Abstract
Cell-based screening methods for nuclear receptor ligands that use transgenic plant cells expressing a single human NR may have advantages over other eukaryotic systems which express multiple NRs. For example, signal-to-noise ratio might be improved because ligands would be less likely to bind to other NRs and/or less likely to cause confounding functional changes in plant cells. As a first step toward this aim we have expressed in plants truncated human estrogen receptor (ER) constructs linked to reporters, or selective markers such as luciferase, green fluorescent protein (GFP) and hygromycin. A variety of ligands for the ER (including estradiol and known phytoestrogens) have then been tested for their ability to over-express the linked marker gene(s) which could be measured (luciferase activity), visualized under fluorescent microscopy (GFP activity), or selected on antibiotic-containing media (Hygromycin B). Our results show a close association between the effects of ER ligands in the transgenic plant roots and their effects on native ERs in mammalian cells. With the stable expression of an ERalpha-GFP ligand detection system in A. thaliana, the estradiol- mediated response in transgenic roots is inhibited by an ER partial agonist (tamoxifen) and an antagonist (fulvestrant) at concentrations relevant to their use in breast cancer. We conclude that it is possible to express human NRs in plants in a form that can report on exogenous or endogenous ER ligands and that these constructs have a pharmacology which is relevant to ligands for the native NRs in human cells.
Keywords: Estrogen receptor, genetic switch, ligand, hairy root, transgenic
Introduction
The current pharmaceutical industry approach to drug discovery is to generate large “libraries” of related synthetic compounds by combinatorial chemistry. These are then tested in ultra HTPS, usually based on interactions with specific proteins (Demain 2002). The evidence that this approach will generate valuable new drugs is not convincing (Raskin et al. 2002) and it may not be the best approach to identify new nuclear receptor ligands for cancer therapy. Plants, on the other hand, have been sources for a number of important anti-cancer agents (vinca alkaloids and taxoids being the examples par excellence). Many of these plant-based products appear to have evolved receptor interactions with a broader spectrum of molecular targets than synthetic drugs. This non-specificity is a potential advantage, particularly if the right spectrum of actions can be found for specific types of clinical use.
Nuclear receptors regulate a wide variety of signaling pathways through their activation by endogenous agonists. The clinical importance of nuclear receptors may be best demonstrated by the role of estrogen receptors (ER) in breast cancer, where the effective treatment currently used is tamoxifen, a “partial agonist/antagonist” to ER. Both subtypes of ER (ERalpha and ERbeta) have similar affinities for endogenous agonist 17β-estradiol, but differences exist for other ligands and phytoestrogens. The abundance and distribution of these receptors also vary in different tissue types of mammals which determine particular effect of a given ligand (Pearce and Jordan 2004).
The best-known source of dietary phytoestrogen is the isoflavone, genistein, in the legume soybean (Glycine max). Genistein has many different actions on ERs and ER-mediated pathways which may contribute to its effects in reducing hormone-responsive cancers, including breast and prostate cancer (Banerjee et al. 2008). However, there are many other dietary plant compounds that may have equal potential value, including the plant sterol, 8-prenylnaringenin, from hops, which appears to act as a partial agonist at ERalpha, and is capable of inducing apoptosis in MCF-7 breast cancer cells (Brunelli et al. 2007). Similarly, the phytoestrogenic sesquiterpene, ferutinin, from Ferula hermonis (Ikeda et al. 2002), and the simple stilbene, resveratrol, found in grapevine and many other plants, act as weak partial agonists at ERalpha and have been reported to inhibit growth of MCF-7 cells (Levenson et al. 2003; Maggiolini et al. 2005). Phytoestrogen guggelsterone was shown to be an antagonist at ERalpha using a yeast-based screen similar to the plant-based screen we used here (Xu et al. 2008). Interestingly guggelsterone has also been shown to be an antagonist at the farnesoid X receptor (Ahn et al. 2008) another example of multifunctionality in plant metabolites of direct relevance to anti-cancer properties.
Screening for new molecules with ER ligand activity from plants thus represents a promising approach. However, crude plant extracts are often not compatible with conventional mammalian cell-based screens. This is due in part to the presence of metabolites that are cytotoxic to mammalian cells in plant crude extracts, thus confounding assays for ER activity. This has prompted our group to develop plant based ER assays by expressing the human ER binding domain linked to a reporter construct in whole plants (Arabidopsis thaliana) as well as in transformed hairy root systems and evaluated its functionality based on the expression of the linked reporter/selectable markers. We found that the transgenic plants and hairy roots harboring ER/reporter or marker constructs are able to retain the pharmacological functions of estrogen receptor and can be used to detect phytoestrogens in the medicinal plant extracts.
