Abstract
Few studies have examined the built environment's role in recruitment to and adherence in dietary intervention trials. Using data from a randomized dietary modification trial of urban Latina breast cancer survivors, we tested the hypotheses that neighborhood produce access could act as a potential barrier and/or facilitator to recruitment, and that a participant's produce availability would be associated with increased fruit/vegetable intake, one of the intervention's targets. Eligible women who lived within a higher produce environment had a non-significant trend towards being more likely to enroll in the trial. Among enrollees, women who had better neighborhood access to produce had a non-significant trend toward increasing fruit/vegetable consumption. As these were not a priori hypotheses to test, we consider these analyses to be hypothesis generating and not confirmatory. Results suggest that participants’ food environment should be considered when recruiting to and assessing the adherence of dietary intervention studies.
Keywords: Dietary interventions, Breast cancer, Geographic Information Systems (GIS), Food environments, Nutrition
1. INTRODUCTION
Dietary modification trials are an important tool used to assess the relationships between diet and disease because they allow investigators to manipulate dietary patterns and assess changes on a range of biomarker and clinical endpoints. Understanding the factors that affect both recruitment of eligible participants and adherence to the intervention allow investigators to more completely account for potential selection bias and effect measure modification in their trials. Knowledge of these external influences can lead to better designed and more externally valid interventions. Despite the growing body of evidence showing an association between an individual's food access within their neighborhood and fruit/vegetable consumption in adults [1, 2], fruit/vegetable consumption in children [3], obesity levels [4-11], higher levels of dietary quality in pregnant women [12], differences in eating patterns [13-15], and the identification of the built environment as a predictor of adherence in physical activity interventions [16], few studies have examined the role of neighborhood food access in dietary modification trials.
Factors related to enrollment in dietary intervention trials and adherence to the interventions itself ultimately affect the interpretability and generalizability of trial results. Studies examining factors related to enrollment in clinical trials has largely focused on participant demographics [17-20], socioeconomic status [17, 19], and participant feelings towards clinical trials [17, 20]. To our knowledge, the association between an eligible participant's food environment and their decision to enroll in a dietary modification trial has not been examined. The literature on predictors of dietary intervention adherence has largely focused on demographic characteristics, such as participant education level, fruit and vegetable affordability [21-24], patient baseline dietary patterns [25], and whether or not the taste preferences of the participants overlap with the intervention nutrient end point [21, 26, 27]. A few studies have identified socio-cultural barriers to adherence such as decision making, cultural context [22, 28], and familial support [28]. To our knowledge, only two studies have examined the food environment as a possible effect measure modifier in dietary interventions [29, 30].
¡Cocinar Para Su Salud! (Cook For Your Health!) was a National Cancer Institute (NCI) funded randomized controlled trial that examined the effects of a community-based dietary modification intervention on fruit, vegetable and fat intake among Latina breast cancer survivors, the majority of whom lived in Northern Manhattan. The main trial results have been previously reported [31]. Briefly, the intervention group attended a short-term in-person 9-session dietary intervention program (24 hours in total) and the control group received written materials. After 6 months, the intervention group compared to the control group reported an increase in targeted fruits and vegetables (+2.7 servings vs. +0.5 servings, P=0.002), a nonsignificant decrease in percent calories from fat (−7.5% vs. −4.4%; P=0.23), and a nonsignificant decrease in weight (−2.5 kg vs. +3.8kg; P=0.22). Using data from this trial, we tested the hypotheses that a participant's food environment, and specifically their access to produce, could pose a barrier to participation in a dietary intervention trial, and that a participant's produce availability would be associated with adherence to the trial. To test these hypotheses we compared characteristics of eligible women who did and did not enroll in the trial, and among participants randomized to the intervention group we examined adherence to the intervention by a participant's local food environment. As these were not a priori hypotheses to test, we consider these analyses to be hypothesis generating and not confirmatory.
2. METHODS AND MATERIALS
2.1 Participant recruitment, consent and enrollment
¡Cocinar Para Su Salud! was a culturally tailored randomized controlled trial comparing the effects of a nine-session (24 hours over 12 weeks) dietary intervention vs. standard of care written materials on dietary intake for cancer survivors [31]. Spanish-speaking patients from the Columbia University Medical Center (CUMC) Breast Oncology Clinic with non-metastatic stage 0-III cancer were recruited by a native Spanish speaker between January 2011 and March 2012. Eligibility criteria were defined as: ≥21 years of age, Spanish language fluency and Hispanic ethnicity, controlled comorbidities if present, non-smoker, fewer than five servings of fruit and vegetables daily as measured by the Block Fruit and Vegetable Screener, and no current involvement with a dietary change program. Trial eligibility was initially assessed by medical record review and participants provided written informed consent to be further screened for trial eligibility. An interviewer administered screening interview was conducted by telephone or in person to obtain data on participant demographics and treatment history. Patients who met the eligibility criteria were invited to participate in the trial and were scheduled for a baseline interview and a clinic visit to assess detailed demographic data, medical history, reproductive history, family history, demographics, physical activity, medication use, acculturation, anthropomorphic measures, physical examination and 24 hour dietary recall. Participants were randomized into the trial following the baseline clinic visit. Participants provided written informed consent and the study was approved by the Columbia University Medical Center and Columbia University Teachers College Institutional Review Boards.
A total of 102 women were screened for the trial and were eligible for participation. Ultimately, 70 women enrolled in the trial. Twenty-two percent (n=21) of the participants screened reported that family members (children, parents or “someone else”) were doing the majority of their shopping. Since many of these family members were using personal cars to procure the groceries, women who did not do their own grocery shopping were excluded from these analyses in order to isolate the effect of the immediate food environment on shopping decisions.
