Abstract
Parathion and chlorpyrifos are organophosphorus insecticides (OPs) that elicit acute toxicity by inhibiting acetylcholinesterase (AChE). The endocannabinoids (eCBs, N-arachidonoylethanolamine, AEA; 2-arachidonoylglycerol, 2AG) are endogenous neuromodulators that regulate presynaptic neurotransmitter release in neurons throughout the central and peripheral nervous systems. While substantial information is known about the eCBs, less is known about a number of endocannabinoid-like metabolites (eCBLs, e.g., N-palmitoylethanolamine, PEA; N-oleoylethanolamine, OEA). We report the comparative effects of parathion and chlorpyrifos on AChE and enzymes responsible for inactivation of the eCBs, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), and changes in the eCBs AEA and 2AG and eCBLs PEA and OEA, in rat striatum. Adult, male rats were treated with vehicle (peanut oil, 2 ml/kg, sc), parathion (27 mg/kg) or chlorpyrifos (280 mg/kg) 6-7 days after surgical implantation of microdialysis cannulae into the right striatum, followed by microdialysis two or four days later. Additional rats were similarly treated and sacrificed for evaluation of tissue levels of eCBs and eCBLs. Dialysates and tissue extracts were analyzed by LC-MS/MS. AChE and FAAH were extensively inhibited at both time-points (85-96%), while MAGL activity was significantly but lesser affected (37-62% inhibition) by parathion and chlorpyrifos. Signs of toxicity were noted only in parathion-treated rats. In general, chlorpyrifos increased eCB levels while parathion had no or lesser effects. Early changes in extracellular AEA, 2AG and PEA levels were significantly different between parathion and chlorpyrifos exposures. Differential changes in extracellular and/or tissue levels of eCBs and eCBLs could potentially influence a number of signaling pathways and contribute to selective neurological changes following acute OP intoxications.
Keywords: acetylcholinesterase, organophosphorus, cholinergic, cannabinoid, endocannabinoid-like, anandamide, 2-arachidonoyl glycerol, N-palmitoylethanolamine, N-oleoylethanolamine
Introduction
Parathion and chlorpyrifos are both organophosphorus insecticides (OPs). Parathion is no longer registered for use in the US, but remains in use in many other countries. Chlorpyrifos is one of the most commonly used OPs in the United States and throughout the world (Grube et al., 2011). OPs elicit acute toxicity through a common mechanism initiated by acetylcholinesterase (AChE) inhibition (Mileson et al. 1998). Extensive inhibition of AChE leads to elevated levels of acetylcholine in cholinergic synapses throughout the body and consequent cholinergic signs of toxicity including tremors, excessive parasympathetic end organ secretions (e.g., lacrimation), vomiting, miosis and various other signs and symptoms (Pope et al., 2005; Wilson, 2010).
Endocannabinoids (eCBs, e.g., arachidonoylethanolamine [AEA] and 2-arachidonoylglycerol [2AG]) are global neuromodulators produced from membrane lipids and released by postsynaptic neurons following depolarization, as well as through receptor-mediated signaling through cholinergic muscarinic M1 and M3 and other neurotransmitter receptors (Castillo et al., 2012). Once released, the eCBs diffuse across the synapse and activate cannabinoid (CB1) receptors on the presynaptic neuron terminal. CB1 activation generally leads to the inhibition of neurotransmitter release in a wide variety of neurons and signaling pathways, in both the central and peripheral nervous systems. The other primary cannabinoid receptor, CB2, is more prominently located on immune cells with lesser putative role in neuromodulation. While the neuromodulatory role of AEA and 2AG has been well documented, a number of eCB-like lipid metabolites (eCBLs) have been more recently reported to have effects in the nervous system (see review of Fezza et al., 2014). The eCBLs (e.g., N-palmitoylethanolamine, PEA; N-oleolyethanolamine, OEA) have no direct effects on CB1 or CB2 receptors but can activate other macromolecular receptors including the peroxisome proliferator-activated receptors and/or the orphan G-protein coupled receptors GPR55 and GPR119 (Godlewski et al., 2009; Hansen, 2010; Pistis and Melis, 2010; Liu et al., 2015).
Disruption of acetylcholine hydrolysis and consequent elevation of synaptic acetylcholine levels is sine qua non for the development of cholinergic toxicity following OP exposure (DuBois et al., 1949). As eCBs can decrease acetylcholine release via activation of CB1 receptors (Gifford and Ashby, 1996; Gifford et al., 2000, Tzavara et. al., 2003; De Groot et al., 2006), and CB1 antagonists can increase acetylcholine release (Gifford and Ashby, 1996; Kathmann et al., 2001), we proposed that eCBs can play a role in OP toxicity. Indeed, we previously reported that acute toxicity of both paraoxon (the active metabolite of parathion) and diisopropylfluorophosphate, is reduced by CB1 receptor agonists (Nallapaneni et al., 2006, 2008; Wright et al., 2010). More recently, the CB1 receptor antagonist AM251 was shown to increase the acute toxicity of paraoxon and chlorpyrifos oxon (the active metabolite of chlorpyrifos; Liu and Pope, 2015).
