H. Fischer, J. Nielsen. Participating countries and physicians Argentina: M. Losso, C. Elias (Hospital JM Ramos Mejia, Buenos Aires).
Austria: N. Vetter (Pulmologisches Zentrum der Stadt Wien, Vienna); R. Zangerle (Medical University Innsbruck, Innsbruck). Belarus: I. Karpov, A. Vassilenko (Belarus State Medical University, Minsk); V. M. Mitsura (Gomel State Medical University, Gomel); O. Suetnov (Regional AIDS Centre, Svetlogorsk). Belgium: N. Clumeck, S. De Wit, M. Delforge (Saint-Pierre Hospital, Brussels); R. Colebunders (Institute of Tropical Medicine, Antwerp); L. Vandekerckhove (University Ziekenhuis Gent, Gent). Bosnia-Herzegovina: V. Hadziosmanovic (Klinicki Centar Univerziteta Sarajevo, Sarajevo). Bulgaria: K. Kostov (Infectious Diseases Hospital, Sofia). Croatia: J. Begovac (University Hospital of Infectious Diseases, Zagreb). Czech Republic: L. Machala, D. Jilich (Faculty Hospital Bulovka, Prague); D. Sedlacek (Charles University Hospital, Plzen). Denmark: J. Nielsen, G. Kronborg, T. Benfield, M. Larsen (Hvidovre Hospital, Copenhagen); J. Gerstoft, T. Katzenstein, A.-B. E. Hansen, P. Skinhøj (Rigshospitalet, Copenhagen); C. Pedersen (Odense University Hospital, Odense); L. Ostergaard (Skejby Hospital, Aarhus). Estonia: K. Zilmer (West-Tallinn Central Hospital, Tallinn); Jelena Smidt, Nakkusosakond Siseklinik (Kohtla-Järve). Finland: M. Ristola (Helsinki University Central Hospital, Helsinki). France: C. Katlama (Hôpital de la Pitié-Salpétière, Paris); J.-P. Viard (Hôpital Necker-Enfants Malades, Paris); P.-M. Girard (Hospital Saint-Antoine, Paris); J. M. Livrozet (Hôpital Edouard Herriot, Lyon); P. Vanhems (University Claude Bernard, Lyon); C. Pradier (Hôpital de l'Archet, Nice); F. Dabis, D. Neau (Unité INSERM, Bordeaux). Germany: J. Rockstroh (Universitäts Klinik Bonn); R. Schmidt (Medizinische Hochschule Hannover); J. van Lunzen, O. Degen (University Medical Center Hamburg-Eppendorf, Infectious Diseases Unit, Hamburg); H. J. Stellbrink (IPM Study Center, Hamburg); S. Staszewski, J. W. (Goethe University Hospital, Frankfurt); Markus Bickel (Medizinische Poliklinik, Munich); G. Fätkenheuer (Universität Köln, Cologne). Greece: J. Kosmidis, P. Gargalianos, G. Xylomenos, J. Perdios (Athens General Hospital); G. Panos, A. Filandras, E. Karabatsaki (1st IKA Hospital); H. Sambatakou (Ippokration Genereal Hospital, Athens). Hungary: D. Banhegyi (Szent Lásló Hospital, Budapest). Ireland: F. Mulcahy (St. James's Hospital, Dublin). Israel: I. Yust, D. Turner, M. Burke (Ichilov Hospital, Tel Aviv); S. Pollack, G. Hassoun (Rambam Medical Center, Haifa); S. Maayan (Hadassah University Hospital, Jerusalem). Italy: S. Vella (Istituto Superiore di Sanità, Rome); R. Esposito, I. Mazeu, C. Mussini (Università Modena, Modena); C. Arici (Ospedale Riuniti, Bergamo); R. Pristera (Ospedale Generale Regionale, Bolzano); F. Mazzotta, A. Gabbuti (Ospedale Santa Maria Annunziata, Firenze); V. Vullo, M. Lichtner (University di Roma la Sapienza, Rome); A. Chirianni, E. Montesarchio, M. Gargiulo (Presidio Ospedaliero AD Cotugno, Monaldi Hospital, Napoli); G. Antonucci, A. Testa, P. Narciso, C. Vlassi, M. Zaccarelli (Istituto Nazionale Malattie Infettive Lazzaro Spallanzani, Rome); A. Lazzarin, A. Castagna, N. Gianotti (Ospedale San Raffaele, Milan); M. Galli, A. Ridolfo (Osp. L. Sacco, Milan); A. d′Arminio Monforte (Istituto Di Clinica