Materials and methods
DNA constructs
For constructing ER-beta ligand detection system, the GAL4-VP16 fused fragment was PCR amplified from the clone kindly provided by Dr. Tavva (2006) with the gene-specific primers containing Xho I and EcoR V restriction sites in the forward (CTCGAGAGTTTAAACAATGGCAGAT) and reverse (GATATCAATTGGATCCCCACCGTACT) primers respectively. The GAL4-VP16 DNA fragment was initially cloned into pBlueScript (pBS) cloning vector to creat pBS-GV. For the ERbeta source, we used pRST7-ERbeta plasmid purchased from Addgene (plasmid#11356). The hormone binding domain (hbd) of estrogen receptor beta was PCR amplified using gene specific primers with added restriction sites EcoR V and Xba I in the forward (GCCAAGGATATCGGCGGCCACGCGCCC) and reverse (TCATCTAGACTGTGGGTTCTGGGAGCC) primers, respectively. The amplicon of ERbeta-hbd was then ligated in-frame into pBS-GV to create pBS-GVE. The complete in-frame fused cassette of GVE was excised out from pBS-GVE using Xho I/Xba I and cloned into pKYLX80 downstream to 35S promoter.
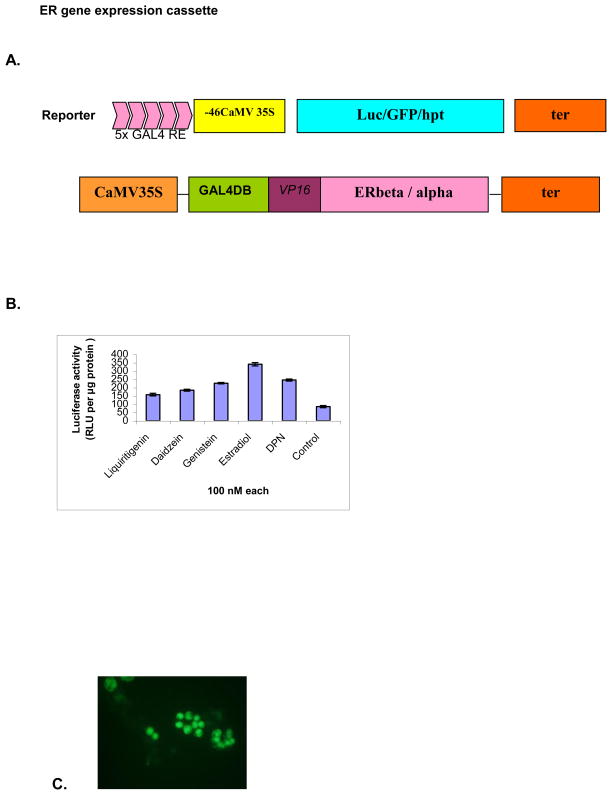
Similarly, the different parts of the reporter construct were sub-cloned initially into a separate pBS cloning vector. To make the reporter construct, the five copies of GAL4 RE (GAL4 responsive element) and minimal promoter (-46) from 35S promoter were PCR amplified from our previously used reporter system and subcloned to make pBS-GRE-46. The luciferase (luc) gene was amplified using gene specific primers with added Xho I/Sac I restriction sites and cloned into pBS-GRE-46 to make the final construct pBS-GRE-46-luc for transient functional analysis. The green fluorescent protein gene (GFP) was also PCR amplified and cloned into pBS-GRE-46 using same strategy. A schematic diagram is presented in figure 1A.
Figure 1.
Diagrammatic representation of ER ligand detection system and its transient expression: (A) The chimeric gene GAL4 DBD:VP16:ER is under the control of 35S constitutive promoter. This fusion protein transactivates the target reporter gene placed under the control of 5X GAL4 response elements and a minimal 35S promoter (-46 nucleotides upstream) in the presence of ligands.
(B) Comparison of luciferase induction levels with different ligands on ERbeta-lucifearse detection system. Tobacco protoplasts were electroporated with about 15 μg of purified plasmid DNA of pKYLX80-GVE and luciferase reporter constructs and exposed to different ligands @ 100 nM each concentration. Luciferase activity was measured after 24 h of incubation and the values were expressed as relative light units (RLU) per microgram protein ±SD.
