Summary
Extracellular electron transfer (EET) is a microbial metabolism that enables efficient electron transfer between microbial cells and extracellular solid materials. Microorganisms harbouring EET abilities have received considerable attention for their various biotechnological applications, including bioleaching and bioelectrochemical systems. On the other hand, recent research revealed that microbial EET potentially induces corrosion of iron structures. It has been well known that corrosion of iron occurring under anoxic conditions is mostly caused by microbial activities, which is termed as microbiologically influenced corrosion (MIC). Among diverse MIC mechanisms, microbial EET activity that enhances corrosion via direct uptake of electrons from metallic iron, specifically termed as electrical MIC (EMIC), has been regarded as one of the major causative factors. The EMIC‐inducing microorganisms initially identified were certain sulfate‐reducing bacteria and methanogenic archaea isolated from marine environments. Subsequently, abilities to induce EMIC were also demonstrated in diverse anaerobic microorganisms in freshwater environments and oil fields, including acetogenic bacteria and nitrate‐reducing bacteria. Abilities of EET and EMIC are now regarded as microbial traits more widespread among diverse microbial clades than was thought previously. In this review, basic understandings of microbial EET and recent progresses in the EMIC research are introduced.
Introduction
Acquisition of energy is an indispensable activity for all living organisms. Most organisms, including human beings, conserve energy through respiration, with organic compounds and oxygen gas as the electron donor and acceptor respectively. In contrast, many microorganisms have the ability to utilize diverse inorganic compounds as the substrates for respiration. Furthermore, some particular microorganisms have the ability to acquire energy through transferring electrons to or from extracellular solid compounds. This microbial metabolism is specifically termed as ‘extracellular electron transfer (EET)’ (Gralnick and Newman, 2007; Richter et al., 2012). In addition to naturally occurring metal minerals, microorganisms harbouring EET abilities can utilize artificial conductive materials, including graphite and metal electrodes, as the electron donor or acceptor (Bond and Lovley, 2003; Gregory et al., 2004). This microbial activity has received considerable attention for biotechnological applications, including bioremediation of toxic metals and diverse bioelectrochemical systems (Arends and Verstraete, 2012; Logan and Rabaey, 2012; Kato, 2015). Besides, recent studies have disclosed that some microorganisms utilize zero‐valent metallic iron as their electron donor via EET metabolisms, which stimulates iron corrosion under anoxic conditions. This review introduces the basic knowledge in microbial EET metabolisms and the recent research progresses on the relevance of microbial EET to iron corrosion.
Microbial EET
As for commonly characterized energy metabolisms, microorganisms first must incorporate substrates for respiration into their cells, where oxidation and reduction of substrates proceed. In contrast, special molecular mechanisms are required for EET‐based energy metabolisms, since microorganisms cannot incorporate solid materials into their cells. Mechanisms of microbial EET can be categorized into either direct or indirect manners. In the direct EET, microorganisms attach to solid surfaces, to or from which they directly transfer electrons (Fig. 1A). The molecular mechanisms for the direct EET have been intensively investigated for some iron‐reducing and iron‐oxidizing bacteria, including Geobacter sulfurreducens, Shewanella oneidensis and Acidithiobacillus ferrooxidans (Stams et al., 2006; Weber et al., 2006; Shi et al., 2007; Castelle et al., 2008). These microorganisms utilize metal‐containing redox proteins, such as c‐type cytochromes and rusticyanin, to electrically connect intracellular respiratory chains and extracellular solid materials. Furthermore, G. sulfurreducens and S. oneidensis were reported to produce conductive filamentous apparatus (pili and outer membrane extensions, respectively) that are specifically termed as ‘nanowires’ (Reguera et al., 2005; Gorby et al., 2006; Pirbadian et al., 2014). These microorganisms have the ability to transfer electrons to or from distantly located solid materials using the filaments as ‘electric wire’ (Fig. 1B).
Figure 1.
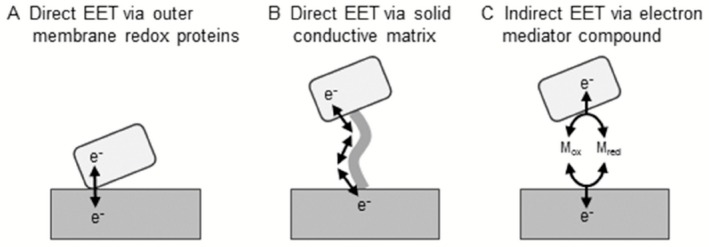
The schematic images of three microbial EET mechanisms.