Malattie Infettive e Tropicale, Milan). Latvia: B. Rozentale, I. Zeltina (Infectology Centre of Latvia, Riga). Lithuania: S. Chaplinskas (Lithuanian AIDS Centre, Vilnius). Luxembourg: R. Hemmer, T. Staub (Centre Hospitalier, Luxembourg). Netherlands: P. Reiss (Academisch Medisch Centrum bij de Universiteit van Amsterdam, Amsterdam). Norway: V. Ormaasen, A. Maeland, J. Bruun (Ullevål Hospital, Oslo). Poland: B. Knysz, J. Gasiorowski (Medical University, Wroclaw); A. Horban, E. Bakowska (Centrum Diagnostyki i Terapii AIDS, Warsaw); A. Grzeszczuk, R. Flisiak, Medical University, Bialystok; A. Boron-Kaczmarska, M. Pynka, M. Parczewski (Medical Univesity, Szczecin); M. Beniowski, E. Mularska (Osrodek Diagnostyki i Terapii AIDS, Chorzow); H. Trocha (Medical University, Gdansk); E. Jablonowska, E. Malolepsza, K. Wojcik (Wojewodzki Szpital Specjalistyczny, Lodz). Portugal: F. Antunes, M. Doroana, L. Caldeira (Hospital Santa Maria, Lisbon); K. Mansinho (Hospital de Egas Moniz, Lisbon); F. Maltez (Hospital Curry Cabral, Lisbon). Romania: D. Duiculescu (Spitalul de Boli Infectioase si Tropicale Dr. Victor Babes, Bucarest). Russia: A. Rakhmanova (Medical Academy Botkin Hospital, St. Petersburg); N. Zakharova (St. Petersburg AIDS Centre, St. Peterburg); S. Buzunova (Novgorod Centre for AIDS, Novgorod). Serbia: D. Jevtovic (The Institute for Infectious and Tropical Diseases, Belgrade). Slovakia: M. Mokráš, D. Staneková (Dérer Hospital, Bratislava). Slovenia: J. Tomazic (University Clinical Centre Ljubljana, Ljubljana). Spain: J. González-Lahoz, V. Soriano, P. Labarga, J. Medrano (Hospital Carlos III, Madrid); S. Moreno, J. M. Rodriguez (Hospital Ramon y Cajal, Madrid); B. Clotet, A. Jou, R. Paredes, C. Tural, J. Puig, I. Bravo (Hospital Germans Trias i Pujol, Badalona); J. M. Gatell, J. M. Miró (Hospital Clinic i Provincial, Barcelona); P. Domingo, M. Gutierrez, G. Mateo, M. A. Sambeat (Hospital Sant Pau, Barcelona). Sweden: A. Karlsson (Venhaelsan-Sodersjukhuset, Stockholm); L. Flamholc (Malmö University Hospital, Malmö). Switzerland: B. Ledergerber, R. Weber (University Hospital, Zürich); P. Francioli, M. Cavassini (Centre Hospitalier Universitaire Vaudois, Lausanne); B. Hirschel, E. Boffi (Hospital Cantonal Universitaire de Geneve, Geneve); H. Furrer (Inselspital Bern, Bern); M. Battegay, L. Elzi (University Hospital Basel). Ukraine: E. Kravchenko, N. Chentsova (Kiev Centre for AIDS, Kiev); V. Frolov, G. Kutsyna (Luhansk State Medical University, Luhansk); S. Servitskiy (Odessa Region AIDS Center, Odessa); M. Krasnov (Kharkov State Medical University, Kharkov). United Kingdom: S. Barton (St. Stephen's Clinic, Chelsea and Westminster Hospital, London); A. M. Johnson, D. Mercey (Royal Free and University College London Medical School, London [University College Campus]); A. Phillips, M. A. Johnson, A. Mocroft (Royal Free and University College Medical School, London [Royal Free Campus]); M. Murphy (Medical College of Saint Bartholomew's Hospital, London); J. Weber, G. Scullard (Imperial College School of Medicine at St. Mary's, London); M. Fisher (Royal Sussex County Hospital, Brighton); C. Leen (Western General Hospital, Edinburgh).
HivBivus (Sweden):
Central coordination: L. Morfeldt, G. Thulin, A. Sundström. Participating physicians: B. Åkerlund (Huddinge); K. Koppel, A. Karlsson (Stockholm); L. Flamholc, C. Håkangård (Malmö).