The enzymatic degradation of AEA is primarily mediated by the enzyme fatty acid amide hydrolase (FAAH, Cravatt et al, 1996, 2001; Egertova et al., 2003). FAAH is also critical in the breakdown of the eCBLs PEA and OEA. While the primary and often most sensitive macromolecular target for many OPs is AChE, a number of OPs including parathion and chlorpyrifos (in vivo) and paraoxon and chlorpyrifos oxon (in vitro) are potent inhibitors of FAAH (Quistad et al., 2001, 2006; Nomura et al., 2008; Nallapaneni et al., 2006, 2008; Liu et al., 2013) and can elevate brain levels of AEA (Nomura et al., 2008; Carr et al., 2013). Monoacylglycerol lipase (MAGL) is the main enzyme involved in 2AG hydrolysis (Blankman et al., 2007; Hashimotodani et al., 2007; Savinainen et al., 2012). MAGL is also inhibited by a number of OPs but, in general, MAGL is less sensitive than FAAH to inhibition by OPs (Quistad et al., 2001, 2006; Nomura et al., 2008; Nomura and Casida, 2011; Liu et al., 2013). OP-mediated inhibition of MAGL has been reported to increase brain levels of 2AG in mice and rats (Nomura et al., 2008; Nomura and Casida, 2011; Carr et al., 2013). We previously reported that in vivo exposure to either parathion or chlorpyrifos led to similar degrees of inhibition in both FAAH and MAGL activities in the hippocampus, with concomitant increases (more so with chlorpyrifos) in extracellular AEA levels. Hippocampal 2AG level was only increased by chlorpyrifos, however (Liu et al., 2013).
In this study, we evaluated the effects of high in vivo dosages of parathion and chlorpyrifos on rat striatal AChE, FAAH and MAGL, and both extracellular and tissue levels of AEA, 2AG, PEA and OEA. As eCBs and eCBLs can be involved in a variety of processes including neuromodulation, gene expression, inflammation and others, OP- and regional-selective changes in eCBs and eCBLs could potentially contribute to cholinergic and non-cholinergic mechanisms involved in acute and long-term consequences of OP intoxication.
Methods
Chemicals and Reagents
Parathion (O,O′-diethyl-p-nitrophenyl-phosphorothioate) and chlorpyrifos (O,O′-diethyl-3,5,6-trichloro-2-pyridinyl-phosphorothioate), >99% pure; GC/MS analysis, were purchased from Chem Service (West Chester, PA). Acetylcholine iodide (acetyl-3H, specific activity 76.0 Ci/mmol) was purchased from Perkin Elmer (Wellesley, MA). [3H]Anandamide (ethanolamine 1-3H) specific activity 60 Ci/mmol), was purchased from American Radiolabeled Chemicals (St. Louis, MO). Arachidonoyl-1-thio-glycerol and anandamide were purchased from Cayman Chemical (Ann Arbor, MI). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Guide cannulae (MD 2250) and microdialysis probes (MD 2204, 4 mm membrane) were purchased from Bioanalytical Systems Inc. (BAS, West Lafayette, IN).
Animals and Treatments
Male, Sprague-Dawley rats (approximately 3 months of age) were purchased from Harlan (Indianapolis, IN) and maintained in the AAALAC-accredited Animal Resources facility at Oklahoma State University. Animals were housed in polycarbonate cages with a 12-h:12-h light:dark cycle, allowed free access to food (PMI® Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN) and water throughout, and acclimated to the facility for 5-7 days prior to beginning the study. Surgical procedures and routine animal care were in accordance with protocols established in the NIH/NRC Guide for the Care and Use of Laboratory Animals and approved by the local Institutional Animal Care and Use Committee.
OP compounds were dissolved in peanut oil (100% pure; Lou-Ana brand, Ventura Foods, Opelousas, LA) and injected subcutaneously (sc) at a volume of 2 ml/kg. Rats were treated with vehicle, parathion (27 mg/kg) or chlorpyrifos (280 mg/kg). Functional signs of toxicity were recorded essentially as described earlier (Liu et al., 2013) by a trained observer “blinded” to treatment groups. Autonomic signs (i.e., SLUD, an acronym for salivation, lacrimation, urination and defecation) were graded as: 1 = normal (no secretions); 2 = mild one or multiple secretions; 3 = moderate multiple secretions; 4 = severe multiple secretions. Involuntary movements were scored as: 2 = normal quivering of vibrissae and head; 3 = fine head and neck tremors; 3.25 = more consistent tremors in head, neck and forelimbs; 3.5 = consistent tremors extending caudally from head to the midbody; 3.75 = tremors extending caudally to the hindlimbs; 4 = whole body tremors; 5 = myoclonic jerks.
Stereotaxic Surgery, Microdialysis and Metabolite Analysis
A guide cannula was surgically implanted into the right striatum. Animals were first anesthetized with a ketamine/xylazine (9:1) mixture (0.6 ml/kg, ip). The scalp was shaved and the head was positioned into a stereotaxic apparatus (Stoelting Co., Wood Dale, IL). The cannula was positioned using the coordinates: anterior-posterior, 1.2 mm, medial-lateral, -2.2 mm; dorsal-ventral, -3.4 mm from bregma. Two screws were placed on each side of the cannula and dental cement was used to secure the cannula. Animals were allowed to recover for 6-7 days prior to OP treatment.
On the day of microdialysis, rats were lightly anesthetized with isoflurane and the probe was rapidly inserted into the guide cannula. Rats were then transferred into a Raturn® animal chamber (BAS, West Lafayette, IN) and dialysis tubing was stabilized with a plastic collar. The dialysis probe was equilibrated for five hours by perfusion with artificial cerebrospinal fluid (aCSF: NaCl, 149 mM; KCl, 2.8 mM; CaCl2, 1.2 mM; MgCl2, 1.2 mM; ascorbic acid, 0.25 mM; D-glucose, 5.4 mM; hydroxypropyl-β-cyclodextrin, 30% [to increase eCB capture, Caille et al., 2007]) at flow rate of 0.8 μl/min using a syringe pump (MD 1101, BAS). Following equilibration, five fractions (15 min each) were collected into a refrigerated fraction collector. All fractions were stored at -80°C until analysis. Cannula/probe placement was verified in all tissues by H&E staining.