2.2 Intervention
Development of the intervention has been described in detail elsewhere [32]. Briefly, eligible participants were randomized into either a nine-session intervention program or the control group of written materials detailing dietary guidelines for cancer survivors. The nine sessions were tailored specifically for the dietary habits of Hispanic populations with the goal of decreasing dietary fat and increasing fruit/vegetable consumption. The sessions included nutrition education, hands-on cooking classes and food shopping field trips. The control group received standard care, a 22-page Spanish language written dietary recommendation booklet for breast cancer survivors [33]. The entire intervention was conducted in Spanish and all study staff were bilingual (Spanish/English).
2.3 Data collection
2.3.1 Dietary data
The Block Fruit, Vegetable and Fiber Screener was used during the screening interview to determine if patients met the eligibility criterion of consuming fewer than 5 servings of fruits and vegetables daily [34]. Afterwards, enrolled participants’ diets were assessed at baseline, 3 months and 6 months via three 24-hour dietary recalls using the multiple pass approach [35]. The multiple pass approach allows for quality control of dietary data because first the participants are asked to name everything they have eaten in a given day without interviewer interruption, second they are probed about forgotten foods, and then they are asked to specify the time and occasion they were eating the aforementioned items. Afterwards, they are asked again about any potential forgotten foods. Dietary data were entered into the University of Minnesota Nutrition Data System for Research (NDSR) database [36].
2.3.2 Spatial location data
Participant spatial location (i.e., residential address) was obtained through medical records, and the location of the medical center was obtained through Google maps. These data were geocoded using Lion, an address coder from the New York City (NYC) Department of City Planning [37].
We defined the produce environment as places participants could feasibly purchase fruits and non-starchy vegetables, specifically: Green Carts, farmers’ markets, grocery stores, health food stores, and retail produce stores. Green Carts are New York City street vendors who are only licensed to sell whole fruit and vegetable in areas considered to be food deserts. Green Cart spatial location data was obtained from the NYC Department of Health and Mental Hygiene (NYCDOHMH). While carts are permitted to move around large licensing areas, Karp Resources (New York, NY), a small-business consulting group maintains a database with approximate locations of the intersections where Green Carts can be found. The Green Cart locations were identified by Karp Resources employees who regularly maintain regular contact with Green Cart vendors and then submit the locations to NYCDOMH for reporting purposes. Green cart data was received from the NYCDOMH in Keyhole Markup Language format in a Google map and was transformed into a shapefile using ArcGIS 10.1 (Redlands, California). Grocery stores, health food stores and retail produce outlets’ spatial coordinate data were obtained through Ref USA (Papillion, NE), and were downloaded in October 2012. RefUSA obtains spatial location data by visiting locations across the US to verify addresses. RefUSA data is updated every Thursday. We chose to download the data in October 2012, so that it matched the time the participants were in the study. Farmers’ market spatial location data was obtained from the United States Department of Agriculture (USDA) website, which is maintained by the Agricultural Marketing Services (AMS) [38]. These data are self-reported by the Famers’ Market managers and other market personnel. All spatial files were transformed using the “project” tool into State Plane, Long Island projections in ArcGIS.
2.3.3 Buffer analysis
Using the buffer tool in ArcGIS 10.1, a .5 kilometer (km) buffer was created around the 74 participant addresses, based upon previously used methods [8, 39]. Using the select by location tool, a count column was created in the buffer attribute file. A produce density variable was created for each participant by dividing the number of produce options by the total area of the buffer (.79 kilometers squared). This variable was broken into six categories indicating the number of specific types of produce retail locations in a .5 km area surrounding the participant's home, including number of total fruit/vegetable locations, green carts, farmers markets, health food stores, retail produce stores, and grocery stores.
The buffer of 1 km has been established by NYC transportation geographers and other investigators as being the distance where walking can be used as a mode of transportation and as a reasonable approximation of the neighborhood [8, 39]. Because a trip to the store requires carrying groceries home, and people might not be willing to travel as far with groceries as to other destinations, we used a radius of half a kilometer (5 blocks), which would total a one kilometer round trip. This density variable was split at the mean into “high” and “low” produce density environments to create a dichotomous outcome.
2.4 Statistical analysis
A chi-squared test was used to examine the differences of the produce environment between eligible women who did and did not enroll in the trial. A student's t-test was used to test for an association related to distance to the medical center between eligible women who chose to participate and those who did not in order to ensure that we were capturing the effect of produce access on participation rather than proximity to the clinic where the intervention was taking place. Among enrolled women in the intervention arm of the trial, two-sided student's t-tests were used to examine the association between produce density and the amount of change in fruits/vegetables during the first 6 months of the trial. We considered p-values below .05 to be statistically significant. Non-significant trends are described as those where the two groups have differences in characteristics, but where the p-values are larger than .05. Sensitivity analyses were conducted to isolate the effect of participant income on produce access by comparing the produce access among enrollees and non-enrollees among participants with annual incomes below $15,000. All analyses were conducted using Stata 12.1 (College Station, TX).
3. RESULTS
3.1 Participant characteristics
Compared to eligible women who did their own grocery shopping and who did not enroll in the trial, enrolled women who did their own grocery shopping tended to be younger (57 years vs. 61 years p=.07) and were more likely to have full time jobs (28% vs. 5% p=.01) (Table 1). Race and nationality between the two groups were similar, as was degree of acculturation and number of comorbid conditions. There was a non-significant trend towards eligible women who chose not to participate having lower household incomes and being more likely to be currently enrolled in a public food assistance program (91% vs. 61% p=.14; 71% vs. 56% p=.24). Lastly, eligible women who participated in the study lived further away from the study medical center compared to eligible women who did not enroll (3.7 km vs. 2.5 km p=.12).