Induction of GFP in tobacco protoplasts expressing ERbeta construct. After electroporation of the mixed plasmids of pKYLX80-GVE and GFP reporter construct, the protoplasts were treated with estradiol (100 nM) and incubated for 24 h.
Electroporation and transient induction of ERbeta construct in tobacco protoplasts About 15 μg of pKYLX80-GVE plasmid DNA and the reporter construct plasmid DNAs were isolated from the respective bacterial culture for functional studies in the tobacco protoplasts. The isolation of protoplasts from the cell suspension cultures of tobacco (Nicotiana tabacum cv. Xanthi-Brad) and their electroporation were performed using the protocol described by Tavva et al. (2006). Equal quantities of pKYLX80-GVE and a reporter construct were mixed in mannitol solution and delivered into tobacco protoplasts (2 × 106/ml) by electroporation. After co-electroporation, the protoplasts were washed twice and re-suspended into 1 ml of protoplast culture media. Various ligands were then added directly to the media. Similarly, the GFP reporter construct was also used for transient expression studies in tobacco protoplasts. Luciferase activity was measured in a luminometer plate reader (Fluoroscan Ascent FL, Thermo Labsystem, Milford, MA, USA) using a luciferase assay system (Promega Corporation, USA).
Development of a positive selection system for ERbeta ligands in tobacco hairy roots The full-length cDNA of Hygromycin (hpt) gene was PCR amplified from pCAMBIA binary vector 1301. The Xho I and Sac I restriction sites were added in the forward and reverse primers to facilitate their cloning at the place of luciferase gene into pBS-GRE-46 construct. Both GVE and GRE-46-hpt cassettes were then sub-cloned into a pKYLX71 binary vector for plant transformation. The final construct was named as pK71-GVEH (referred to hereafter as ERbeta-hpt). About 5 μg of ERbeta-hpt plasmid DNA was mobilized into Agrobacterium rhizogenes strain R1000 by the freeze-thaw method and used to infect aseptically grown tobacco leaves. Hairy roots appeared after 2 weeks and were cultured on fresh media plates.
Construction of ERalpha ligand detection system and stable transformation of A. thaliana
The DNA construct having human estrogen-receptor-alpha (ERalpha) hormone binding domain fused with GAL4-DNA binding domain and VP16 transactivation domain was kindly provided by D. Picard (University of Geneva) (Louvion et al. 1993). The DNA fragment encoding this cassette was produced by PCR amplification and cloned upstream of the constitutive cauliflower mosaic virus CaMV35S promoter (35S). The thermostable mutant of green fluorescent protein, mGFP5 (Siemering et al. 1996) was cloned and inserted in-frame with 35S minimal promoter-GAL4 upstream activation sequence (GAL4 UAS). Plasmid derivatives of pBI101 were used as a vector for cloning and introduced into Agrobacterium tumefaciens strain GV3101 for transformation of Arabidopsis thaliana. Transgenic Arabidopsis thaliana -ecotype Columbia plants were produced by the floral dip method (Clough and Bent 1998). Primary transgenic plants were allowed to self-fertilize and seeds were collected. Transgenic progeny were selected on kanamycin (100 mg/L) containing MS medium (Murashige and Skoog 1962) supplemented with 1% sucrose and 0.8% agar. T3 homozygous plants grown on the same agar medium for one week after germination were used for various treatments. In all cases, plants were grown at 20 ± 2 °C with 10 h light (70–80 μmoles/m2 s) and 14 h darkness.
Treatments with chemicals and plant extracts
All chemicals were purchased from Sigma Chemicals, St Louis, MO, USA and dissolved in DMSO or ethanol. The chemicals were added to liquid MS media before treatment of plants. Roots of plants were submerged into this media and kept in growth chamber for 24 – 48 h. Control treatments consisted of adding the same volume of solvent without chemicals. Native plant species were collected under permit from a variety of habitats, including swamps, fens, bogs, marshes, vernal pools and riparian areas. These accessions were transported on dry ice to the laboratory, where they were lyophilized and macerated with mortar and pestle. Aqueous extract solutions were agitated for 18–24 hours, and filtered. A second extraction was then performed on the material collected from the filter paper. The extract solutions were combined to provide a final extract solution (25 mg/ml) for screening and 500 μl of these extracts were added to liquid MS medium as treatment.