AEA, 2AG, PEA and OEA in microdialysates and tissue extracts were analyzed essentially by the method of Caille and coworkers (2007) as described previously (Liu et al., 2013). Deuterated internal standards (d4-AEA, d4-PEA, d4-OEA, and d5-2AG) and the stable isotope dilution quantification method were used with LC-MS/MS (Agilent 6410) multiple reaction monitoring in positive ion mode (Buczynski et al., 2013).
Fatty Acid Amide Hydrolase, Acetylcholinesterase and Monoacylglycerol Lipase Assays
FAAH was measured by a modification of the method of Long and coworkers (2009). PBS or striatal homogenates in PBS (approximately 75 μg protein) were incubated at 37°C for 20 min with [3H]anandamide (10 μM final concentration) in a final reaction volume of 0.4 ml. The reaction was stopped by adding 0.4 ml chloroform:methanol (1:1) and vortexing. Tubes were then centrifuged in a microcentrifuge (3,300 rpm for 10 min, Marathon 13K/M, Fisher, Chicago, IL). An aliquot of the upper aqueous phase (200 μl) was removed, added to 4 ml Scintisafe® scintillation fluid, and counted. AChE and MAGL were measured by a modification of the Ellman method (Ellman et al., 1961), including modifications based on studies by Casida and coworkers (2010) and Ulloa and Deutsch (2010). PBS or striatal homogenates in PBS (AChE, 10 μg protein; MAGL, approximately 30 μg protein) were added to individual wells of a 96-well plastic plate on ice. A solution (175 μl) containing either acetylthiocholine (1 mM final) or arachidonoyl-1-thioglycerol (100 μM final) and the color reagent 5,5′-dithiobis-(2-nitrobenzoic acid, 100 μM final) was then added and the plate was immediately placed into the reader (SPECTRAmax 340PC, Molecular Devices, Sunnyvale, CA). The reaction was conducted in kinetic mode at 412 nm, at either 37°C (AChE) or 30°C (MAGL) for 5 min, with an initial lag time of 60 sec for equilibration. Data were collected every 30 sec and average reaction rates were determined. Rate of hydrolysis was calculated based on the molar extinction coefficient of the thiolate product (14,150 M-1 cm-1, Ulloa and Deutsch, 2010), which is produced in direct proportion to substrate hydrolysis.
Total protein was estimated using the Bio-Rad (Bradford) protein assay with bovine serum albumin as the standard. Enzyme activities were plotted as nmole substrate hydrolyzed min-1mg-1 protein (AChE and MAGL) or pmol substrate hydrolyzed min-1 mg-1 protein (FAAH).
Statistical Analysis
All statistical analyses were conducted using the Prism statistical package (version 6.0, GraphPad Software, La Jolla, CA). Functional (toxicity) data were analyzed by Kruskall-Wallis test and reported as median ± interquartile range (IQR). Enzyme activity and metabolite data were analyzed at both 2 and 4 days after dosing using two-way ANOVA and Tukey's multiple comparisons post-hoc tests.
Results
Similar to a number of our previous studies (Pope et al., 1992; Liu and Pope, 1998; Karanth et al., 2007; Liu et al., 2013), parathion (27 mg/kg) elicited extensive involuntary movements (tremors), starting about 24 hours after dosing. At two days after dosing, rats treated with parathion showed significantly more involuntary movements (H(2,18) = 16.6, p=0.0002; median score = 3.5 ±0). By four days after dosing, one parathion-treated rat had died and involuntary movements were still significantly greater than controls (H(2,17) = 16.0, p=0.0002; median score = 4.0 ± 0). No signs of involuntary movements were noted in any control or chlorpyrifos-treated rats at either time-point. Few autonomic signs were noted (i.e., all three treatment groups had median scores of 1.0 at both time-points). Only one out of six parathion-treated rats showed autonomic signs (score of 2) two days after dosing (this rat died before functional observations on day 4), and two more parathion-treated rats were given a score of 2 at the four day time-point. No control or chlorpyrifos-treated rats showed any autonomic signs.
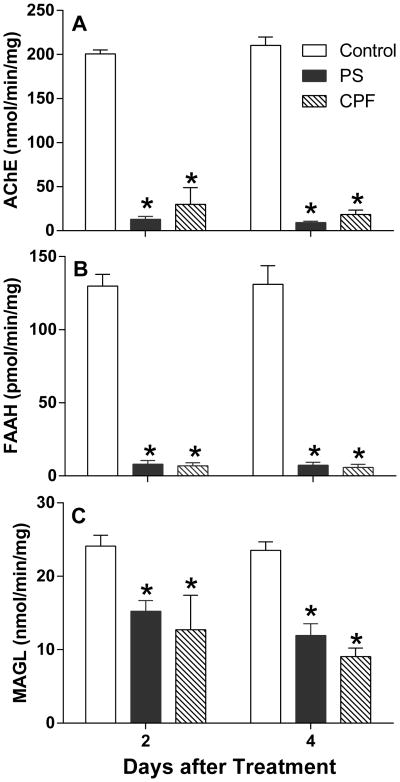
Figure 1 shows striatal AChE (Figure 1A), FAAH (Figure 1B) and MAGL (Figure 1C) activity at 2 and 4 days after dosing. Striatal AChE was extensively inhibited by both parathion and chlorpyrifos (main effect of treatment, F(2,30)= 1,662, p<0.0001). There was no significant effect of time but a significant interaction between treatment and time was noted (F(2,30)= 3.944, p = 0.03). At 2 days after dosing, rats treated with parathion showed 96% inhibition compared to 85% inhibition following chlorpyrifos. Relatively similar changes were noted at 4 days after dosing (parathion, 96% inhibition; chlorpyrifos, 91% inhibition).