The ICONA Foundation (Italy):
Board of Directors: M. Moroni (Chair), G. Angarano, A. Antinori, O. Armignacco, A. d′Arminio Monforte, F. Castelli, R. Cauda, G. Di Perri, M. Galli, R. Iardino, G. Ippolito, A. Lazzarin, C. F. Perno, F. von Schloesser, P. Viale. Scientific Secretary: A. d′Arminio Monforte, A. Antinori, A. Castagna, F. Ceccherini-Silberstein, A. Cozzi-Lepri, E. Girardi, S. Lo Caputo, C. Mussini, M. Puoti. ICONA Steering Committee: M. Andreoni, A. Ammassari, A. Antinori, A. d′Arminio Monforte, C. Balotta, P. Bonfanti, S. Bonora, M. Borderi, R. Capobianchi, A. Castagna, F. Ceccherini-Silberstein, A. Cingolani, P. Cinque, A. Cozzi-Lepri, A. De Luca, A. Di Biagio, E. Girardi, N. Gianotti, A. Gori, G. Guaraldi, G. Lapadula, M. Lichtner, S. Lo Caputo, G. Madeddu, F. Maggiolo, G. Marchetti, S. Marcotullio, L. Monno, C. Mussini, M. Puoti, E. Quiros Roldan, S. Rusconi. Statistical and Monitoring Team: A. Cozzi-Lepri, P. Cicconi, I. Fanti, T. Formenti, L. Galli, P. Lorenzini. Participating Physicians and Centers: A. Giacometti, A. Costantini (Ancona); G. Angarano, L. Monno, C. Santoro (Bari); F. Maggiolo, C. Suardi (Bergamo); P. Viale, E. Vanino, G. Verucchi (Bologna); F. Castelli, E. Quiros Roldan, C. Minardi (Brescia); T. Quirino, C. Abeli (Busto Arsizio); P. E. Manconi, P. Piano (Cagliari); J. Vecchiet, K. Falasca (Chieti); L. Sighinolfi, D. Segala (Ferrara); F. Mazzotta, S. Lo Caputo (Firenze); G. Cassola, G. Viscoli, A. Alessandrini, R. Piscopo, G. Mazzarello (Genova); C. Mastroianni, V. Belvisi (Latina); P. Bonfanti, I. Caramma (Lecco); A. P. Castelli (Macerata); M. Galli, A. Lazzarin, G. Rizzardini, M. Puoti, A. d′Arminio Monforte, A. L. Ridolfo, R. Piolini, A. Castagna, S. Salpietro, L. Carenzi, M. C. Moioli, P. Cicconi, G. Marchetti (Milano); C. Mussini, C. Puzzolante (Modena); A. Gori, G. Lapadula (Monza); N. Abrescia, A. Chirianni, M. G. Guida, M. Onofrio (Napoli); F. Baldelli, D. Francisci (Perugia); G. Parruti, T. Ursini (Pescara); G. Magnani, M. A. Ursitti (Reggio Emilia); R. Cauda, M. Andreoni, A. Antinori, V. Vullo, A. Cingolani, A. d′Avino, A. Ammassari, L. Gallo, E. Nicastri, R. Acinapura, M. Capozzi, R. Libertone, G. Tebano (Roma); A. Cattelan (Rovigo); M. S. Mura, G. Madeddu (Sassari); P. Caramello, G. Di Perri, G. C. Orofino, S. Bonora, M. Sciandra (Torino); G. Pellizzer, V. Manfrin (Vicenza).
Nice HIV Cohort (France):
Central coordination: C. Pradier, E. Fontas, K. Dollet, C. Caissotti. Participating physicians: P. Dellamonica, E. Bernard, E. Cua, F. De Salvador-Guillouet, J. Durant, S. Ferrando, V. Mondain-Miton, A. Naqvi, I. Perbost, B. Prouvost-Keller, S. Pillet, P. Pugliese, V. Rahelinirina, P. M. Roger.
Swiss HIV Cohort Study, Switzerland (SHCS):
V. Aubert, M. Battegay, E. Bernasconi, J. Böni, H. C. Bucher, C. Burton-Jeangros, A. Calmy, M. Cavassini, G. Dollenmaier, M. Egger, L. Elzi, J. Fehr, J. Fellay, H. Furrer (Chairman of the Clinical and Laboratory Committee), C. A. Fux, M. Gorgievski, H. Günthard (President of the SHCS), D. Haerry (Deputy of “Positive Council”), B. Hasse, H. H. Hirsch, M. Hoffmann, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, T. Klimkait, R. Kouyos, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, K. Metzner, N. Müller, D. Nadal, D. Nicca, G. Pantaleo, A. Rauch (Chairman of the Scientific Board), S. Regenass, M. Rickenbach (Head of Data Center), C. Rudin (Chairman of the Mother & Child Substudy), F. Schöni-Affolter, P. Schmid, J. Schüpbach, R. Speck, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, R. Weber, S. Yerly.
Financial support. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study was supported by the Highly Active Antiretroviral Therapy Oversight Committee, a collaborative committee with representation from academic institutions, the European Agency for the Evaluation of Medicinal Products, the United States Food and Drug Administration, the patient community, and pharmaceutical companies with licensed anti-human immunodeficiency virus (HIV) drugs in the European Union: AbbVie, Bristol-Myers Squibb, Gilead Sciences Inc., ViiV Healthcare, Merck & Co Inc., and Janssen Pharmaceuticals. This work was also funded by a grant from the Danish National Research Foundation (CHIP & PERSIMUNE; grant number DNRF126); a grant from the Dutch Ministry of Health, Welfare and Sport (ATHENA); a grant from the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (Action Coordonnée no. 7, Cohortes; to the Aquitaine Cohort); The Australian HIV Observational Database (AHOD) is funded as part of the Asia Pacific HIV Observational Database, a program of The Foundation for AIDS Research, amfAR, and is supported in part by a grant from the US National Institutes of Health's National Institute of Allergy and Infectious Diseases (grant number U01-AI069907) and by unconditional grants from Merck Sharp & Dohme, Gilead Sciences, Bristol-Myers Squibb, Boehringer Ingelheim Roche, Pfizer, GlaxoSmithKline, and Janssen Pharmaceuticals. The Kirby Institute is funded by The Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, The University of New South Wales. This work was additionally supported by grants from the Fondo de Investigación Sanitaria (grant number FIS 99/0887) and Fundación para la Investigación y la Prevención del SIDA en Espanã (grant number FIPSE 3171/00; to the Barcelona Antiretroviral Surveillance Study); the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants numbers 5U01AI042170-10 and 5U01AI046362-03; to the Terry Beirn Community Programs for Clinical Research on AIDS); grants from the BIOMED 1 (grant number CT94-1637) and BIOMED 2 (grant number CT97-2713) programs; the 5th Framework Program (grant number QLK2-2000-00773); the 6th Framework Program (grants number LSHP-CT-2006-018632), and the 7th Framework (FP7/2007-2013, EuroCoord n° 260694) programmes of the European Commission and unrestricted grants by Janssen R&D, Merck and Co. Inc., Pfizer Inc., GlaxoSmithKline LLC (the participation of centres from Switzerland is supported by The Swiss National Science Foundation [grant 108787]) to the EuroSIDA study; unrestricted educational grants from AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Pfizer, Janssen Pharmaceuticals to the Italian Cohort Naive to Antiretrovirals (The ICONA Foundation); and by a grant from the Swiss National Science Foundation (grant 148522) to the Swiss HIV Cohort Study.