Fluorescence microscopy
After treatment, roots were washed with distilled water to remove chemicals and kept on a glass slide. Treated protoplasts were directly used for visualization. An inverted fluorescence microscope (Nikon Eclipse TE200) fitted with filter sets (Nikon; excitation filter 355 – 490 nm), suitable for UV or blue light excitation of GFP, was used to obtain all fluorescent images.
Results and Discussion
Transient expression studies of ERbeta-ligand detection system
The efficiency of ligand binding to ERbeta and subsequent activation of the luciferase reporter gene was tested by co-electroporating the reporter plus pKYLX80-GVE constructs into protoplasts isolated from tobacco cell suspension cultures. The electroporated protoplasts were treated with different ligands and with only DMSO (control). Luciferase activity was measured after 24 h incubation period. Luciferase induction was highest with estradiol treatment (4.4- fold increase) among other ligands used when compared with the control (Fig. 1B). Liquiritigenin and Diarylpropionitrile (DPN) are selective agonists to ERbeta (Mersereau et al. 2008; Connell and Saleh 2011) whereas daidzein is selective to ERbeta at low dose only (Wilde et al. 2004). Based on this finding, other reporter constructs were also evaluated for induction with pKYLX80-GVE in tobacco protoplasts transient expression system. For the induction of GFP, we used estradiol to treat the electroporated protoplasts and visualized under fluorescent microscope (Fig. 1C). All reporter genes were efficiently induced by the activation of ERbeta.
ER ligand-induced positive selection in tobacco hairy roots
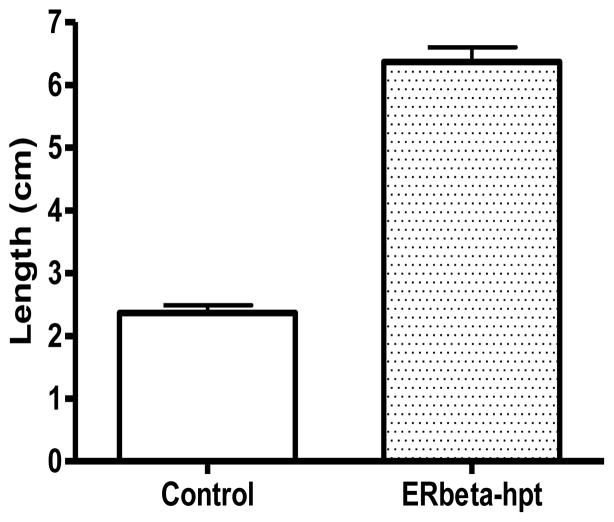
To develop a facile positive selection system to indicate the presence of ER ligands, a construct containing ERbeta whose ligand-induced activation is linked to the expression of hygromycin resistance gene was designed and transformed into tobacco leaves and hairy root cultures. Transgenic hairy roots that appeared from the infected sites were separated from the explants and cultured onto fresh agar media to evaluate the induction of hpt gene via hygromycin resistance upon ligand treatments. Several concentrations of hygromycin were tested to establish that mutant hairy roots could be efficiently selected with 15 mg/L of hygromycin during ERbeta induction with 10 μM estradiol. One-to-two-cm long transformed hairy roots were cultured in MS media containing 15 mg/L hygromycin and estradiol. Root lengths were measured after 2 weeks (Fig. 2). Tobacco hairy roots transformed with the ERbeta-hpt construct did not grow in hygromycin-containing media without exogenous ligand present whereas hairy roots proliferated in the estradiol-inducing media. The data showed that the hpt gene was sufficiently expressed to detoxify the hygromycin in the media in the presence of estradiol. In the absence of ERbeta ligand, hairy roots failed to survive in selective media, suggesting that hpt was not adequately expressed and unable to detoxify hygromycin in the culture media. Different mutagenesis approaches are being applied on several medicinal plant species capable of synthesizing ER ligands (such as soybean) to generate ligand-overproducer lines using this survival selection strategy.
Figure 2.
Stable expression of ERbeta-hpt construct in tobacco (Nicotiana tabacum var. SR1) hairy roots:
Treatment of tobacco ERbeta-hpt hairy roots with estradiol in the media and selection under hygromycin antibiotic. Tobacco hairy roots were generated after A. rhizogenes infection transforming ERbeta-hygromycin selective construct. Hairy roots were produced from the infected explants cultured on MS media containing kanamycin and cefotaxime (without hygromycin). One-cm long hairy roots were then transferred to ER inducible/hygromycin selectable media [100 nM estradiol + 15 mg/L hygromycin, and only hygromycin 15 mg/L]. Measurements were made after 2 weeks of culture: Only estradiol treated hairy root cultures were able to grow and survive in hygromycin containing media.