Figure 1. Effects of parathion and chlorpyrifos on A) acetylcholinesterase (AChE), B) fatty acid amide hydrolase (FAAH) and C) monoacylglycerol lipase (MAGL) activity in rat striatum.
Rats (n=6/treatment group) were treated as in Methods and sacrificed 2 or 4 days later. Striatum was rapidly dissected and frozen at -70°C until assay. Tissues were homogenized in PBS and assayed for enzyme activity as described in Methods. Data were calculated as nmol substrate hydrolyzed min-1 mg protein-1 (AChE and MAGL) or pmol substrate hydrolyzed min-1 mg protein-1 (FAAH). Values are reported as mean ± SEM. Control values for acetylcholinesterase activity were: 2 days = 200.7 ± 1.8nmol·min-1·mg protein-1; 4 days = 210.2 ± 3.9nmol·min-1·mg protein-1. Control values for FAAH activity were: 2 days = 130±4pmol·min-1 mg protein-1 ;4 days = 131 ±5 pmol·min-1·mg protein-1. Control values for MAGL activity were: 2 days= 24.1 ± 0.6 nmol·min-1·mg protein-1; 4 days = 23.5 ± 0.5 nmol·min-1·mg protein-1. An asterisk indicates a significant difference compared to controls.
FAAH, the enzyme primarily responsible for metabolic degradation of AEA, was also extensively inhibited by parathion and chlorpyrifos (94-95%; main effect of treatment, F(2,30) = 1,471, p<0.0001). While both OPs elicited extensive FAAH inhibition, there were no significant differences between the OPs, nor was there any significant effect of time or an interaction.
MAGL, the principal enzyme involved in hydrolysis of 2AG, was lesser but significantly inhibited by both parathion and chlorpyrifos (main effect of treatment, F(2,30)= 106.1, p < 0.0001). There was a main effect of time (F(2,30)= 10.71, p = 0.0027), but no significant interaction. There were significant pairwise comparisons between controls and both parathion and chlorpyrifos at both 2 and 4 days, but no significant differences between parathion and chlorpyrifos on either day. At 2 days after dosing, rats treated with parathion showed 37% inhibition of MAGL activity, compared to 47% with chlorpyrifos. A similar trend towards greater inhibition by chlorpyrifos was seen at 4 days (parathion, 49% inhibition; chlorpyrifos, 62% inhibition).
Figure 2 shows the effects of parathion and chlorpyrifos on striatal extracellular AEA (A,B) and 2AG (C,D) levels. In general, chlorpyrifos exposure led to increases in both AEA and 2AG compared to parathion, and 2AG levels were relatively higher at four days after dosing compared to the 2-day time-point. At two days (Figure 2A), chlorpyrifos increased AEA levels while parathion had little effect (main effect of treatment, F(2,65)=58.35, p<0.0001). Significant pairwise comparisons were noted between chlorpyrifos and both the control and the parathion groups. It must be noted that there were no differences in the extent of FAAH inhibition between the two groups (Figure 1B). Four days after dosing (Figure 2B), there was also a main effect of treatment on AEA levels (F(2,50)=6.194, p=0.004) but no significant pairwise comparisons. At two days after dosing, there was a main effect of treatment on extracellular 2AG levels (Figure 2C, F(2,65)= 20.81, p<0.0001), as well as significant pairwise differences between parathion and chlorpyrifos treatments. Extracellular 2AG appeared somewhat lower than control levels after parathion, but were higher than controls after chlorpyrifos. Four days after dosing (Figure 2D), 2AG levels were increased by OP treatment (F(2,50)=28.54, p<0.0001), with a significant difference between control and chlorpyrifos among all fractions and a difference between parathion and chlorpyrifos in the first fraction. There was no significant effect of fraction or a significant treatment-fraction interaction with either AEA or 2AG at either time-point. Thus while a trend towards increased eCBs was noted four days after parathion exposure, only chlorpyrifos significantly affected extracellular AEA and 2AG levels.
Figure 2. In vivo effects of parathion and chlorpyrifos on extracellular levels of anandamide (AEA) and 2-arachidonoyl glycerol (2AG) in rat striatum.
A microdialysis cannula was surgically implanted into striatum 6-7 days prior to exposure to vehicle (n=4), parathion (n=6) or chlorpyrifos (n=4-6) as described in the Methods section. At day 2 (Figure 2A and C) or day 4 (Figure 2B and D) after dosing, a dialysis probe was inserted into the cannula and tissue perfused (0.8 μl/min) with artificial cerebrospinal fluid (aCSF) containing 30% hydroxypropyl-β-cyclodextrin. After 5 hours, five 15-min fractions were collected into a refrigerated fraction collector. Dialysates were frozen until analysis by LC/MS-MS as described in Methods. Values are reported as mean ± SEM. Symbols represent: filled circle, controls; open square, parathion-treated; filled triangle, chlorpyrifos-treated. Mean control values for AEA were: 2 days = 5.24 nM ± 0.81 nM, 4 days = 6.98 ± 1.08 nM. Mean control values for 2AG were: 2 days = 4.8 ± 0.69 nM; 4 days = 3.83 ± 0.46 nM. An asterisk indicates a difference between chlorpyrifos and both control and parathion treatment groups, a pound sign indicates a significant difference between parathion and chlorpyrifos, while a dollar sign signifies a difference between control and chlorpyrifos.