A. Direct EET via outer membrane redox proteins.
B. Direct EET via solid conductive matrix (e.g. conductive pili).
C. Indirect EET via an electron mediator compound (M red/M ox).
In contrast, some microorganisms indirectly transfer electrons to or from solid compounds using diffusible redox chemicals, referred to as electron mediators (Watanabe et al., 2009). Microorganisms reduce (or oxidize) intracellular electron mediators, after which the reduced (or oxidized) electron mediators diffuse out of the cell to solid surfaces and donate (or accept) electrons, and then the oxidized (or reduced) mediators return back into the cells and are again utilized as respiratory substrates (Fig. 1C). Some microorganisms have the ability to synthesize low‐molecular‐weight organic compounds that work as electron mediators, including phenazine compounds and flavin derivatives (Rabaey et al., 2004; Marsili et al., 2008). Naturally occurring (e.g. humic substances) and artificial (e.g. quinone derivatives, ferrocene derivatives) redox chemicals can also work as electron mediators to propel microbial EET (Jiang and Kappler, 2008; Watanabe et al., 2009; Nishio et al., 2013). The reader is referred to several excellent review articles on the mechanisms of direct and indirect EET (Hernandez and Newman, 2001; Shi et al., 2007; Lovley, 2008; Thrash and Coates, 2008; Watanabe et al., 2009; Richter et al., 2012).
Microorganisms and corrosion of iron
Corrosion of iron is an electrochemical process consisting of oxidation of metallic iron to ferrous ion (anodic reaction, Eq. (1)) and reduction of electron acceptor compounds (cathodic reaction):
| (1) |
where −0.47 V references the standard hydrogen electrode (the same applies hereafter). In oxic environments, the cathodic reaction is reduction of oxygen (Eq. (2)) and corrosion rate is relatively high (Fig. 2A):
| (2) |
In contrast, the most common electron consuming reaction under anoxic condition is proton reduction to hydrogen gas (Eq. (3)) (Fig. 2B):
| (3) |
Theoretically, iron corrosion in anoxic environments does not become a serious problem, since the proton reduction reaction on iron surfaces is usually a particularly slow reaction. However, iron corrosion under anoxic conditions has often been reported, and in most cases it is thought to be mediated by microbial metabolic activities (Lee et al., 1995; Hamilton, 2003; Beech and Sunner, 2004; Videla and Herrera, 2005).
Figure 2.
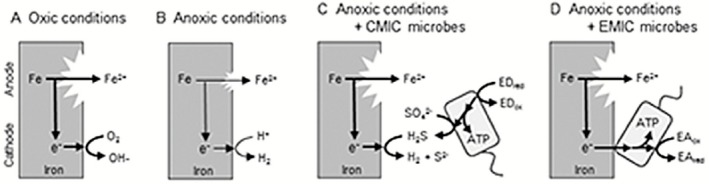
The schematic images of the iron corrosion mechanisms. Spontaneous, oxidative dissolution of metallic iron into ferrous iron (the anodic reaction) is the first step of corrosion in all of the cases.
A. Under oxic conditions, electrons derived from iron oxidation are consumed by reduction of O2 (the cathodic reaction).
B. Under anoxic conditions, the possible cathodic reaction is the reduction of H + into H 2, while the corrosion rate is quite low.
C. CMIC‐inducing SRB reduce sulfate into corrosive H2S, which stimulate the cathodic reaction and hence iron corrosion. In this case, SRB requires exogenous electron donors (ED, such as lactate and H 2) to reduce sulfate and acquire energy.
D. EMIC‐inducing microorganisms directly utilize electrons in metallic iron as the electron donor to stimulate iron corrosion. Sulfate reduction, methanogenesis, acetogenesis and nitrate reduction were reported as the electron‐accepting reactions (EAred/EAox).
Corrosion of iron caused by microbial metabolism is specifically termed as microbiologically influenced corrosion (MIC) or biocorrosion. Microbiologically influenced corrosion under anaerobic conditions often damages energy infrastructures, including crude oil reservoir tanks and underground oil and gas pipelines, causing enormous economic losses. The microorganisms first identified as MIC‐inducers were sulfate‐reducing bacteria (SRB) (Gaines, 1910). Sulfate‐reducing bacteria produce hydrogen sulfide through reduction of sulfate (Eq. (4)) with various organic compounds and often H2 as the source of reducing equivalents:
| (4) |
Hydrogen sulfide is a highly oxidative chemical and is known to react with metallic iron to generate iron sulfide (Eq. (5)):
| (5) |
This process enhances corrosion of iron (Fig. 2C). Similarly, other microorganisms that secrete corrosive chemicals such as organic acids also stimulate corrosion of iron (Little et al., 2001; Usher et al., 2014). This manner of MIC, i.e. stimulation of the cathodic reaction by microbial metabolic end‐products, is referred to as chemical MIC (CMIC; Enning et al., 2012).
In contrast, acceleration of cathodic reactions via consumption of cathodic electrons, often referred to as cathodic depolarization, had been believed as one of the most prominent causes of MIC (Wolzogen Kühr, 1961). Microorganisms consuming H2 abiotically generated on iron surfaces (Eq. (3)) coupled with sulfate reduction (Eq. (4)) or methanogenesis (Eq. (6)) were considered to induce cathodic depolarization (King and Miller, 1971; Daniels et al., 1987):
| (6) |
However, stimulation of anaerobic corrosion via H2 consumption has been viewed critically (Hardy, 1983), and recent studies revealed that H2 consumption itself is not sufficient to induce fatal iron corrosion as often observed in actual fields (Dinh et al., 2004; Mori et al., 2010). These studies implied that only microorganisms with EET ability, which enables efficient uptake of electrons from metallic iron, can stimulate the cathodic reaction and hence iron corrosion (Fig. 2D). This manner of MIC, i.e. stimulation of the cathodic reaction by consuming cathodic electrons as the metabolic energy sources, is referred to as electrical MIC (EMIC) (Enning et al., 2012). In the following sections, specific examples of microorganisms causing EMIC are introduced and their putative EET mechanisms are discussed.