Responses of ERapha-GFP transgenic A. thaliana roots to exogenous agonists and antagonists
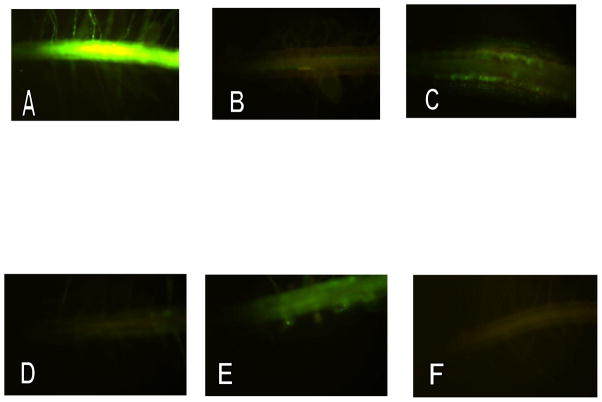
In a first step to gauge the activation of ERalpha by way of GFP expression, we used different concentrations of estradiol, one of the most potent ER ligands. Young transgenic plants were grown on agar plates and transferred to liquid MS medium containing 0.01, 0.1, 0.2 and 1 μM estradiol. After 48 h on induction medium, roots were washed with distilled water to remove estradiol and visualized under UV light. As a negative control, the seedlings were transferred from plates to liquid media containing the same amount of ethanol instead of estradiol. Our result indicates that estradiol is capable of producing a detectable GFP response at 10 nM and almost a full response at a concentration of 100 nM within 24 h (Fig. 3-A). This is a higher concentration than would be required for isolated mammalian cells, but is quite compatible with the predicted affinity of the ER construct. It is highly likely that the diffusion barriers imposed by intact plant roots account for the slightly lower sensitivity of our system. GFP expression continues over several days if the agonist remains present in the medium. After removal of the agonist GFP expression was still detectable for 48 h (data not shown). The truncated ERalpha used here is able to distinguish between agonist and antagonist actions in relation to expression of GFP. To confirm functionality of the truncated human estrogen receptor in a transgenic plant, we used the ER partial agonist tamoxifen and the ER antagonist Fulvestrant to block the induction effect of estradiol. Roots of young seedlings were treated with 1 μM tamoxifen for 48 h in liquid media and visualized under UV light. In another experiment the roots were treated with 1μM tamoxifen for 24 h and transferred into liquid media containing 5μM estradiol for 24h. Low μM concentrations of the partial agonist tamoxifen behaved similarly as in mammalian cells expressing the native ER; that is they generate a weak signal alone but largely prevent the “full agonist response” as estradiol (Fig. 3-B & C). Similarly, Fulvestrant, a supposed “pure competitive antagonist” also acted as predicted, with almost no GFP signal generated at 5μM, but almost completely prevents GFP response to estradiol (Fig. 3-D & E). No fluorescent signal was detected in non-ligand control treatments (Fig. 3-F).
Figure 3.
Induction of GFP in transgenic A. thaliana roots expressing truncated human ER-alpha linked with GFP: The roots of 6-day-old seedlings were treated with different agonist and antagonist synthetic compounds for 24 – 48 hrs and visualized in the microscope under UV light:
A. 17beta-estradiol (100nM) treated for 24 hrs
B. Tamoxifen (1 μM) treated for 48 hrs
C. Tamoxifen (1 μM) for 24 hrs then estradiol (1 μM) for 24 hrs
D. Fulvestrant (5 μM) for 48 hrs
E. Fulvestrant (5 μM) for 24 hrs then estradiol (1 μM) for 24 hrs
F. Control roots treated with either ethanol or DMSO
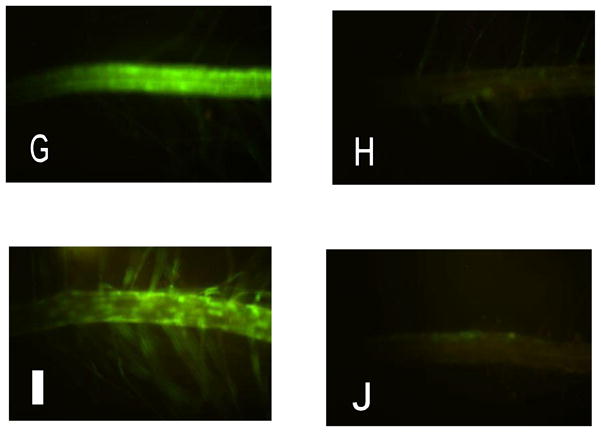
GFP induction in roots by treatment with known commercially available phytoestrogen
G. Genistein (10 μM),
H. Kaempferol (10 μM).
Screening of plant extracts to detect the presence of phytoestrogen: roots were treated with different plant extracts for 24 h and GFP was visualized under a fluorescence microscope.