Effects of parathion and chlorpyrifos on striatal extracellular PEA and OEA levels are shown in Figure 3. At two days after dosing (Figure 3A), there was a significant main effect of treatment on striatal PEA levels (F(2,65)=20.09, p=<0.0001). Interestingly, PEA levels were similarly affected as noted with extracellular 2AG, i.e., there was a significant difference between the OPs, with lower levels after parathion but higher with chlorpyrifos, relative to controls. Significant pairwise differences were noted between parathion and chlorpyrifos at the 2 day time-point, but no significant effect of OP treatment on extracellular PEA levels was observed four days after dosing (Figure 3B). With OEA, a significant OP treatment effect was observed at both two days (Figure 3; main effect of treatment, F(2,65)=8.925, p=0.0004) and four days(Figure 3D; main effect of treatment, F(2,50)=5.083, p=0.0098) after dosing, but no significant pairwise differences were noted. There were no main effects of fraction, and there were no significant interactions between fraction and time with either eCBL. In control dialysates there was a trend (t=2.42, df=6, p=0.052,) towards an increase in extracellular PEA, and a significant increase in extracellular OEA (t=3.25, df=6, p=0.0175), at four days compared to two days after treatment. The reason for an increase in control levels of eCBLs with time is unclear, but could possibly be due to time-dependent changes in membrane disruption/inflammation elicited by cannula implantation.
Figure 3. In vivo effects of parathion and chlorpyrifos on extracellular levels of N-palmitoylethanolamide (PEA) and N-oleoylethanolamide (OEA) in rat striatum.
Rats were treated and microdialysis and eCBL analysis conducted as described in Figure 2 legend and the Methods section. Values are reported as mean ± SEM. Chlorpyrifos exposure was associated with a significant increase in PEA levels at 2 days after dosing (Figure 3A), while PEA levels were decreased following parathion. No significant treatment related effects were noted at 4 days after exposure (Figure 3B). OEA levels were increased by both parathion and chlorpyrifos at both two days (Figure 3C) and four days (Figure 3D) after OP exposure. Symbols represent: filled circle, controls; open square, parathion-treated; filled triangle, chlorpyrifos-treated. Mean control values for PEA were: 2 days = 77.3 ± 7.6 nM; 4 days = 126.8 ± 19.0 nM. Mean control values for OEA were: 2 days = 9.2 ± 1.9 nM; 4 days = 16.9 ± 1.4 nM. A pound sign indicates a significant difference between parathion and chlorpyrifos treatment groups.
Figure 4 shows the effects of parathion and chlorpyrifos on striatal tissue eCB levels at two and four days after treatment. With AEA (Figure 4A), there was a significant main effect of treatment (F(2,52)=3.583, p=0.0349), but no significant pair-wise comparisons. With 2AG (Figure 4B), there was also a significant effect of treatment (F(2,51)=8.709, p=0.0005), with a significant difference between control and chlorpyrifos treatment at four days after dosing.
Figure 4. In vivo effects of parathion and chlorpyrifos on striatal tissue levels of A) anandamide (AEA) and B) 2-arachidonoyl glycerol (2AG).
Both AEA and 2AG were affected by treatment. No significant pair-wise comparisons were noted with AEA, but 2AG levels were significantly increased 4 days after chlorpyrifos. An asterisk indicates a significant difference (p<0.05) between control and chlorpyrifos. Mean control values for tissue AEA were: 2 days = 7.8 ±1.9 pmol/g; 4 days = 9.8 ± 1.7 pmol/g. Mean control values for tissue 2AG were: 2 days = 6.3 ± 0.4 pmol/g; 4 days = 6.2 ± 0.5 pmol/g. An asterisk indicates a significant difference compared to controls.
Figure 5 shows the effects of parathion and chlorpyrifos on tissue levels of PEA and OEA at two and four days after treatment. With PEA (Figure 5A), a main effect of treatment was noted (F(2,52)=37.0, p<0.0001), along with significant differences between parathion and controls, as well as between chlorpyrifos and controls, at both two and four days after dosing. Similarly, with OEA (Figure 5B), a main effect of treatment was observed (F(2,51)=25.04, p<0.0001) and significant differences were noted between parathion and controls, and between chlorpyrifos and controls at both two and four days after dosing. No significant difference between OPs was noted with either metabolite at either time-point.
Figure 5. Effect of parathion and chlorpyrifos on tissue levels of the eCBLs A) palmitoylethanolamide (PEA), and B) oleoylethanolamide (OEA).
Generally robust increases in tissue levels were noted with both PEA and OEA after either parathion or chlorpyrifos exposure. An asterisk indicates a significant difference (p<0.05) between control and OP treatment. Mean control values for tissue PEA were: 2 days = 260 ± 12.0 pmol/g; 4 days = 292 ± 12.6 pmol/g. Mean control values for OEA were: 2 days= 178± 11.0 pmol/g; 4 days = 216 ± 11.1 pmol/g. An asterisk indicates a significant difference compared to controls.