Table 1.
Demographic characteristics of Latina breast cancer survivors who were eligible to participate in a dietary intervention trial and who do their own grocery shopping
| Total (n=74) | Eligible enrolled participants (n=53) | Eligible non-enrolled participants (n=21) | P-value* | |
|---|---|---|---|---|
| Age, years | 0.07 | |||
| Means | 57.8 (9.4) | 56.6 (9.4) | 60.9 (9.1) | |
| Median | 57.7 | 56.7 | 61 | |
| Range | 40.2 - 80.7 | 40.2 - 77.5 | 46.3 - 80.7 | |
| Race, n (%) | 0.08 | |||
| Black | 17 (23.0) | 11 (20.8) | 6 (28.6) | |
| White | 31 (41.9) | 22 (41.5) | 9 (42.9) | |
| Native American | 5 (6.8) | 2 (3.8) | 3 (14.3) | |
| Mixed Race | 9 (12.2) | 9 (17) | 0 (0) | |
| Missing/Refused | 12 (16.2) | 9 (17) | 3 (14.3) | |
| Nationality, n (%) | 0.98 | |||
| Dominican | 56 (75.7) | 39 (73.6) | 17 (81) | |
| Puerto Rican | 8 (10.8) | 5 (9.4) | 3 (14.3) | |
| Ecuadorian | 5 (6.8) | 4 (7.5) | 1 (4.8) | |
| Colombian | 1 (1.4) | 1 (1.9) | 0 (0) | |
| Cuban | 1 (1.4) | 1 (1.9) | 0 (0) | |
| Honduran | 1 (1.4) | 1 (1.9) | 0 (0) | |
| Mexican | 1 (1.4) | 1 (1.9) | 0 (0) | |
| Other | 1 (1.4) | 1 (1.9) | 0 (0) | |
| Education, n (%) | 0.28 | |||
| High school or below | 44 (59.5) | 30 (56.6) | 14 (66.7) | |
| College or above | 27 (36.5) | 22 (41.5) | 5 (23.8) | |
| Missing/Refused | 3 (4.1) | 1 (1.9) | 2 (9.5) | (A) |
| Employment status, n (%) | 0.09 | |||
| Full-time | 16 (21.6) | 15 (28.3) | 1 (4.8) | |
| Part-time | 11 (14.9) | 8 (15.1) | 3 (14.3) | |
| Retired | 6 (8.1) | 5 (9.4) | 1 (4.8) | |
| Homemaker | 12 (16.2) | 11 (20.8) | 1 (4.8) | |
| Unemployed | 6 (8.1) | 3 (5.7) | 3 (14.3) | |
| Disabled | 23 (31.1) | 11 (20.8) | 12 (57.1) | |
| Annual household income, n (%) | 0.01 | |||
| $0 - $15,000 | 52 (70.3) | 33 (62.3) | 19 (90.5) | |
| $15,001 - $30,000 | 11 (14.9) | 11 (20.8) | 0 (0) | |
| $30,000 - $60,000 | 6 (8.1) | 5 (9.4) | 1 (4.8) | |
| Missing/Refused | 17 (23) | 16 (30.2) | 1 (4.8) | (C) |
| Currently receiving food assistance, n (%) | 0.24 | |||
| Yes | 45 (60.8) | 30 (56.6) | 15 (71.4) | |
| No | 29 (39.2) | 23 (43.4) | 6 (28.6) | |
| Acculturation index, n (%)*** | 0.66 | |||
| Means | 1.6 (0.6) | 1.6 (0.6) | 1.5 (0.5) | |
| Median | 1.4 | 1.4 | 1.4 | |
| Range | 0.7 - 3.5 | 0.7 - 3.5 | 1.0 - 3.0 | |
| Number of comorbid conditions, n (%)**** | 0.67 | |||
| Means | 1.2 (1.4) | 1.2 (1.4) | 1.0 (1.4) | |
| Median | 1 | 1 | 0 | |
| Range | 0.0 - 7.0 | 0.0 - 7.0 | 0.0 - 5.0 | |
| Distance from home to medical center, km | 0.12 | |||
| Means | 3.8 (4.1) | 3.7 (4.6) | 2.5 (2.0) |
The values presented are number, percentage. When means are noted they are presented as the mean with the S.D. in parentheses. The total sample presented is 74 broken down into 53 eligible enrolled women, and 21 eligible unenrolled women.
**P-values were calculated using t-tests to compare means, and chi-squared tests (employment and food assistance) and Fisher's exact tests (race, nationality, education and income) to compare proportions. Fisher's exact test for employment status compared between participants currently working (part-time or full-time) vs. not working (retired, homemaker, unemployed, or disabled). Fisher's exact test for household income compared between low ($0-$15,000) vs. high (>$15,000) income.
The acculturation index was the Short Acculturation Scale for Hispanics, ranging from 1 to 5 (high).
The comorbidity index was created based on the methods of Charlston et al. and Patterson et al., and included the following conditions: ulcer, diabetes, neurological problems, gastrointestinal problems, respiration problems, risk factors for heart disease (weight=1); kidney disease, heart problems, chest pain, physical limitation (weight=2) HIV/AIDS, and cancer other than breast cancer (weight=3).
****Values don't all add up to 100% because of missing values. They were excluded from analyses, but left in the tables to show the limitations of our data.