Microorganisms inducing EMIC
So far microorganisms in four different metabolic groups (SRB, methanogenic archaea, acetogenic bacteria and nitrate‐reducing bacteria) are elucidated as EMIC‐inducers (Table 1). In this section, specific examples of the EMIC‐inducing microorganisms are introduced.
Table 1.
Isolated strains identified as EMIC‐inducing microorganisms
|
Metabolic group Strain name |
Isolation sources | Phylogeny | References | |
|---|---|---|---|---|
| Phylum/class | Family | |||
| Sulfate‐reducing bacteria | ||||
| ‘Desulfopila corrodens’ IS4 | Marine sediment | Deltaproteobacteria | Desulfobulbaceae | Dinh and colleagues (2004) |
| ‘Desulfovibrio ferrophilus’ IS5 | Marine sediment | Deltaproteobacteria | Desulfovibrionaceae | Dinh and colleagues (2004) |
| Methanogenic archaea | ||||
| Methanobacterium sp. IM1 | Marine sediment | Euryarchaeota | Methanobacteriaceae | Dinh and colleagues (2004) |
| Methanococcus maripaludis KA1 | Crude‐oil storage tank | Euryarchaeota | Methanococcaceae | Uchiyama and colleagues (2010) |
| Methanococcus maripaludis Mic1c10 | Crude‐oil storage tank | Euryarchaeota | Methanococcaceae | Mori and colleagues (2010) |
| Acetogenic bacteria | ||||
| Sporomusa sp. GT1 | Rice paddy field | Firmicutes | Veillonellaceae | Kato and colleagues (2015) |
| Nitrate‐reducing bacteria | ||||
| Bacillus licheniformis ATCC 14580 | Soil | Firmicutes | Bacillaceae | Xu and colleagues (2013) |
| Prolixibacter sp. MIC1‐1 | Crude‐oil well | Bacteroidetes | Prolixibacteraceae | Iino and colleagues (2015) |
SRB
As introduced in the previous section, SRB is known as one of the most prominent bacteria to cause anaerobic MIC, mainly via production of corrosive chemical H2S. In addition to the indirect mechanisms, recent research disclosed that some SRB strains stimulate corrosion via more direct manners. Dinh and colleagues (2004) reported enrichment and isolation of two novel SRB strains from marine sediment using metallic iron as the sole electron donor. These isolated strains, subsequently denominated as ‘Desulfopila corrodens’ strain IS4 and ‘Desulfovibrio ferrophilus’ strain IS5 (Enning et al., 2012), reduced sulfate with concomitant oxidation of metallic iron much faster than abiotic H2 generation in an organic matter‐free medium. In contrast, the closely related SRB with efficient H2‐utilizing abilities did not show such activities (Dinh et al., 2004; Gittel et al., 2010). So far, EMIC‐inducing abilities in SRB have been identified in only a limited number of strains in the deltaproteobacterial families Desulfovibrionaceae and Desulfobulbaceae (Enning and Garrelfs, 2014). These reports suggest that the EMIC‐inducing SRBs have special molecular mechanisms for electron uptake from metallic iron, and the ability is a species and/or strains‐specific trait rather than universal in SRB. Under sulfate‐reducing conditions, black mineral crust mainly consisting of iron sulfide minerals is generally formed as corrosion products (Enning et al., 2012) (Fig. 3).
Figure 3.
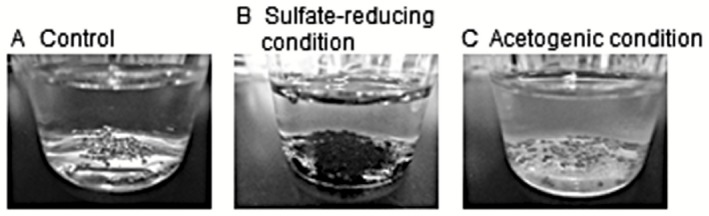
Corrosion products formed by EMIC‐inducing microorganisms.
A. Non‐inoculated control.
B. An iron‐corroding enrichment culture under sulfate‐reducing conditions.
C. An iron‐corroding enrichment culture under acetogenic conditions.
Methanogenic archaea
Methane production by methanogenic archaea (Eq. (6)) is a major microbial metabolism in anoxic environments, especially in sulfate‐depleted zones. Participation of microbial methanogenesis to cathodic depolarization and anaerobic MIC was proposed in the 1980s (Daniels et al., 1987). The first direct evidence for an EMIC‐inducing methanogen was reported by Dinh and colleagues (2004). A new methanogenic strain, Methanobacterium sp. strain IM1, isolated from marine sediment, was shown to grow and produce methane with metallic iron as the sole electron source, and stimulate corrosion of iron (Dinh et al., 2004). Another research group in Japan also isolated EMIC‐inducing methanogenic strains, namely Methanococcus maripaludis strain KA1 and strain Mic1c10, from crude oil reservoir tanks (Mori et al., 2010; Uchiyama et al., 2010). Interestingly, authentic hydrogenotrophic methanogens closely related to these EMIC‐inducing methanogens did not grow well on and produce methane from metallic iron, indicating that the corrosive ability is a limited trait for certain methanogenic archaea.