Discussion
We studied the comparative effects of two OPs on AChE, the target enzyme for acute OP toxicity, and FAAH and MAGL, the two principal enzymes involved in the degradation of AEA, 2AG, PEA and OEA. OP effects on enzyme activity were compared with changes in both extracellular and tissue levels of these metabolites. Both parathion and chlorpyrifos elicited extensive inhibition of striatal AChE and FAAH at two and four days after dosing, with lesser effects on MAGL (Figure 1). The relative degree of enzyme inhibition observed was very similar to results noted in the hippocampus with the same experimental conditions (Liu et al., 2013). Chlorpyrifos significantly increased extracellular levels of AEA at 2 days as well as 2AG at 4 days after dosing, while parathion had no statistically significant effects on either AEA or 2AG, compared to controls (Figure 2). Chlorpyrifos also significantly increased striatal tissue levels of 2AG four days after exposure (Figure 4B). While there were no significant changes in extracellular PEA and OEA levels relative to controls (Figure 3), striatal tissue levels of both PEA and OEA were substantially increased by both parathion and chlorpyrifos (Figure 5). Of particular interest, extracellular 2AG and PEA levels were significantly different between parathion and chlorpyrifos two days after exposure. As MAGL and FAAH activities were both significantly inhibited by both OPs, the selective increases in 2AG and PEA by chlorpyrifos were unanticipated and could be due to other factors besides enzymatic degradation. It should be noted that eCB signaling modulates acetylcholine release in the hippocampus but has relatively little influence on acetylcholine release in the striatum (Gifford et al., 2000; Kathmann et al., 2001). Moreover, cholinergic signaling in the striatum may play an important role in motor signs associated with OP toxicity (Slater and Dickinson, 1982, Espinola et al., 1999; O'Donnell et al., 2010). We previously reported that extracellular AEA in the hippocampus was increased by both chlorpyrifos and parathion, with significantly greater elevation after chlorpyrifos. An early increase in extracellular 2AG in the hippocampus was also noted following chlorpyrifos, but not parathion (Liu et al. 2013). Since eCBs are involved in modulating acetylcholine release in the hippocampus, the more robust and selective changes in hippocampal AEA and 2AG may contribute to the lack of signs of cholinergic toxicity with chlorpyrifos by reducing acetylcholine release. In contrast, the selective increases in striatal AEA and 2AG by chlorpyrifos noted herein may influence OP toxicity through non-cholinergic pathways.
Endocannabinoid signaling was initially characterized in the phenomenon of depolarization-induced suppression of inhibition, a neurophysiological process mediated by inhibition of GABA release via CB1 receptor activation (Ohno-Shosaku et al., 2001; Wilson and Nicholl, 2001). Enhancing GABAergic signaling with benzodiazepines has been a strategy in the treatment of OP intoxications for decades (Marrs, 1993), thus eCB-mediated changes in GABA release may be important in the context of OP toxicity. The release of other non-cholinergic transmitters, e.g., glutamate, can also be influenced by eCBs (Krietzer and Regher, 2001). Recruitment of glutamatergic signaling is proposed to contribute to some of the neurological dysfunction associated with OP toxicity (Lallement et al., 1991; Shih et al., 1991; Shih and McDonough, 1997; Weissman and Raveh, 2008). Thus eCB-mediated regulation of non-cholinergic signals may be important in the ultimate expression of toxicity. AEA is considered the primary eCB in the control of glutamate input to striatal neurons (Gubellini et. al., 2002), whereas 2AG has been coupled to GABAergic synapses, regulating inhibitory input to the striatum (Maccarrone et. al., 2008).
Endocannabinoid-mediated modulation of neurotransmitter release by activation of presynaptic CB1 receptors has been extensively studied. In contrast, activation of non-cannabinoid (e.g., peroxisome proliferator-activated receptors, PPARs) receptors by eCBs and eCBLs has received less attention. AEA is an agonist at both PPARα and PPARγ, while PEA and OEA selectively activate PPARα (O'Sullivan, 2007; Pistis and Melis, 2010, Fezza et al., 2014). Early selective increases in extracelluar levels of AEA and PEA in the striatum following chlorpyrifos exposure (Figures 2A and 3A) could thus play a potential role in differential toxicity through PPARα and PPARγ.
Intracerebroventricular administration of PEA facilitated the hypnotic effect of pentobarbital in mice (Sasso et al., 2010). This behavioral change was associated with a marked increase in brainstem levels of the neurosteroid allopregnanolone, and was absent in PPARα knockout mice. It should be noted that allopregnanolone, similar to benzodiazepines used to treat OP toxicity, is a GABAA agonist and provides improved treatment for the status epilepticus (Rogawski et al., 2013). Using a hyperalgesia model in rats, Romero and colleagues (2012) reported that exogenously administered PEA elicited an antinociceptive response that was reversed by a neuronal nitric oxide synthase (nNOS) inhibitor. The work of Romero and colleagues is particularly interesting in the context of previous studies demonstrating potentiation of parathion toxicity by a nNOS inhibitor (Liu et al., 2007). PPARγ agonists have neuroprotective actions via anti-inflammatory and antioxidative pathways (Heneka et al., 2000; Yi et al., 2008; Quinn et al., 2008; Schintu et al., 2009; Lee et al., 2012). Lee and coworkers (2014) recently reported that the PPARγ agonist rosiglitazone blocked apoptosis in SH-SY5Y cells exposed to chlorpyrifos. These studies all suggest the potential role of eCBs and eCBLs in modulating OP toxicity through non-cholinergic mechanisms.