3.2 Food environment characteristics
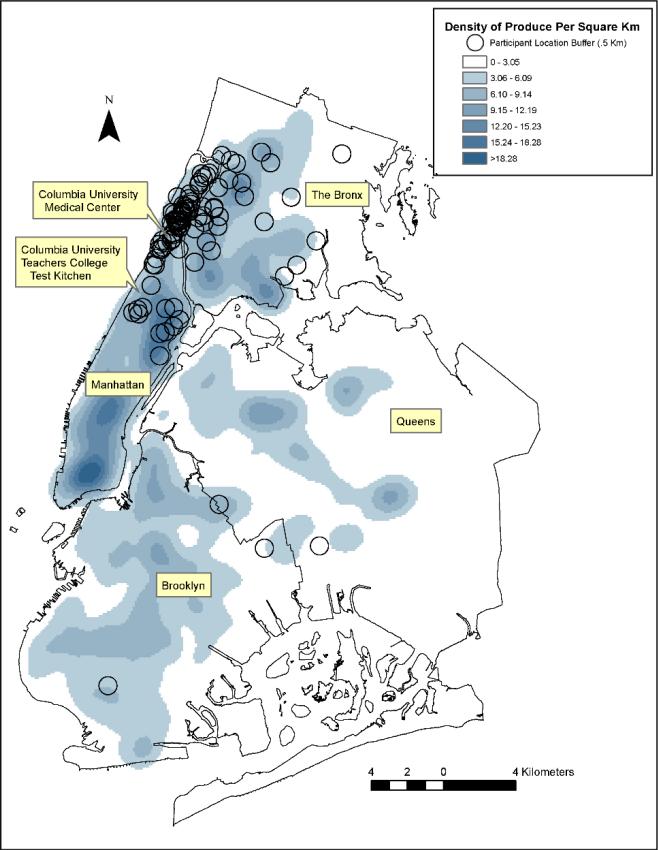
The majority of women who were eligible for participation were residents of northern Manhattan and a small number lived in other NYC boroughs, all of which are highly urban areas (Figure 1). On average, women had 12 [standard deviation (SD) 7.4] produce retail outlets within a .5 km buffer around their home, with a range of 0-35 produce retail outlets (Table 2). The most common produce retail outlets within each study participant's food environment were green carts [mean 3.1 (SD 4.7)], while the least common produce retail outlets were health food stores [mean 0.8 (SD 1.1)].
Figure 1. Density of Produce in New York City Per Square Kilometer.
Map of the produce outlets per square kilometer in New York City relative to the homes of the patient population as well as the Columbia University Medical Center and Columbia University Teacher's College Test Kitchen. Each circle represents a .5 kilometer buffer around each eligible woman's residence. We used ArcGIS Spatial Analyst (ESRI,Redlands, CA) to estimate a continuous surface of produce outlets with a kernel function using a one mile bandwidth.
Table 2.
1: Neighborhood fruit and vegetable density**, by participation among Latina breast cancer survivors who shop for themselves (n=74)
| Total (n=74) |
Eligible enrolled participants (n=53) |
Eligible non-enrolled participants (n=21) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Means (S.D.) | Median | Range | Means (S.D.) | Median | Range | Means (S.D.) | Median | Range | P-value | |
| All participants | ||||||||||
| Total produce density | 11.9 (7.4) | 10.2 | 0.0 - 35.7 | 12.7 (8.0) | 11.5 | 1.3 - 35.7 | 9.7 (5.3) | 8.9 | 0.0 - 17.8 | 0.07 |
| Grocery store density | 1.2 (1.8) | 0.0 | 0.0 - 6.4 | 1.1 (1.8) | 0.0 | 0.0 - 6.4 | 1.5 (2.0) | 0.0 | 0.0 - 5.1 | 0.42 |
| Health food store density | 0.8 (1.1) | 0.0 | 0.0 - 3.8 | 1.0 (1.1) | 1.3 | 0.0 - 3.8 | 0.4 (0.8) | 0.0 | 0.0 - 2.5 | 0.03 |
| Produce store density | 2.8 (2.1) | 2.5 | 0.0 - 7.6 | 3.1 (2.0) | 2.5 | 0.0 - 7.6 | 2.2 (2.4) | 2.5 | 0.0 - 7.6 | 0.15 |
| Farmers’ market density | 1.2 (1.8) | 0.0 | 0.0 - 6.4 | 1.1 (1.8) | 0.0 | 0.0 - 6.4 | 1.5 (2.0) | 0.0 | 0.0 - 5.1 | 0.42 |
| Green Cart density | 3.1 (4.7) | 1.3 | 0.0 - 22.9 | 3.5 (5.4) | 1.3 | 0.0 - 22.9 | 2.0 (1.2) | 2.5 | 0.0 - 3.8 | 0.07 |
| Total (n=52) | Eligible enrolled participants (n=33) | Eligible non-enrolled participants | (n=19) | |||||||
| Income $0-$15,000 | ||||||||||
| Total produce density | 11 (7.1) | 10.2 | 0.0-35.7 | 11.7 (8.1) | 10.2 | 1.3-35.7 | 9.7 (5.1) | 8.9 | 0.0-17.8 | 0.28 |
| Grocery store density | 1.2 (1.8) | 0.0 | 0.0-6.4 | 1.0 (1.6) | 0.0 | 0.0-6.4 | 1.7 (2.1) | 1.3 | 0.0-5.1 | 0.21 |
| Health food store density | 0.6 (1.0) | 0.0 | 0.0-3.8 | 0.8 (1.1) | 0.0 | 0.0-3.8 | 0.4 (0.9) | 0.0 | 0.0-2.5 | 0.18 |
| Produce store density | 2.6 (2.1) | 2.5 | 0.0-7.6 | 2.9 (2.1) | 2.5 | 0.0-7.6 | 2.0 (2.1) | 2.5 | 0.0-6.4 | 0.15 |
| Farmers’ market density | 1.2 (1.8) | 0.0 | 0.0-6.4 | 1.0 (1.6) | 0.0 | 0.0-6.4 | 1.7 (2.1) | 1.3 | 0.0-5.1 | 0.21 |
| Green Cart density | 2.8 (4.3) | 1.3 | 0.0-22.9 | 3.2 (5.3) | 1.3 | 0.0-22.9 | 2.1 (1.3) | 2.5 | 0.0-3.8 | 0.24 |
*P-values were calculated using t-tests.