Acetogenic bacteria
Acetogenic bacteria have the abilities to conserve energy via the reductive acetyl‐CoA pathway, by which acetate is produced through reduction of carbon dioxide (Eq. (7)):
| (7) |
Acetogenic bacteria generally utilize hydrogen gas or certain organic compounds as the source of reducing equivalents. While engagement of microbial acetogenesis for anaerobic MIC in low‐sulfate environments was postulated (Suflita et al., 2008), the direct evidence had not been demonstrated. Recently, Mand and colleagues (2014) demonstrated acetate production in microbial enrichment cultures with metallic iron as the sole electron source, from which bacteria closely related to known acetogenic bacterium (Acetobacterium sp.) were detected. Our research group also enriched acetogenic microbial communities with metallic iron as the sole energy source (Kato et al., 2015). The enriched communities produced acetate coupled with oxidation of metallic iron and produced significantly larger amount of ferrous iron than the abiotic controls. We eventually isolated Sporomusa sp. strain GT1 from the enrichments and demonstrated that the isolate acetogenetically grew on metallic iron and enhanced iron corrosion, while other well‐known acetogenic bacteria did not. In addition to the direct effects, the metabolism of acetogenic bacteria should cause another peripheral effects on iron corrosion. Most SRB cannot grow autotrophically and require organic compounds such as acetate as the carbon source. Hence, it is postulated that acetate generated by acetogenic bacteria indirectly enhances iron corrosion via stimulation of growth of CMIC‐ and/or EMIC‐inducing SRB (Mand et al., 2014).
Nitrate‐reducing bacteria (NRB)
Reduction of nitrate (Eq. (8)) is also a major microbial metabolism under anoxic conditions:
| (8) |
Recent studies revealed that particular NRB also causes EMIC. For instance, Xu and colleagues (2013) demonstrated that Bacillus licheniformis ATCC 14580, a facultative NRB strain, stimulates corrosion of carbon steel under nitrate‐reducing conditions. Iino and colleagues (2015) isolated another EMIC‐causing NRB, Prolixibacter sp. strain MIC1‐1, which is the first corrosive representative belonging to the phylum Bacteroidetes. In addition to the ability of Prolixibacter sp. strain MIC1‐1 to utilize cathodic electrons in metallic iron as the electron donor, the metabolic end‐product, nitrite, which is known as a strong corrosive compound (Lin et al., 2008), appears to induce severe corrosion (Iino et al., 2015). Nitrate injection into oil and gas reservoirs has been used for mitigation of souring and MIC caused by SRB, since it promotes growth of NRB and in turn suppresses growth of SRB (Gieg et al., 2011). However, the discovery of MIC‐inducing NRB led to the revelation that supplementation of nitrate may risk inducing NRB‐assisted corrosion. In contrast to sulfate‐reducing conditions, greyish mineral crust mainly containing iron carbonate and iron phosphate is generally formed as corrosion products under methanogenic, acetogenic and nitrate‐reducing conditions (Uchiyama et al., 2010; Iino et al., 2015) (Fig. 3).
How do corrosive microorganisms uptake electrons from metallic iron?
The EMIC‐inducing microorganisms should have either direct or indirect EET abilities to efficiently uptake electrons from metallic iron. Physiological and electrochemical analyses have disclosed that the corrosive SRB strains (Desulfopila corrodens strain IS4 and Desulfovibrio ferrophilus strain IS5) appear to directly uptake electrons from metallic iron (Enning et al., 2012; Venzlaff et al., 2013). Production of the mineral crust (containing FeS, FeCO3 and Mg/CaCO3) with semiconductor‐like properties by the EMIC‐inducing SRB also supports this assumption. Deng and colleagues (2015) electrochemically demonstrated that the corrosive SRB Desulfovibrio ferrophilus strain IS5 is capable of extracting electrons from an inert electrode without consuming H2 as an electron carrier. Similarly, Beese‐Vasbender and colleagues (2015) reported that the corrosive methanogen Methanobacterium sp. strain IM1 generates cathodic current and produces methane at an electrode potential of −0.4 V, which is much more positive than the potential required for H2 production on a graphite cathode. Furthermore, Iino and colleagues (2015) confirmed that the Prolixibacter sp. strain MIC1‐1 does not have ability to oxidize H2, while this strain showed high EMIC activities coupled with nitrate reduction. These studies indicate that at least a part of EMIC‐causing microorganisms have the abilities for direct EET, while the molecular mechanisms remain unknown.