Disclaimer. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Potential conflicts of interest. H. K. received independent scientific grant support from Gilead Sciences, travel grants from Merck Sharp & Dohme and Gilead Sciences, and attended advisory boards for Gilead Sciences. C. A. S. received funding from Gilead Sciences, ViiV Healthcare, Janssen-Cilag, and MSD for the membership of Data Safety and Monitoring Boards and Advisory Boards, for participation in speaker bureaus, and for the development of educational materials. B. L. received travel grants or honoraria from Bristol-Myers Squibb, Gilead, ViiV, Merck Sharp & Dohme, and Janssen. P. R. received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals Inc., Merck & Co., Bristol-Myers Squibb, and ViiV Healthcare; he has served on scientific advisory board for Gilead Sciences; he serves on Data Safety Monitoring Committee for Janssen Pharmaceuticals Inc.; he chaired a scientific symposium by ViiV Healthcare, for which his institution has received remuneration. M. L. received unrestricted research grants from Boehringer Ingelheim, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, and ViiV HealthCare, and DSMB siting fees from Sirtex Pty Ltd. C. P. received honoraria from Pfizer, Merck Sharp & Dome, Gilead Sciences, and ViiV HealthCare. O. K. received honoraria, consultancy, lecture fees, and travel grants from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Roche, and ViiV Healthcare, and served on Advisory Boards for Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare. R. W. received travel grants from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dome, Pfizer, Roche, TRB Chemedica, and Tibotec, the clinic has received unrestricted educational grants from GlaxoSmithKline, ViiV, and Gilead Sciences.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: on behalf of the D:A:D Study group, B. Powderly, N. Shortman, C. Moecklinghoff, G. Reilly, X. Franquet, C.I. Hatleberg, L. Ryom, C.A. Sabin, D. Kamara, C. Smith, A. Phillips, A. Mocroft, A. Bojesen, J. Nielsen, C. Matthews, D. Raben, J.D. Lundgren, R. Salbøl Brandt, M. Rickenbach, I. Fanti, E. Krum, M. Hillebregt, S. Geffard, Jaohar Mourabi, A. Sundström, M. Delforge, E. Fontas, F. Torres, H. McManus, S. Wright, J. Kjær, A. Sjøl, P. Meidahl, J. Helweg-Larsen, J. Schmidt Iversen, L. Ryom, A. Mocroft, O. Kirk, P. Reiss, M. Ross, C.A. Fux, P. Morlat, O. Moranne, A.M. Kesselring, D.A. Kamara, C. Smith, J.D. Lundgren, C. Smith, L. Ryom, A. Phillips, R. Weber, P. Morlat, C. Pradier, P. Reiss, N. Friis-Møller, J. Kowalska, J.D. Lundgren, C. Sabin, M. Law, A. d'Arminio Monforte, F. Dabis, M. Bruyand, P. Reiss, C. Smith, D.A. Kamara, M. Bower, G. Fätkenheuer, A. Grulich, L. Ryom, J.D. Lundgren, P. Reiss, S. Zaheri, M. Hillebregt, L. Gras, J.M. Prins, T.W. Kuijpers, H.J. Scherpbier, J.T.M. van der Meer, F.W.M.N. Wit, M.H. Godfried, P. Reiss, T. van der Poll, F.J.B. Nellen, J.M.A. Lange, S.E. Geerlings, M. van Vugt, D. Pajkrt, J.C. Bos, M. van der Valk, M.L. Grijsen, W.J. Wiersinga, A. Goorhuis, J.W.R. Hovius, S. Lowe, A. Oude Lashof, D. Posthouwer, M.J.H. Pronk, H.S.M. Ammerlaan, M.E. van der Ende, T.E.M.S. de Vries-Sluijs, C.A.M. Schurink, J.L. Nouwen, A. Verbon, B.J.A. Rijnders, E.C.M. van Gorp, M. van der Feltz, G.J.A. Driessen, A.M.C. van Rossum, J. Branger, E.F. Schippers, C. van Nieuwkoop, E.P. van Elzakker, P.H.P. Groeneveld, J.W. Bouwhuis, R. Soetekouw, R.W. ten Kate, F.P. Kroon, J.T. van Dissel, S.M. Arend, M.G.J. de Boer, H. Jolink, H.J.M. ter Vollaard, M.P. Bauer, J.G. den Hollander, K. Pogany, G. van Twillert, W. Kortmann, J.W.T. Cohen Stuart, B.M.W. Diederen, E.M.S. Leyten, L.B.S. Gelinck, G.J. Kootstra, C.E. Delsing, K. Brinkman, W.L. Blok, P.H.J. Frissen, W.E.M. Schouten, G.E.L. van den Berk, M.E.E. van