Density variable was calculated by summing the total number of produce outlets available within .5 km of a participant's home and dividing
3.3 Association between food environment and study participation
There were differences in the food environments between eligible women who did and did not enroll in the trial (Table 2). There was a trend towards total access to produce retail outlets being greater among eligible women who enrolled in the trial compared to those who chose not to enroll (12.7 outlets vs. 9.7 outlets, P=0.07). Differences were also observed between enrolled and non-enrolled women in the number of Green Carts (3.5 vs. 2.0, respectively, P=0.07) and health food stores (1.0 vs. 0.4, respectively, P=0.03). We did not observe a difference between groups when we restricted the population of participants to those with income <$15,000 per year and compared those who did (n=33) and did not (n=21) enroll (Table 2).
3.4 Intervention adherence among enrolled women
As previously reported in the trial's main outcomes paper, enrolled women in the intervention arm of the trial increased their fruit/vegetable consumption by 2.0 (SD 2.8) servings, compared to women in the control group who decreased their intake by 0.1 (SD 2.9) servings (P<0.01) [31]. In the present analysis, at three months, women in the high total produce density environment had double the increased fruits/vegetable intake of women in low produce density neighborhoods (3.1 servings vs. 1.6 servings, P=0.24) (Table 3), though this value did not reach statistical significance. Non-statistically significant differences remained when the produce environment was examined by type. Women in areas of high grocery store, health food store, produce store, farmer's market, or green cart density consumed approximately .5 servings more of the targeted fruit/vegetables than those in low density environments (all P>0.05). At six months, the women in both high density and low density areas had increased their fruit and vegetable consumption almost the same amount, and the only difference of a half a serving or more that persisted was that women who had higher produce store density reported trend towards a higher intake compared to women with lower produce store density (3.9 servings vs. 2.7 servings, P=0.17).
Table 3.
Adherence to the Intervention based on food environment, among participants who shop for themselves (n=24)
| Baseline |
3 Month |
6 month |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Daily servings of fruit/vegetables, means (S.D.) | P-value* | n | Absolute change in fruit/vegetable intake from baseline, means (S.D.) | P-value* | n | Absolute change in fruit/vegetable intake from baseline, means (S.D.) | P-value* | |
| Total produce density | 0.44 | 0.24 | 0.92 | ||||||
| High total produce density | 11 | 2.4 (2.4) | 11 | 3.1 (2.5) | 10 | 3.3 (1.3) | |||
| Low total produce density | 13 | 3.1 (1.7) | 11 | 1.6 (2.9) | 11 | 3.2 (2.6) | |||
| Grocery store density | 0.95 | 0.68 | 0.74 | ||||||
| High grocery store density | 10 | 2.8 (2.7) | 10 | 2.6 (3.1) | 9 | 3.4 (1.4) | |||
| Low grocery store density | 14 | 2.7 (1.5) | 12 | 2.1 (2.6) | 12 | 3.1 (2.5) | |||
| Health food store density | 0.60 | 0.76 | 0.70 | ||||||
| High health food store density | 13 | 3.0 (2.2) | 11 | 2.6 (3.1) | 11 | 3.4 (1.9) | |||
| Low health food store density | 11 | 2.5 (1.9) | 11 | 2.2 (2.6) | 10 | 3.0 (2.3) | |||
| Produce store density | 0.95 | 0.66 | 0.17 | ||||||
| High produce store density | 11 | 2.8 (2.4) | 10 | 2.7 (3.1) | 9 | 3.9 (1.3) | |||
| Low produce store density | 13 | 2.7 (1.7) | 12 | 2.1 (2.6) | 12 | 2.7 (2.4) | |||
| Farmers’ market density | 0.95 | 0.68 | 0.74 | ||||||
| High farmers’ market density | 10 | 2.8 (2.7) | 10 | 2.6 (3.1) | 9 | 3.4 (1.4) | |||
| Low farmers’ market density | 14 | 2.7 (1.5) | 12 | 2.1 (2.6) | 12 | 3.1 (2.5) | |||
| Green Cart density | 0.69 | 0.59 | 0.48 | ||||||
| High Green Cart density | 7 | 2.5 (1.9) | 7 | 2.8 (2.5) | 7 | 2.7 (2.6) | |||
| Low Green Cart density | 17 | 2.9 (2.1) | 17 | 2.1 (3.0) | 14 | 3.5 (1.8) | |||
| Household income | 0.34 | 0.90 | 0.57 | ||||||
| High income | 9 | 2.0 (1.0) | 8 | 2.7 (1.9) | 8 | 3.5 (2.0) | |||
| Low income | 12 | 2.6 (1.8) | 12 | 2.6 (3.0) | 11 | 3.0 (2.2) | |||
| Employment status | 0.07 | 0.21 | 0.52 | ||||||
| Working | 8 | 1.8 (1.1) | 8 | 3.3 (1.6) | 8 | 3.6 (1.6) | |||
| Not working | 15 | 3.2 (2.3) | 13 | 2.0 (3.3) | 12 | 3.0 (2.4) | |||
P-values calculated were using t-tests.