There are some reports on the relevance of indirect EET to EMIC. In the indirect EET, electron mediator molecules are required to achieve electron transfer from metallic iron to microbial cells. Zhang and colleagues (2015) demonstrated that exogenous addition of electron mediators [riboflavin and flavin adenine dinucleotide (FAD)] accelerates corrosion of stainless steel by Desulfovibrio vulgaris. Some EET‐harbouring microorganisms such as Shewanella spp. were reported to produce and excrete riboflavin and FAD to facilitate their EET activities (Marsili et al., 2008; Brutinel and Gralnick, 2012). These findings suggest that electron mediators secreted by microorganisms themselves facilitate EMIC, although there has been no direct evidence yet. Naturally occurring inorganic chemicals are another candidate of electron mediators facilitating EMIC. Manganese‐oxidizing bacteria were reported to conduct this type of corrosion (Dickinson et al., 1997; Ashassi‐Sorkhabi et al., 2012). In this case, oxidized forms of manganese [Mn(III/IV)] consume cathodic electrons in metallic iron and is reduced to Mn(II), which is oxidized back to Mn(III/IV) by manganese‐oxidizing bacteria. Iodide‐oxidizing bacteria isolated from brine in an iodide production facility stimulate iron corrosion in a similar manner with redox coupling of I−/I2 (Wakai et al., 2014).
Hydrogen gas is also a possible candidate of an electron mediator molecule. As discussed above, however, a number of studies showed that microorganisms that simply consume H2 do not induce significant corrosion of iron. Recently, Deutzmann and colleagues (2015) demonstrated a novel mechanism for electron uptake from metallic iron with hydrogen gas (and possibly formate) as an important intermediate. They discovered that cell‐free spent culture medium of a corrosive methanogen Methanococcus maripaludis strain MM901 accelerated H2 (and formate) generation from metallic iron, while that of a hydrogenase‐deficient mutant did not. This study suggests that surface‐associated redox enzymes, such as hydrogenases and formate dehydrogenase, are sufficient to mediate an apparent electron uptake that enhances iron corrosion.
The EET mechanisms for EMIC remains controversial, and it may be highly possible that each EMIC‐inducing microorganism utilizes completely different EET strategies. Also in the research field of bioelectrochemistry, the molecular mechanisms of microbial electron uptake from cathodes are much more enigmatic rather than the opposite reaction, i.e. microbial electron injection to anodes (Rosenbaum et al., 2011). Further research with multiple viewpoints, including microbial physiology, genomics, molecular biology and electrochemistry, is required to understand how EMIC‐inducers efficiently uptake electrons from metallic iron.
Concluding remarks
The studies on MIC in the last decades have demonstrated that physiologically and phylogenetically diverse microorganisms, including SRB (Proteobacteria), methanogens (Euryarchaeota), acetogens (Firmicutes) and NRB (Firmicutes and Bacteroidetes), stimulate iron corrosion via their EET metabolisms. Further investigation on these microorganisms, especially on their EET mechanisms, and also identification of novel EMIC‐causing microorganisms will lead to technological development for MIC mitigation. In addition, EMIC‐causing microorganisms can potentially be utilized as novel biocatalysts. Microbial electron uptake from solid materials is being pursued as biocatalysts for cathode reactions in diverse microbial electrochemical systems (Rabaey and Rozendal, 2010; Erable et al., 2012; Kim et al., 2015). Based on this idea, cathodic methane production by a corrosive methanogen has already been examined (Beese‐Vasbender et al., 2015). Close relatives of a corrosive acetogen are also being investigated as biocatalysts for conversion on electrical energy into liquid fuels (Nevin et al., 2011).
Considering that almost all metallic iron has been recently introduced into environments by human activities, EMIC reaction, namely utilization of metallic iron as an electron donor of respiration, is an enigmatic metabolism from the viewpoint of evolution. One plausible explanation is that EMIC reaction is due to the promiscuous usage of other analogous metabolisms. Our group recently demonstrated that electrically conductive metal minerals, which abundantly exist in natural environments (Hochella et al., 2008; Nakamura et al., 2010a), serve as an electron source and sink for some EET‐harbouring microorganisms (Nakamura et al., 2009; 2010b; Kato et al., 2010, 2013). Furthermore, a novel microbial symbiotic metabolism, in which electrons released by one microorganism are transferred to another via electric current flowing through biological and mineralogical solid materials, has recently been demonstrated (Summers et al., 2010; Kato et al., 2012a). Diverse kinds of microorganisms, including NRB, ferric iron reducers, methanogens and dehalorespirators, have been reported to participate in the electron‐consuming part of the symbiotic process (Summers et al., 2010; Kato et al., 2012a,b; Aulenta et al., 2013). Further studies on EMIC will also give novel insights for ecological and evolutional aspects of microbial EET.
Conflict of Interest
None declared.
Acknowledgement
I thank Dr. Mia Terashima for critical reading of the manuscript.
Funding Information No funding information provided.