Kasteren, A.E. Brouwer, J. Veenstra, K.D. Lettinga, J.W. Mulder, S.M.E. Vrouenraets, F.N. Lauw, A. van Eeden, D.W.M. Verhagen, H.G. Sprenger, R. Doedens, E.H. Scholvinck, S. van Assen, W.F.W. Bierman, P.P. Koopmans, M. Keuter, A.J.A.M. van der Ven, H.J.M. ter Hofstede, A.S.M. Dofferhoff, A. Warris, R. van Crevel, A.I.M. Hoepelman, T. Mudrikova, M.M.E. Schneider, P.M. Ellerbroek, J.J. Oosterheert, J.E. Arends, M.W.M. Wassenberg, R.E. Barth, M.A. van Agtmael, R.M. Perenboom, F.A.P. Claessen, M. Bomers, E.J.G. Peters, S.P.M. Geelen, T.F.W. Wolfs, L.J. Bont, C. Richter, J.P. van der Berg, E.H. Gisolf, M. van den Berge, A. Stegeman, M.G.A. van Vonderen, D.P.F. van Houte, S. Weijer, R. el Moussaoui, C. Winkel, F. Muskiet, R. Voigt, F. Bonnet, F. Dabis, F. Bonnet, S. Bouchet, D. Breilh, G. Chêne, F. Dabis, M. Dupon, H. Fleury, V. Gaborieau, D. Lacoste, D. Malvy, P. Mercié, P. Morlat, D. Neau, I. Pellegrin, JL. Pellegrin, S. Reigadas, S. Tchamgoué, M. Bruyand, G. Chêne, F. Dabis, C. Fagard, S. Lawson-Ayayi, L. Richert, R. Thiébaut, L. Wittkop, K. André, F. Bonnet, N. Bernard, L. Caunègre, C. Cazanave, J. Ceccaldi, I. Chossat, C. Courtault, F.A. Dauchy, S. De Witte, D. Dondia, M. Dupon, A. Dupont, P. Duffau, H. Dutronc, S. Farbos, I. Faure, V. Gaborieau, Y. Gerard, C. Greib, M. Hessamfar-Joseph, Y. Imbert, D. Lacoste, P. Lataste, E. Lazaro, D. Malvy, J. Marie, M. Mechain, JP. Meraud, P. Mercié, E. Monlun, P. Morlat, D. Neau, A. Ochoa, J.L. Pellegrin, M. Pillot-Debelleix, T. Pistone, I. Raymond, M.C. Receveur, P. Rispal, L. Sorin, S. Tchamgoué, C. Valette, M.A. Vandenhende, MO. Vareil, J.F. Viallard, H. Wille, G. Wirth, J.F. Moreau, I. Pellegrin, H. Fleury, M.E. Lafon, S. Reigadas, P. Trimoulet, S. Bouchet, D. Breilh, F. Haramburu, G. Miremont-Salamé, M.J. Blaizeau, I. Crespel, M. Decoin, S. Delveaux, F. Diarra, C. D'Ivernois, C. Hanappier, D. Lacoste, S. Lawson-Ayayi, O. Leleux, F. Le Marec, E. Lenaud, J. Mourali, E. Pernot, A. Pougetoux, B. Uwamaliya-Nziyumvira, A. Tsaranazy, A. Valdes, V. Conte, I. Louis, G. Palmer, V. Sapparrart, D. Touchard, M. Law, K. Petoumenos, H. McManus, S. Wright, C. Bendall, R. Moore, S. Edwards, J. Hoy, K. Watson, N. Roth, J. Nicholson, M. Bloch, T. Franic, D. Baker, R. Vale, A. Carr, D. Cooper, J. Chuah, M. Ngieng, D. Nolan, J. Skett, G. Calvo, F. Torres, S. Mateu, P. Domingo, M.A. Sambeat, J. Gatell, E. Del Cacho, J. Cadafalch, M. Fuster, C. Codina, G. Sirera, A. Vaqué, S. De Wit, N. Clumeck, M. Delforge, C. Necsoi, N. Clumeck, S. De Wit, A.F. Gennotte, M. Gerard, K. Kabeya, D. Konopnicki, A. Libois, C. Martin, M.C. Payen, P. Semaille, Y. Van Laethem, J. Neaton, G. Bartsch, W.M. El-Sadr, E. Krum, G. Thompson, D. Wentworth, R. Luskin-Hawk, E. Telzak, W.M. El-Sadr, D.I. Abrams, D. Cohn, N. Markowitz, R. Arduino, D. Mushatt, G. Friedland, G. Perez, E. Tedaldi, E. Fisher, F. Gordin, L.R. Crane, J. Sampson, J. Baxter, J. Lundgren, O. Kirk, A. Mocroft, A. Cozzi-Lepri, D. Grint, D. Podlekareva, J. Kjær, L. Peters, J. Reekie, J. Kowalska, J. Tverland, A. H. Fischer, J. Nielsen, C. Elias, N. Vetter, R. Zangerle, I. Karpov, A. Vassilenko, V.M. Mitsura, O. Suetnov, N. Clumeck, S. De Wit, M. Delforge, R. Colebunders, L. Vandekerckhove, V. Hadziosmanovic, K. Kostov, J. Begovac, L. Machala, D. Jilich, D. Sedlacek, J. Nielsen, G. Kronborg, T. Benfield, M. Larsen, J. Gerstoft, T. Katzenstein, A-B. E. Hansen, P. Skinhøj, C. Pedersen, L. Ostergaard, K. Zilmer, M. Ristola, C. Katlama, J-P. Viard, P-M. Girard, J.M. Livrozet, P. Vanhems, C. Pradier, F. Dabis, D. Neau, J. Rockstroh, R. Schmidt, J. van