The relative ability of OPs to modify the release and/or clearance of eCBs and eCBLs may provide a molecular basis for differential expression of OP toxicity. OP- selective and brain regional-specific changes in eCB and eCBL metabolism, and the participation of cannabinoid and non-cannabinoid receptors in the cellular actions of eCBs and eCBLs, constitute more complex considerations in the adverse outcome pathway for anticholinesterases.
Acknowledgments
This research was supported by grant R01ES009119 from National Institute of Environmental Health Sciences, NIH, the Oklahoma State University Board of Regents, and the Oklahoma State University Interdisciplinary Toxicology Program. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS. The authors declare that there are no conflicts of interest. We appreciate the efforts of Dr. Melanie Breshears, Anatomic Pathologist, Department of Veterinary Pathobiology, Oklahoma State University, in the confirmation of cannula/probe placement.
Footnotes
Conflict of Interest: The authors declare there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Polis IY, Parsons LH. The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology. 2013;38:574–584. doi: 10.1038/npp.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Adams AL, Kepler DR, Ward AB, Ross MK. Induction of endocannabinoid levels in juvenile rat brain following developmental chlorpyrifos exposure. Toxicol Sci. 2013;135:193–201. doi: 10.1093/toxsci/kft126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Gulevich AG, Sarpong R, Bunnelle EM. S-Arachidonoyl-2-thioglycerol synthesis and use for fluorimetric and colorimetric assays of monoacylglycerollipase. Bioorg Med Chem. 2010;18:1942–1947. doi: 10.1016/j.bmc.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Köfalvi A, Wade MR, Davis RJ, Rodrigues RJ, Rebola N, Cunha RA, Nomikos GG. CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol Pharmacol. 2006;70:1236–1245. doi: 10.1124/mol.106.024661. [DOI] [PubMed] [Google Scholar]
- DuBois KP, Doull J, Salerno PR, Coon JM. Studies on the toxicity and mechanism of action of p-nitrophenyl diethyl thionophosphate (parathion) Pharmacol Exp Ther. 1949;95:79–91. [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapidcolorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Espínola EB, Oliveira MG, Carlini EA. Differences in central and peripheral responses to oxotremorine in young and aged rats. Pharmacol Biochem Behav. 1999;62:419–423. doi: 10.1016/s0091-3057(98)00192-0. [DOI] [PubMed] [Google Scholar]
- Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19:17078–17106. doi: 10.3390/molecules191117078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford AN, Ashby CR., Jr Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J Pharmacol Exp Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Volkow ND. Cannabinoid receptor-mediated inhibition of acetylcholine release from hippocampal and cortical synaptosomes. Br J Pharmacol. 2000;131:645–50. doi: 10.1038/sj.bjp.0703599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Offertáler L, Wagner JA, Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009;89:105–111. doi: 10.1016/j.prostaglandins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube A, Kiely T, Donaldson D, Wu L. EPA's Biological and Economic Analysis Division, Office of Pesticide Programs, and Office of Prevention, Pesticides, and Toxic Substances. U.S. Environmental Protection Agency; Washington, DC: 2011. Pesticide Industry Sales and Usage: 2006 and 2007 Market Estimates. http://www.epa.gov/opp00001/pestsales/07pestsales/table_of_contents2007.htm. [Google Scholar]
- Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agrò A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22(16):6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp Neurol. 2010;224:48–55. doi: 10.1016/j.expneurol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci. 2000;20:6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth S, Liu J, Ray A, Pope C. Comparative in vivo effects of parathion onstriatal acetylcholine accumulation in adult and aged rats. Toxicology. 2007;239:167–79. doi: 10.1016/j.tox.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Weber B, Zimmer A, Schlicker E. Enhanced acetylcholine release in the hippocampus of cannabinoid CB(1) receptor-deficient mice. Br J Pharmacol. 2001;132:1169–1173. doi: 10.1038/sj.bjp.0703987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lallement G, Carpentier P, Collet A, Pernot-Marino I, Baubichon D, Sentenac-Roumanou H, Blanchet G. Involvement of glutamatergic system of amygdala in generalized seizures induced by soman: comparison with the hippocampus. C R Acad Sci III. 1991;313:421–426. [PubMed] [Google Scholar]
- Lee EY, Lee JE, Park JH, Shin IC, Koh HC. Rosiglitazone, a PPAR-γ agonist, protects against striatal dopaminergic neurodegeneration induced by 6-OHDA lesions in the substantia nigra of rats. Toxicol Lett. 2012;213:332–344. doi: 10.1016/j.toxlet.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Lee JE, Park JH, Jang SJ, Koh HC. Rosiglitazone inhibits chlorpyrifos-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. Toxicol Appl Pharmacol. 2014;278:159–171. doi: 10.1016/j.taap.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Liu J, Pope CN. Comparative presynaptic neurochemical changes in rat striatum following exposure to chlorpyrifos or parathion. J Toxicol Environ Health A. 1998;53:531–44. doi: 10.1080/009841098159123. [DOI] [PubMed] [Google Scholar]