References
- Arends, J.B. , and Verstraete, W. (2012) 100 years of microbial electricity production: three concepts for the future. Microb Biotechnol 5: 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashassi‐Sorkhabi, H. , Moradi‐Haghighi, M. , and Zarrini, G. (2012) The effect of Pseudoxanthomonas sp. as manganese oxidizing bacterium on the corrosion behavior of carbon steel. Mater Sci Eng C 32: 303–309. [Google Scholar]
- Aulenta, F. , Rossetti, S. , Amalfitano, S. , Majone, M. , and Tandoi, V. (2013) Conductive magnetite nanoparticles accelerate the microbial reductive dechlorination of trichloroethene by promoting interspecies electron transfer processes. ChemSusChem 6: 433–436. [DOI] [PubMed] [Google Scholar]
- Beech, I.B. , and Sunner, J. (2004) Biocorrosion: towards understanding interactions between biofilms and metals. Curr Opin Biotechnol 15: 181–186. [DOI] [PubMed] [Google Scholar]
- Beese‐Vasbender, P.F. , Grote, J.P. , Garrelfs, J. , Stratmann, M. , and Mayrhofer, K.J. (2015) Selective microbial electrosynthesis of methane by a pure culture of a marine lithoautotrophic archaeon. Bioelectrochemistry 102: 50–55. [DOI] [PubMed] [Google Scholar]
- Bond, D.R. , and Lovley, D.R. (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69: 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutinel, E.D. , and Gralnick, J.A. (2012) Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl Microbiol Biotechnol 93: 41–48. [DOI] [PubMed] [Google Scholar]
- Castelle, C. , Guiral, M. , Malarte, G. , Ledgham, F. , Leroy, G. , Brugna, M. , and Giudici‐Orticoni, M.T. (2008) A new iron‐oxidizing/O2‐reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile Acidithiobacillus ferrooxidans . J Biol Chem 283: 25803–25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, L. , Belay, N. , Rajagopal, B.S. , and Weimer, P.J. (1987) Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 237: 509–511. [DOI] [PubMed] [Google Scholar]
- Deng, X. , Nakamura, R. , Hashimoto, K. , and Okamoto, A. (2015) Electron extraction from an extracellular electrode by Desulfovibrio ferrophilus strain IS5 without using hydrogen as an electron carrier. Electrochemistry 83: 529–531. [Google Scholar]
- Deutzmann, J.S. , Sahin, M. , and Spormann, A.M. (2015) Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio 6: e00496–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, W.H. , Caccavo, F. , Olesen, B. , and Lewandowski, Z. (1997) Ennoblement of stainless steel by the manganese‐depositing bacterium Leptothrix discophora . Appl Environ Microbiol 63: 2502–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh, H.T. , Kuever, J. , Mussmann, M. , Hassel, A.W. , Stratmann, M. , and Widdel, F. (2004) Iron corrosion by novel anaerobic microorganisms. Nature 427: 829–832. [DOI] [PubMed] [Google Scholar]
- Enning, D. , and Garrelfs, J. (2014) Corrosion of iron by sulfate‐reducing bacteria: new views of an old problem. Appl Environ Microbiol 80: 1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enning, D. , Venzlaff, H. , Garrelfs, J. , Dinh, H.T. , Meyer, V. , Mayrhofer, K. , et al (2012) Marine sulfate‐reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ Microbiol 14: 1772–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erable, B. , Féron, D. , and Bergel, A. (2012) Microbial catalysis of the oxygen reduction reaction for microbial fuel cells: a review. ChemSusChem 5: 975–987. [DOI] [PubMed] [Google Scholar]
- Gaines, R. (1910) Bacterial activity as a corrosive influence in the soil. J Ind Eng Chem 2: 128–130. [Google Scholar]
- Gieg, L.M. , Jack, T.R. , and Foght, J.M. (2011) Biological souring and mitigation in oil reservoirs. Appl Microbiol Biotechnol 92: 263–282. [DOI] [PubMed] [Google Scholar]
- Gittel, A. , Seidel, M. , Kuever, J. , Galushko, A.S. , Cypionka, H. , and Könneke, M. (2010) Desulfopila inferna sp. nov., a sulfate‐reducing bacterium isolated from the subsurface of a tidal sand‐flat. Int J Syst Evol Microbiol 60: 1626–1630. [DOI] [PubMed] [Google Scholar]
- Gorby, Y.A. , Yanina, S. , McLean, J.S. , Rosso, K.M. , Moyles, D. , Dohnalkova, A. , et al (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR‐1 and other microorganisms. Proc Natl Acad Sci USA 103: 11358–11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick, J.A. , and Newman, D.K. (2007) Extracellular respiration. Mol Microbiol 65: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, K.B. , Bond, D.R. , and Lovley, D.R. (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6: 596–604. [DOI] [PubMed] [Google Scholar]
- Hamilton, W.A. (2003) Microbially influenced corrosion as a model system for the study of metal microbe interactions: A unifying electron transfer hypothesis. Biofouling 19: 65–76. [DOI] [PubMed] [Google Scholar]