Lunzen, O. Degen, H.J. Stellbrink, S. Staszewski, G. Fätkenheuer, J. Kosmidis, P. Gargalianos, G. Xylomenos, J. Perdios, G. Panos, A. Filandras, E. Karabatsaki, H. Sambatakou, D. Banhegyi, F. Mulcahy, I. Yust, D. Turner, M. Burke, S. Pollack, G. Hassoun, S. Maayan, S. Vella, R. Esposito, I. Mazeu, C. Mussini, C. Arici, R. Pristera, F. Mazzotta, A. Gabbuti, S. Maria, V. Vullo, M. Lichtner, A. Chirianni, E. Montesarchio, M. Gargiulo, G. Antonucci, A. Testa, P. Narciso, C. Vlassi, M. Zaccarelli, A. Lazzarin, A. Castagna, N. Gianotti, M. Galli, A. Ridolfo, L. Sacco, B. Rozentale, I. Zeltina, S. Chaplinskas, R. Hemmer, T. Staub, P. Reiss, V. Ormaasen, A. Maeland, J. Bruun, B. Knysz, J. Gasiorowski, A. Horban, E. Bakowska, A. Grzeszczuk, R. Flisiak, A. Boron-Kaczmarska, M. Pynka, M. Parczewski, M. Beniowski, E. Mularska, H. Trocha, E. Jablonowska, E. Malolepsza, K. Wojcik, F. Antunes, M. Doroana, L. Caldeira, K. Mansinho, F. Maltez, D. Duiculescu, A. Rakhmanova, N. Zakharova, S. Buzunova, D. Jevtovic, M. Mokráš, D. Staneková, J. Tomazic, J. González-Lahoz, V. Soriano, P. Labarga, J. Medrano, S. Moreno, J.M. Rodriguez, B. Clotet, A. Jou, R. Paredes, C. Tural, J. Puig, I. Bravo, J.M. Gatell, J.M. Miró, P. Domingo, M. Gutierrez, G. Mateo, M.A. Sambeat, A. Karlsson, L. Flamholc, B. Ledergerber, R. Weber, P. Francioli, M. Cavassini, B. Hirschel, E. Boffi, H. Furrer, M. Battegay, L. Elzi, E. Kravchenko, N. Chentsova, V. Frolov, G. Kutsyna, S. Servitskiy, M. Krasnov, S. Barton, A.M. Johnson, D. Mercey, A. Phillips, M.A. Johnson, A. Mocroft, M. Murphy, J. Weber, G. Scullard, M. Fisher, C. Leen, L. Morfeldt, G. Thulin, A. Sundström, B. Åkerlund, K. Koppel, A. Karlsson, L. Flamholc, C. Håkangård, M. Moroni, G. Angarano, A. Antinori, O. Armignacco, F. Castelli, R. Cauda, G. Di Perri, M. Galli, R. Iardino, G. Ippolito, A. Lazzarin, C.F. Perno, F. von Schloesser, P. Viale, A. Antinori, A. Castagna, F. Ceccherini-Silberstein, A. Cozzi-Lepri, E. Girardi, S. Lo Caputo, C. Mussini, M. Puoti, M. Andreoni, A. Ammassari, A. Antinori, C. Balotta, P. Bonfanti, S. Bonora, M. Borderi, R. Capobianchi, A. Castagna, F. Ceccherini-Silberstein, A. Cingolani, P. Cinque, A. Cozzi-Lepri, A. De Luca, A. Di Biagio, E. Girardi, N. Gianotti, A. Gori, G. Guaraldi, G. Lapadula, M. Lichtner, S. Lo Caputo, G. Madeddu, F. Maggiolo, G. Marchetti, S. Marcotullio, L. Monno, C. Mussini, M. Puoti, E. Quiros Roldan, S. Rusconi, A. Cozzi-Lepri, P. Cicconi, I. Fanti, T. Formenti, L. Galli, P. Lorenzini, A. Giacometti, A. Costantini, G. Angarano, L. Monno, C. Santoro, F. Maggiolo, C. Suardi, P. Viale, E. Vanino, G. Verucchi, F. Castelli, C. Minardi, T. Quirino, C. Abeli, P.E. Manconi, P. Piano, J. Vecchiet, K. Falasca, L. Sighinolfi, D. Segala, F. Mazzotta, S. Lo Caputo, G. Cassola, G. Viscoli, A. Alessandrini, R. Piscopo, G. Mazzarello, C. Mastroianni, V. Belvisi, P. Bonfanti, I. Caramma, A. P. Castelli, M. Galli, A. Lazzarin, G. Rizzardini, M. Puoti, A.L. Ridolfo, R. Piolini, A. Castagna, S. Salpietro, L. Carenzi, M.C. Moioli, P. Cicconi, G. Marchetti, C. Mussini, C. Puzzolante, A. Gori, G. Lapadula, N. Abrescia, A. Chirianni, M.G. Guida, M. Onofrio, F. Baldelli, D. Francisci, G. Parruti, T. Ursini, G. Magnani, M.A. Ursitti, R. Cauda, M. Andreoni, A. Antinori, V. Vullo, A. Cingolani, A. d'Avino, A. Ammassari, L. Gallo, E. Nicastri, R. Acinapura, M. Capozzi, R. Libertone, G. Tebano, A. Cattelan, M.S. Mura, G. Madeddu, P. Caramello, G. Di Perri, G.C. Orofino, S. Bonora, M. Sciandra, G. Pellizzer, V. Manfrin, C. Pradier, E. Fontas, K. Dollet, C. Caissotti, P. Dellamonica, E. Bernard, E. Cua, F. De Salvador-Guillouet, J. Durant, S. Ferrando, V. Mondain-Miton, A. Naqvi, I. Perbost, B. Prouvost-Keller, S. Pillet, P. Pugliese, V. Rahelinirina, P.M. Roger, V. Aubert, M. Battegay, E. Bernasconi, J. Böni, HC. Bucher, C. Burton-Jeangros, A. Calmy, M. Cavassini, G. Dollenmaier, M. Egger, L. Elzi, J. Fehr, J. Fellay, H. Furrer, CA. Fux, M. Gorgievski, H. Günthard, D. Haerry, B. Hasse, HH. Hirsch, M. Hoffmann, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, T. Klimkait, R. Kouyos, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, K. Metzner, N. Müller, D. Nadal, D. Nicca, G. Pantaleo, A. Rauch, S. Regenass, M. Rickenbach, C. Rudin, F. Schöni-Affolter, P. Schmid, J. Schüpbach, R. Speck, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, R. Weber, and S. Yerly