4. DISCUSSION
We conducted two hypothesis generating analyses to test whether an individual's neighborhood produce access could pose a barrier to participation in a dietary intervention trial, and whether produce availability would be associated with adherence to the trial and accept both hypotheses. We found that eligible women who lived within a kilometer of more produce outlets had a non-significant trend towards being more likely to enroll in the trial. When this analysis was restricted to those with incomes <$15,000 a year those who enrolled still showed a non significant trend towards having better access to produce. Among enrollees, women who had better neighborhood access to produce had a non-significant trend toward increasing fruit/vegetable consumption. Our results suggest that we cannot reject our hypotheses and that the food environment potentially may play a role in participants choosing to enroll in dietary intervention trials and in their ability to adhere to the intervention. To our knowledge, this is the first study to examine whether the food environment is associated with participation in a dietary modification trial by eligible potential participants. Our findings suggest that availability of produce can represent a barrier to recruitment in certain populations, and should potentially be considered in recruitment strategies of future behavioral modification trials.
There are a limited number of trials with which we can compare our results. A paper by Gustafson et al. examined the food environment's role in a behavioral intervention, but their population, approach and outcome variables differed significantly from the present analyses [30]. The participants in the Gustafson analyses resided in rural area where cars are the most common mode of transportation, which is quite different compared to an urban area such as northern Manhattan where the majority of inhabitants rely on walking and public transportation. The Gustafson analyses included predominantly white non-Hispanic English speakers, which is very different from the predominantly Spanish speaking Latina breast cancer survivors presented here. Gustafson et al.'s measure of the food environment included convenience stores, whereas we only included stores where participants were most likely to obtain produce. Similarly, a paper by Wedick et al.[29] also examined the relationship between produce access and adherence to a dietary modification trial. However, their study population was also very different from ours. Whereas the majority of woman in the present analyses earned <$15,000 a year and had a high school level education, their population earned >$40,000 and was highly educated. Additionally, their population consisted of almost all white women from suburban Massachusetts. Their measure of food access only included places to purchase healthy foods—and their spatial data was also from RefUSA. Both Gustafson et al. and Wedick et al. found that the food environment influenced intervention adherence. While our results trended in the same direction as theirs, by 6 months both high and low density produce access participants had increased their produce consumption by almost the same amount. We attribute this difference in our results to the fact that our sample included women with an average of 12 produce outlets within five blocks of their houses.
Because we initially observed a univariate correlation between annual household income and study participation, we conducted sensitivity analyses to isolate the effects of income and the produce environment on study participation. In the sensitivity analyses a non significant trend remained, showing that women who participated had more produce outlets in their immediate neighborhood (10.2 vs. 8.9 p=.28). The relationship between income, produce availability and produce cost is complex. While Farmers’ markets can accept EBT, a recent report showed that only 27% of NYC Green Carts have this capability [40]. In the same report, randomized sample of Green Cart customers cited prices as their reason for shopping at the carts, however the analysis did not provide a head to head comparison of prices with other retail outlets. The best data on comparative produce cost comes from the Northeast Organic Farming Association of Vermont, which suggests that prices can be less expensive, particularly for organic produce at farmers’ markets vs. grocery stores [41]. A more in depth discussion is outside of the scope of this paper.
Northern Manhattan, the area where the majority of ¡Cocinar Para Su Salud! participants live, represents a unique neighborhood food environment compared to other parts of New York City. In 2008, as part of a multipronged produce initiative, the NYC mayor's office created a special license class for mobile vegetable vendors in poor neighborhoods, specifically northern Manhattan, in an effort to increase fresh produce consumption in poorer neighborhoods. On average, women who completed the screening questionnaire for this study had three of these carts within a .5 km area around their residences. Previous studies have shown that northern Manhattan has fewer produce outlets than other parts of New York City, but to our knowledge, this is the first study to include the green carts as part of the produce environment.
Strengths of this analysis include the use of three 24-hour dietary recalls using the multiple pass approach to assess dietary change, which is the gold standard in nutrition research, and the novel use of GIS methods to assess the food environment of dietary intervention participants. Our generated hypotheses were also novel as few researchers have examined the potential role of neighborhood food access in dietary modification trials. However, there are important limitations to address. A major study limitation is that our sample was small and relatively homogeneous, which limited power and analysis options. Despite our small sample size, we were able to show a marginally statistically significant relationship between whether or not an eligible woman ultimately enrolled in the study and her access to fresh produce. A second limitation in our study was the measurement of the food environment. Several studies have shown that measurements of the food environment using databases such as Ref USA and Dun and Bradstreet often differ when compared to canvassing the streets in a given neighborhood[42, 43]. As such, our measurement of the produce environment could be subject to measurement error. Additionally, dietary data were collected via a 24 hour recall which were self-reported and are potentially subject to measurement error. The sample size in many of our analyses is small and were not a priori aims of the parent study. Our results need to be considered hypothesis generating and should be tested in larger trials.
Our findings generate the hypothesis that in an urban sample, the food environment might warrant future attention as a barrier to recruitment as well as an effect measure modifier supporting intervention adherence. Having this knowledge would allow researchers to take into account potential issues of selection bias in the design phase of their studies by either discussing the food environment during recruitment, stratifying during randomization and/or including a discussion of how to navigate difficult food environments as part of the intervention itself. If the bias was unavoidable, researchers could control for it during the analysis phase.
We found that there was a non-significant trend towards eligible women who chose to participate in ¡Cocinar Para Su Salud! having higher quality food environments compared eligible women who declined enrollment. While adherence to the intervention was not statistically significantly related to participant food environments, trends were observed in the hypothesized direction. Power was limited by our sample size and may have been further affected by limited exposure variance given that women with better quality food environments were more likely to enroll in our trial. Our results contribute to the growing body of research on the food environment's effect on health and uniquely assess the potential relationship between produce access in a dietary modification trial and both trial recruitment and level of dietary intervention adherence. Further research is needed in this area to better ascertain how the food environment affects the ability for individuals to make and maintain sustained dietary change.