- Liu J, Pope C. The cannabinoid receptor antagonist AM251 increases paraoxon and chlorpyrifos oxon toxicity in rats. Neurotoxicology. 2015;46:12–18. doi: 10.1016/j.neuro.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gupta RC, Goad JT, Karanth S, Pope C. Modulation of parathion toxicity by glucose feeding: Is nitric oxide involved? Toxicol Appl Pharmacol. 2007;219:106–113. doi: 10.1016/j.taap.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Liu J, Parsons L, Pope C. Comparative effects of parathion and chlorpyrifos on extracellular endocannabinoid levels in rat hippocampus: influence on cholinergic toxicity. Toxicol Appl Pharmacol. 2013;272:608–615. doi: 10.1016/j.taap.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Song S, Jones PM, Persaud SJ. GPR55: from orphan to metabolic regulator? Pharmacol Ther. 2015;145:35–42. doi: 10.1016/j.pharmthera.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVerme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agrò A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–9. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Marrs TC. Organophosphate poisoning. Pharmacol Ther. 1993;58:51–66. doi: 10.1016/0163-7258(93)90066-m. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Modulation of paraoxon toxicity by the cannabinoid receptor agonist WIN 55,212-2. Toxicology. 2006;227:173–183. doi: 10.1016/j.tox.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Pharmacological enhancement of endocannabinoid signaling reduces the cholinergic toxicity of diisopropylfluorophosphate. Neurotoxicology. 2008;29:1037–1043. doi: 10.1016/j.neuro.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Hudak CS, Ward AM, Burston JJ, Issa RS, Fisher KJ, Abood ME, Wiley JL, Lichtman AH, Casida JE. Monoacylglycerol lipase regulates 2-arachidonoylglycerol action and arachidonic acid levels. Bioorg Med Chem Lett. 2008;18:5875–8. doi: 10.1016/j.bmcl.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Casida JE. Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mousebrain. J Agric Food Chem. 2011;59:2808–15. doi: 10.1021/jf101747r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell JC, Acon-Chen C, McDonough JH, Shih TM. Comparison of extracellular striatal acetylcholine and brain seizure activity following acute exposure to the nerve agents cyclosarin and tabun in freely moving guinea pigs. Toxicol Mech Methods. 2010;20:600–608. doi: 10.3109/15376516.2010.521208. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- O′Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistis M, Melis M. From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr Med Chem. 2010;17:1450–1467. doi: 10.2174/092986710790980014. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD. Long-term neurochemical and behavioral effects induced by acute chlorpyrifos treatment. Pharmacol Biochem Behav. 1992;42:251–256. doi: 10.1016/0091-3057(92)90523-i. [DOI] [PubMed] [Google Scholar]
- Pope C, Karanth S, Liu J. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol. 2005;19:433–446. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Quinn LP, Crook B, Hows ME, Vidgeon-Hart M, Chapman H, Upton N, Medhurst AD, Virley DJ. The PPARgamma agonist pioglitazone is effective in the MPTP mouse model of Parkinson's disease through inhibition of monoamine oxidase B. Br J Pharmacol. 2008;154:226–233. doi: 10.1038/bjp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol Appl Pharmacol. 2001;173:48–55. doi: 10.1006/taap.2001.9175. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. Monoacylglycerollipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol Appl Pharmacol. 2006;211:78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54(Suppl 6):93–98. doi: 10.1111/epi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero TR, Galdino GS, Silva GC, Resende LC, Perez AC, Cortes SF, Duarte ID. Involvement of the L-arginine/nitric oxide/cyclic guanosine monophosphate pathway in peripheral antinociception induced by N-palmitoyl-ethanolamine in rats. J Neurosci Res. 2012;90:1474–1479. doi: 10.1002/jnr.22797. [DOI] [PubMed] [Google Scholar]
- Sasso O, La Rana G, Vitiello S, Russo R, D'Agostino G, Iacono A, Russo E, Citraro R, Cuzzocrea S, Piazza PV, De Sarro G, Meli R, Calignano A. Palmitoylethanolamide modulates pentobarbital-evoked hypnotic effect in mice: involvement of allopregnanolone biosynthesis. Eur Neuropsychopharmacol 2010. 2010;20:195–206. doi: 10.1016/j.euroneuro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Laitinen JT. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf) 2012;204:267–276. doi: 10.1111/j.1748-1716.2011.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Shih TM, Koviak TA, Capacio BR. Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci Biobehav Rev. 1991;15:349–362. doi: 10.1016/s0149-7634(05)80028-4. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17:255–264. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Slater P, Dickinson SL. Effects of lesioning basal ganglia nuclei and output pathways on tremorine-induced tremor in rats. J Neurol Sci. 1982;57:235–247. doi: 10.1016/0022-510x(82)90030-2. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J Neurosci. 2003;23:9374–9384. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa NM, Deutsch DG. Assessment of a spectrophotometric assay for monoacylglycerol lipase activity. AAPS J. 2010;12:197–201. doi: 10.1208/s12248-010-9180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman BA, Raveh L. Therapy against organophosphate poisoning: the importance of anticholinergic drugs with antiglutamatergic properties. Toxicol Appl Pharmacol. 2008;232:351–358. doi: 10.1016/j.taap.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Wilson BW. Cholinesterases. In: Krieger R, editor. Handbook of Pesticide Toxicology. 3rd. Vol. 2. Elsevier; Amsterdam: 2010. pp. 1457–1478. [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wright LK, Liu J, Nallapaneni A, Pope CN. Behavioral sequelae following acute diisopropylfluorophosphate intoxication in rats: comparative effects of atropine and cannabinomimetics. Neurotoxicol Teratol. 2010;32:329–35. doi: 10.1016/j.ntt.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]