References
- 1.Kovari H, Weber R. Influence of antiretroviral therapy on liver disease. Curr Opin HIV AIDS 2011; 6:272–7. [DOI] [PubMed] [Google Scholar]
- 2.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci 2008; 53:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovari H, Ledergerber B, Battegay M et al. . Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis B or C virus co-infection. Clin Infect Dis 2010; 50:502–11. [DOI] [PubMed] [Google Scholar]
- 4.Crum-Cianflone N, Collins G, Medina S et al. . Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol 2010; 8:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansi L, Gazzard B, Post F et al. . Biomarkers to monitor safety in people on art and risk of mortality. J Acquir Immune Defic Syndr 2012; 60:51–8. [DOI] [PubMed] [Google Scholar]
- 6.Blanco F, Barreiro P, Ryan P et al. . Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat 2011; 18:11–6. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Mehta SH, Torbenson M et al. . Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS 2005; 19:585–92. [DOI] [PubMed] [Google Scholar]
- 8.McGovern BH, Ditelberg JS, Taylor LE et al. . Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis 2006; 43:365–72. [DOI] [PubMed] [Google Scholar]
- 9.Merchante N, Perez-Camacho I, Mira JA et al. . Prevalence and risk factors for abnormal liver stiffness in HIV-infected patients without viral hepatitis coinfection: role of didanosine. Antivir Ther 2010; 15:753–63. [DOI] [PubMed] [Google Scholar]
- 10.Ryom L, Lundgren JD, De Wit S et al. . Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. AIDS. 2016 Jan 8. PMID 26752282. [DOI] [PubMed]
- 11.Kovari H, Ledergerber B, Peter U et al. . Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis 2009; 49:626–35. [DOI] [PubMed] [Google Scholar]
- 12.Sabin CA, Ryom L, Kovari H et al. . Association between ALT level and the rate of cardio/cerebrovascular events in HIV-positive individuals: the D: A: D Study. J Acquir Immune Defic Syndr 2013; 63:456–63. [DOI] [PubMed] [Google Scholar]
- 13.Ingiliz P, Valantin MA, Duvivier C et al. . Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology 2009; 49:436–42. [DOI] [PubMed] [Google Scholar]
- 14.Morse CG, McLaughlin M, Matthews L et al. . Nonalcoholic steatohepatitis and hepatic fibrosis in HIV-1-Monoinfected adults with elevated aminotransferase levels on antiretroviral therapy. Clin Infect Dis 2015; 60:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friis-Moller N, Sabin CA, Weber R et al. . Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 16.Kowalska JD, Friis-Moller N, Kirk O et al. . The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology 2011; 22:516–23. [DOI] [PubMed] [Google Scholar]
- 17.Sterling RK, Lissen E, Clumeck N et al. . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ et al. . A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–26. [DOI] [PubMed] [Google Scholar]
- 19.Nunez M. Clinical syndromes and consequences of antiretroviral-related hepatotoxicity. Hepatology 2010; 52:1143–55. [DOI] [PubMed] [Google Scholar]
- 20.Rivero A, Mira JA, Pineda JA. Liver toxicity induced by non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother 2007; 59:342–6. [DOI] [PubMed] [Google Scholar]
- 21.Lattuada E, Lanzafame M, Carolo G et al. . Does tenofovir increase efavirenz hepatotoxicity? AIDS 2008; 22:995. [DOI] [PubMed] [Google Scholar]