Acknowledgements
Funding for ¡Cocinar Para Su Salud! was provided by the following grants: NCI/NIH R21CA152903 and in part by Columbia University's CTSA grant No.UL1TR000040 from NCATS/NIH. The authors wish to thank Jeremiah Trinidad-Christensen for assistance with the GIS analyses.
ABBREVIATIONS
- CUMC
Columbia University Medical Center
- DOHMH
Department of Health and Mental Hygiene
- GIS
Geographic Information Systems
- NCI
National Cancer Institute
- NYC
New York City
- NDSR
University of Minnesota Nutrition Data System for Research
- SD
Standard Deviation
- USDA
United States Department of Agriculture
Footnotes
None of the authors had conflicts of interest with regards to the article content.
References
- 1.Haynes-Maslow L, Parsons SE, Wheeler SB, Leone LA. A qualitative study of perceived barriers to fruit and vegetable consumption among low-income populations, North Carolina, 2011. Prev Chronic Dis. 2013;10:E34. doi: 10.5888/pcd10.120206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shohaimi S, Welch A, Bingham S, Luben R, Day N, Wareham N, et al. Residential area deprivation predicts fruit and vegetable consumption independently of individual educational level and occupational social class: a cross sectional population study in the Norfolk cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk). J Epidemiol Community Health. 2004;58:686–91. doi: 10.1136/jech.2003.008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timperio A, Ball K, Roberts R, Campbell K, Andrianopoulos N, Crawford D. Children's fruit and vegetable intake: associations with the neighbourhood food environment. Prev Med. 2008;46:331–5. doi: 10.1016/j.ypmed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang MC, Kim S, Gonzalez AA, MacLeod KE, Winkleby MA. Socioeconomic and food-related physical characteristics of the neighbourhood environment are associated with body mass index. J Epidemiol Community Health. 2007;61:491–8. doi: 10.1136/jech.2006.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings A, Welch A, Jones AP, Harrison F, Bentham G, van Sluijs EM, et al. Local food outlets, weight status, and dietary intake: associations in children aged 9-10 years. Am J Prev Med. 2011;40:405–10. doi: 10.1016/j.amepre.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morland K, Diez Roux AV, Wing S. Supermarkets, other food stores, and obesity: the atherosclerosis risk in communities study. Am J Prev Med. 2006;30:333–9. doi: 10.1016/j.amepre.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Morland KB, Evenson KR. Obesity prevalence and the local food environment. Health Place. 2009;15:491–5. doi: 10.1016/j.healthplace.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rundle A, Neckerman KM, Freeman L, Lovasi GS, Purciel M, Quinn J, et al. Neighborhood food environment and walkability predict obesity in New York City. Environ Health Perspect. 2009;117:442–7. doi: 10.1289/ehp.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smoyer-Tomic KE, Spence JC, Raine KD, Amrhein C, Cameron N, Yasenovskiy V, et al. The association between neighborhood socioeconomic status and exposure to supermarkets and fast food outlets. Health Place. 2008;14:740–54. doi: 10.1016/j.healthplace.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Minaker LM, Raine KD, Wild TC, Nykiforuk CI, Thompson ME, Frank LD. Objective food environments and health outcomes. Am J Prev Med. 2013;45:289–96. doi: 10.1016/j.amepre.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Hattori A, An R, Sturm R. Neighborhood food outlets, diet, and obesity among California adults, 2007 and 2009. Prev Chronic Dis. 2013;10:E35. doi: 10.5888/pcd10.120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laraia BA, Siega-Riz AM, Kaufman JS, Jones SJ. Proximity of supermarkets is positively associated with diet quality index for pregnancy. Prev Med. 2004;39:869–75. doi: 10.1016/j.ypmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Diez-Roux AV, Nieto FJ, Caulfield L, Tyroler HA, Watson RL, Szklo M. Neighbourhood differences in diet: the Atherosclerosis Risk in Communities (ARIC) Study. J Epidemiol Community Health. 1999;53:55–63. doi: 10.1136/jech.53.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curl CL, Beresford SA, Hajat A, Kaufman JD, Moore K, Nettleton JA, et al. Associations of organic produce consumption with socioeconomic status and the local food environment: Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One. 2013;8:e69778. doi: 10.1371/journal.pone.0069778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen KM, Qureshi F, Schaible A, Park S, Gittelsohn J. Environmental factors that impact the eating behaviors of low-income African American adolescents in Baltimore City. J Nutr Educ Behav. 2013;45:652–60. doi: 10.1016/j.jneb.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Zenk SN, Wilbur J, Wang E, McDevitt J, Oh A, Block R, et al. Neighborhood environment and adherence to a walking intervention in African American women. Health Educ Behav. 2009;36:167–81. doi: 10.1177/1090198108321249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JR, Whelan TJ, Schiff S, Dubois S, Crooks D, Haines PT, et al. Why cancer patients enter randomized clinical trials: exploring the factors that influence their decision. J Clin Oncol. 2004;22:4312–8. doi: 10.1200/JCO.2004.01.187. [DOI] [PubMed] [Google Scholar]
- 18.Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst. 1995;87:1747–59. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- 19.Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–17. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Martin A, Negron R, Balbierz A, Bickell N, Howell EA. Recruitment of black and Latina women to a randomized controlled trial. J Health Care Poor Underserved. 2013;24:1102–14. doi: 10.1353/hpu.2013.0125. [DOI] [PubMed] [Google Scholar]