- Hardy, J.A. (1983) Utilisation of cathodic hydrogen by sulphate‐reducing bacteria. Br Corros J 18: 190–193. [Google Scholar]
- Hernandez, M.E. , and Newman, D.K. (2001) Extracellular electron transfer. Cell Mol Life Sci 58: 1562–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochella, M.F., Jr , Lower, S.K. , Maurice, P.A. , Penn, R.L. , Sahai, N. , Sparks, D.L. , and Twining, B.S. (2008) Nanominerals, mineral nanoparticles, and Earth systems. Science 319: 1631–1635. [DOI] [PubMed] [Google Scholar]
- Iino, T. , Ito, K. , Wakai, S. , Tsurumaru, H. , Ohkuma, M. , and Harayama, S. (2015) Iron corrosion induced by nonhydrogenotrophic nitrate‐reducing Prolixibacter sp. strain MIC1‐1. Appl Environ Microbiol 81: 1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , and Kappler, A. (2008) Kinetics of microbial and chemical reduction of humic substances: implications for electron shuttling. Environ Sci Technol 42: 3563–3569. [DOI] [PubMed] [Google Scholar]
- Kato, S. (2015) Biotechnological aspects of microbial extracellular electron transfer. Microbes Environ 30: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, S. , Kai, F. , Nakamura, R. , Watanabe, K. , and Hashimoto, K. (2010) Respiratory interactions of soil bacteria with (semi)conductive iron‐oxide minerals. Environ Microbiol 12: 3114–3123. [DOI] [PubMed] [Google Scholar]
- Kato, S. , Hashimoto, K. , and Watanabe, K. (2012a) Microbial interspecies electron transfer via electric currents through conductive minerals. Proc Natl Acad Sci USA 109: 10042–10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, S. , Hashimoto, K. , and Watanabe, K. (2012b) Methanogenesis facilitated by electric syntrophy via (semi)conductive iron‐oxide minerals. Environ Microbiol 14: 1646–1654. [DOI] [PubMed] [Google Scholar]
- Kato, S. , Hashimoto, K. , and Watanabe, K. (2013) Iron‐oxide minerals affect extracellular electron‐transfer paths of Geobacter spp. Microbes Environ 28: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, S. , Yumoto, I. , and Kamagata, Y. (2015) Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl Environ Microbiol 81: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B.H. , Lim, S.S. , Daud, W.R. , Gadd, G.M. , and Chang, I.S. (2015) The biocathode of microbial electrochemical systems and microbially‐influenced corrosion. Bioresour Technol 190: 390–401. [DOI] [PubMed] [Google Scholar]
- King, R.A. , and Miller, J.D.A. (1971) Corrosion by the sulphate‐reducing bacteria. Nature 233: 491–492. [DOI] [PubMed] [Google Scholar]
- Lee, W. , Lewandowski, Z. , Nielsen, P.H. , and Hamilton, W.A. (1995) Role of sulfate‐reducing bacteria in corrosion of mild steel: A review. Biofouling 8: 165–194. [Google Scholar]
- Lin, K.S. , Chang, N.B. , and Chuang, T.D. (2008) Fine structure characterization of zero‐valent iron nanoparticles for decontamination of nitrites and nitrates in wastewater and groundwater. Sci Technol Adv Mater 9: 025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, B. , Staehle, R. , and Davis, R. (2001) Fungal influenced corrosion of post‐tensioned cables. Int Biodeterior Biodegrad 47: 71–77. [Google Scholar]
- Logan, B.E. , and Rabaey, K. (2012) Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337: 686–690. [DOI] [PubMed] [Google Scholar]
- Lovley, D.R. (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol 19: 564–571. [DOI] [PubMed] [Google Scholar]
- Mand, J. , Park, H.S. , Jack, T.R. , and Voordouw, G. (2014) The role of acetogens in microbially influenced corrosion of steel. Front Microbiol 5: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsili, E. , Baron, D.B. , Shikhare, I.D. , Coursolle, D. , Gralnick, J.A. , and Bond, D.R. (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA 105: 3968–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, K. , Tsurumaru, H. , and Harayama, S. (2010) Iron corrosion activity of anaerobic hydrogen‐consuming microorganisms isolated from oil facilities. J Biosci Bioeng 110: 426–430. [DOI] [PubMed] [Google Scholar]
- Nakamura, R. , Kai, F. , Okamoto, A. , Newton, G.J. , and Hashimoto, K. (2009) Self‐constructed electrically conductive bacterial networks. Angew Chem Int Ed 48: 508–511. [DOI] [PubMed] [Google Scholar]
- Nakamura, R. , Takashima, T. , Kato, S. , Takai, K. , Yamamoto, M. , and Hashimoto, K. (2010a) Electrical current generation across a black smoker chimney. Angew Chem Int Ed 49: 7692–7694. [DOI] [PubMed] [Google Scholar]
- Nakamura, R. , Okamoto, A. , Tajima, N. , Newton, G.J. , Kai, F. , Takashima, T. , and Hashimoto, K. (2010b) Biological iron‐monosulfide production for efficient electricity harvesting from a deep‐sea metal‐reducing bacterium. Chembiochem 11: 643–645. [DOI] [PubMed] [Google Scholar]