- 22.Qayyum S, Dong H, Kovacic D et al. . Combination therapy efavirenz/emtricitabine/tenofovir disoproxil fumarate associated with hepatic failure. Curr Drug Saf 2012; 7:391–3. [DOI] [PubMed] [Google Scholar]
- 23.Fink DL, Bloch E. Liver transplantation for acute liver failure due to efavirenz hepatotoxicity: the importance of routine monitoring. Int J STD AIDS 2013; 24:831–3. [DOI] [PubMed] [Google Scholar]
- 24.Echenique IA, Rich JD. EFV/FTC/TDF-associated hepatotoxicity: a case report and review. AIDS Patient Care STDS 2013; 27:493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallant JE, Staszewski S, Pozniak AL et al. . Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. [DOI] [PubMed] [Google Scholar]
- 26.Barditch-Crovo P, Deeks SG, Collier A et al. . Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 2001; 45:2733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin A, Bloch M, Amin J et al. . Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-Lamivudine: a randomized, 96-week trial. Clin Infect Dis 2009; 49:1591–601. [DOI] [PubMed] [Google Scholar]
- 28.Sax PE, Tierney C, Collier AC et al. . Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis 2011; 204:1191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkus G, Hitchcock MJ, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 2002; 46:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adaramoye OA, Adewumi OM, Adesanoye OA et al. . Effect of tenofovir, an antiretroviral drug, on hepatic and renal functional indices of Wistar rats: protective role of vitamin E. J Basic Clin Physiol Pharmacol 2012; 23:69–75. [DOI] [PubMed] [Google Scholar]
- 31.Taburet AM, Piketty C, Chazallon C et al. . Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 2004; 48:2091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearney BP, Sayre JR, Flaherty JF et al. . Drug-drug and drug-food interactions between tenofovir disoproxil fumarate and didanosine. J Clin Pharmacol 2005; 45:1360–7. [DOI] [PubMed] [Google Scholar]
- 33.Rotger M, Colombo S, Furrer H et al. . Does tenofovir influence efavirenz pharmacokinetics? Antivir Ther 2007; 12:115–8. [PubMed] [Google Scholar]
- 34.Lapadula G, Costarelli S, Chatenoud L et al. . Risk of liver enzyme elevation during treatment with ritonavir-boosted protease inhibitors among HIV-monoinfected and HIV/HCV coinfected patients. J Acquir Immune Defic Syndr 2015; 69:312–8. [DOI] [PubMed] [Google Scholar]
- 35.Torti C, Lapadula G, Antinori A et al. . Hyperbilirubinemia during atazanavir treatment in 2,404 patients in the Italian atazanavir expanded access program and MASTER Cohorts. Infection 2009; 37:244–9. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez JM, Hermida JM, Casado JL et al. . The use of atazanavir in HIV-infected patients with liver cirrhosis: lack of hepatotoxicity and no significant changes in bilirubin values or model for end-stage liver disease score. AIDS 2011; 25:1006–9. [DOI] [PubMed] [Google Scholar]
- 37.Browning JD, Szczepaniak LS, Dobbins R et al. . Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40:1387–95. [DOI] [PubMed] [Google Scholar]
- 38.Crum-Cianflone N, Dilay A, Collins G et al. . Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr 2009; 50:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat 2008; 15:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovari H, Sabin CA, Ledergerber B et al. . Antiretroviral drug-related liver mortality among HIV-positive persons in the absence of hepatitis B or C virus coinfection: the data collection on adverse events of anti-HIV drugs study. Clin Infect Dis 2013; 56:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009; 136:477–85.e11. [DOI] [PubMed] [Google Scholar]
- 42.Lee TH, Kim WR, Benson JT et al. . Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008; 47:880–7. [DOI] [PubMed] [Google Scholar]