- 21.Urban N, White E, Anderson GL, Curry S, Kristal AR. Correlates of maintenance of a lowfat diet among women in the Women's Health Trial. Prev Med. 1992;21:279–91. doi: 10.1016/0091-7435(92)90027-f. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Maintenance of a low-sodium, high-carotene and -vitamin C diet after a 1-year dietary intervention: the Hiraka dietary intervention follow-up study. Prev Med. 2006;43:14–9. doi: 10.1016/j.ypmed.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Newman VA, Thomson CA, Rock CL, Flatt SW, Kealey S, Bardwell WA, et al. Achieving substantial changes in eating behavior among women previously treated for breast cancer--an overview of the intervention. J Am Diet Assoc. 2005;105:382–91. doi: 10.1016/j.jada.2004.12.008. quiz 488. [DOI] [PubMed] [Google Scholar]
- 24.van Gool CH, Penninx BW, Kempen GI, Miller GD, van Eijk JT, Pahor M, et al. Determinants of high and low attendance to diet and exercise interventions among overweight and obese older adults. Results from the arthritis, diet, and activity promotion trial. Contemp Clin Trials. 2006;27:227–37. doi: 10.1016/j.cct.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Kyngas H, Lahdenpera T. Compliance of patients with hypertension and associated factors. J Adv Nurs. 1999;29:832–9. doi: 10.1046/j.1365-2648.1999.00962.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Horn L, Dolecek TA, Grandits GA, Skweres L. Adherence to dietary recommendations in the special intervention group in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr. 1997;65:289S–304S. doi: 10.1093/ajcn/65.1.289S. [DOI] [PubMed] [Google Scholar]
- 27.Crichton GE1HP, Buckley JD, Coates AM, Murphy KJ, Bryan J. Long-term dietary intervention trials: critical issues and challenges. Trials. 2012:13. doi: 10.1186/1745-6215-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman AM, Bowen DJ, Vitolins M, Perri MG, Rosal MC, Sevick MA, et al. Dietary adherence: characteristics and interventions. Control Clin Trials. 2000;21:206S–11S. doi: 10.1016/s0197-2456(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 29.Wedick NM, Ma Y, Olendzki BC, Procter-Gray E, Cheng J, Kane KJ, et al. Access to healthy food stores modifies effect of a dietary intervention. Am J Prev Med. 2015;48:309–17. doi: 10.1016/j.amepre.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafson AA, Sharkey J, Samuel-Hodge CD, Jones-Smith JC, Cai J, Ammerman AS. Food Store Environment Modifies Intervention Effect on Fruit and Vegetable Intake among Low-Income Women in North Carolina. J Nutr Metab. 2012;2012:932653. doi: 10.1155/2012/932653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenlee H, Gaffney AO, Aycinena AC, Koch P, Contento I, Karmally W, et al. inverted exclamation markCocinar Para Su Salud!: Randomized Controlled Trial of a Culturally Based Dietary Intervention among Hispanic Breast Cancer Survivors. Journal of the Academy of Nutrition and Dietetics. 2015 doi: 10.1016/j.jand.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aycinena A, Jennings KA, Gaffney AO, Koch P, Contento I, Gonzalez M, Zullig L, Guidon E, Glassing E, Karmally W, Hershman D, Greenlee H. Development of a culturally-tailored nutrition education curriculum for Latina breast cancer survivors: ¡Cocinar Para Su Salud! . doi: 10.1177/1090198116642236. [DOI] [PubMed] [Google Scholar]
- 33.Deliver GsLW. Every Bite Counts: Nutrition Tips for Breast Cancer Survivors. New York: God's Love We Deliver; 2009. God's Love We Deliver. [Google Scholar]
- 34.Block CG G, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–8. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 35.Dwyer J, Picciano MF, Raiten DJ. Collection of food and dietary supplement intake data: What We Eat in America-NHANES. J Nutr. 2003;133:590s–600s. doi: 10.1093/jn/133.2.590S. [DOI] [PubMed] [Google Scholar]
- 36.University of Minnesota . Nutrition Data System For Research. University of Minnesota; 2009. [Google Scholar]
- 37.Department of City Planning . NYC Planning Open Data. New York City: New York City Department of City Planning; 2011. [Google Scholar]
- 38.US Department of Agriculture. USDA National Directory of Farmers' Markets. USDA. 2012 [Google Scholar]
- 39.Zenk SN, Schulz AJ, Izumi BT, Mentz G, Israel BA, Lockett M. Neighborhood food environment role in modifying psychosocial stress-diet relationships. Appetite. 2013;65:170–7. doi: 10.1016/j.appet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs ESH. Kimberly Bayer and Alexandra Feathers. Innovative Partnership for Public Health: An Evaluation of the New York City Green Cart Initiative to Expand Access to Healthy Produce in Low-Income Neighborhoods. Columbia University School of International and Public Affairs Case Study Series in Global Public Policy; 2014. [Google Scholar]
- 41.Claro J. Vermont Farmers' Markets and Grocery Stores: A Price Comparison. Northest Organic Farming Association of Vermont. 2011 [Google Scholar]
- 42.Liese AD, Barnes TL, Lamichhane AP, Hibbert JD, Colabianchi N, Lawson AB. Characterizing the food retail environment: impact of count, type, and geospatial error in 2 secondary data sources. J Nutr Educ Behav. 2013;45:435–42. doi: 10.1016/j.jneb.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liese AD, Colabianchi N, Lamichhane AP, Barnes TL, Hibbert JD, Porter DE, et al. Validation of 3 food outlet databases: completeness and geospatial accuracy in rural and urban food environments. Am J Epidemiol. 2010;172:1324–33. doi: 10.1093/aje/kwq292. [DOI] [PMC free article] [PubMed] [Google Scholar]