- Nevin, K.P. , Hensley, S.A. , Franks, A.E. , Summers, Z.M. , Ou, J. , Woodard, T.L. , et al (2011) Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microbiol 77: 2882–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio, K. , Nakamura, R. , Lin, X. , Konno, T. , Ishihara, K. , Nakanishi, S. , and Hashimoto, K. (2013) Extracellular electron transfer across bacterial cell membranes via a cytocompatible redox‐active polymer. Chemphyschem 14: 2159–2163. [DOI] [PubMed] [Google Scholar]
- Pirbadian, S. , Barchinger, S.E. , Leung, K.M. , Byun, H.S. , Jangir, Y. , Bouhenni, R.A. , et al (2014) Shewanella oneidensis MR‐1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc Natl Acad Sci USA 111: 12883–12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaey, K. , and Rozendal, R.A. (2010) Microbial electrosynthesis – revisiting the electrical route for microbial production. Nat Rev Microbiol 8: 706–716. [DOI] [PubMed] [Google Scholar]
- Rabaey, K. , Boon, N. , Siciliano, S.D. , Verhaege, M. , and Verstraete, W. (2004) Biofuel cells select for microbial consortia that self‐mediate electron transfer. Appl Environ Microbiol 70: 5373–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera, G. , McCarthy, K.D. , Mehta, T. , Nicoll, J.S. , Tuominen, M.T. , and Lovley, D.R. (2005) Extracellular electron transfer via microbial nanowires. Nature 435: 1098–1101. [DOI] [PubMed] [Google Scholar]
- Richter, K. , Schicklberger, M. , and Gescher, J. (2012) Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl Environ Microbiol 78: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, M. , Aulenta, F. , Villano, M. , and Angenent, L.T. (2011) Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour Technol 102: 324–333. [DOI] [PubMed] [Google Scholar]
- Shi, L. , Squier, T.C. , Zachara, J.M. , and Fredrickson, J.K. (2007) Respiration of metal (hydr)oxides by Shewanella and Geobacter: A key role for multihaem c‐type cytochromes. Mol Microbiol 65: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stams, A.J. , de Bok, F.A. , Plugge, C.M. , van Eekert, M.H. , Dolfing, J. , and Schraa, G. (2006) Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol 8: 371–382. [DOI] [PubMed] [Google Scholar]
- Suflita, J.M. , Phelps, T.J. , and Little, B. (2008) Carbon dioxide corrosion and acetate: a hypothesis on the influence of microorganisms. Corros Sci 64: 854–859. [Google Scholar]
- Summers, Z.M. , Fogarty, H.E. , Leang, C. , Franks, A.E. , Malvankar, N.S. , and Lovley, D.R. (2010) Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330: 1413–1415. [DOI] [PubMed] [Google Scholar]
- Thrash, J.C. , and Coates, J.D. (2008) Review: direct and indirect electrical stimulation of microbial metabolism. Environ Sci Technol 42: 3921–3931. [DOI] [PubMed] [Google Scholar]
- Uchiyama, T. , Ito, K. , Mori, K. , Tsurumaru, H. , and Harayama, S. (2010) Iron‐corroding methanogen isolated from a crude‐oil storage tank. Appl Environ Microbiol 76: 1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher, K.M. , Kaksonen, A.H. , and MacLeod, I.D. (2014) Marine rust tubercles harbour iron corroding archaea and sulphate reducing bacteria. Corros Sci 83: 189–197. [Google Scholar]
- Venzlaff, H. , Enning, D. , Srinivasan, J. , Mayrhofer, K. , Hassel, A.W. , Widdel, F. , and Stratmann, M. (2013) Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate‐reducing bacteria. Corros Sci 66: 88–96. [Google Scholar]
- Videla, H.A. , and Herrera, L.K. (2005) Microbiologically influenced corrosion: looking to the future. Int Microbiol 8: 169–180. [PubMed] [Google Scholar]
- Wakai, S. , Ito, K. , Iino, T. , Tomoe, Y. , Mori, K. , and Harayama, S. (2014) Corrosion of iron by iodide‐oxidizing bacteria isolated from brine in an iodine production facility. Microb Ecol 68: 519–527. [DOI] [PubMed] [Google Scholar]
- Watanabe, K. , Manefield, M. , Lee, M. , and Kouzuma, A. (2009) Electron shuttles in biotechnology. Curr Opin Biotechnol 20: 633–641. [DOI] [PubMed] [Google Scholar]
- Weber, K.A. , Achenbach, L.A. , and Coates, J.D. (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4: 752–764. [DOI] [PubMed] [Google Scholar]
- Wolzogen Kühr, C.A.H. (1961) Unity of anaerobic and aerobic iron corrosion process in the soil. Corrosion 17: 293–299. [Google Scholar]
- Xu, D. , Li, Y. , Song, F. , and Gu, T. (2013) Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis . Corros Sci 77: 385–390. [Google Scholar]
- Zhang, P. , Xu, D. , Li, Y. , Yang, K. , and Gu, T. (2015) Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 101: 14–21. [DOI] [PubMed] [Google Scholar]