I. application of extract from Cacalia plantaginea
J. application of extract from Scirpus acutus
Response to known phytoestrogens
Phytoestrogens are bioactive natural products with a chemical structure similar to the mammalian endogenous estrogen, estradiol. Similarity in chemical structure allows them to interact at ligand binding sites on ERs and impose agonist or antagonist effects. Several compounds tested resulted in a clear fluorescent signal and were ranked based on the extent of their inducibility of the system in transgenic Arabidopsis seedlings. Seedlings exposed to 10 μM of a given compound and examined after 24 h showed root green fluorescence from the following (arranged in order of decreasing inducibility): 17β-estradiol (estrogen), (maximum inducibility) followed by genistein (Fig. 3-G) > apigenin/naringenin > biochanin > kaempferol (Fig. 3-H). The results show that not only do known mammalian hormones induce activity of the system and result in GFP expression, but several plant-derived compounds show inducibility as well.
The specificity of distinct chemical structures on the truncated ER expressed in this transgenic line was further demonstrated by the lack of detectable GFP inducibility displayed by a set of related compounds including progesterone, quercetin, catechin, betamethasone, luteolin, chrysin and campesterol. All of these compounds, except for campesterol, show measurable binding to the human ER in in vitro tests (Miksicek 1995) but they are not agonists.
Application of plant extracts and GFP expression
Many plant-derived compounds are known for their potent pharmacological effects at estrogen receptors. For example, St. John’s wort contains a potent pregnane receptor ligand (Moore et al. 2000). Soy is rich in phytoestrogens such as genistein and daidzein (Usui 2006). Plant-based “nutraceutical” products are a very different proposition from novel pharmaceuticals. Thus, such compounds commonly have a broader spectrum of targets than drugs, for example compounds like resveratrol are anti-oxidants as well as potent phytoestrogens (Moosmann and Behl 1999). In an initial investigation of a library of plant extracts, we selected 25 plant species based on their likelihood of possessing high anti-oxidant activity for phytoestrogenic activity. The seedlings were transferred to 2 ml of growth media containing 500 μl of plant extract and incubated in growth chamber for 24 h. The results show that phytoestrogen activity is not common in these plant species and there is no correlation with their high anti-oxidant activity. Extracts from at least two plant species were able to induce detectable GFP expression. An aqueous plant extract of Cacalia plantigenia produced full GFP response similar to estradiol treatments (Fig. 3-I) whereas extracts from Scirpus acutus showed activity closely to that of Tamoxifen on these ER/GFP screens (Fig. 3-J). Further characterizations of these plant species are under process.
The developed reporter and selection survival approach based on expression of NR linked with inducible reporter or selective marker system provides a conventional primary high throughput screen (HTS) for the presence of ER ligands in crude plant extracts which includes partial agonist or antagonist activity. However, antagonists at ERs also seem capable of causing GFP expression to some extent, and agonists may be more effective. Being based on fluorescence microscopy, the system is not easily quantifiable. Although this is important in that the system cannot indicate either the potency or the concentration of active compounds, it can however, be used for detection of activity. The feasibility study described here shows the efficacy of using an antibiotic gene linking to ER ligand binding. This also opens a way to select or screen for desirable new lines over-expressing compounds with such activity from mutagenized populations of plants. In such a scheme, lines identified by their GFP fluorescence can then be further analyzed by subjecting extracts from a fluorescing line to conventional bio-assays and/or chemical analysis. However, identification of mutants via activation of GFP fluorescence reporter mechanisms may greatly decrease the analytical workload required for characterizing a large mutant population for novel secondary metabolism.
The use of bio-activity as a quantitative measure requires some caveats. Receptor activation at the molecular level is only in part a function of ligand affinity for a known receptor population, and higher affinity ligands tend to be receptor antagonists (Gyling and LeClerq, 1988). Thus the intensity of reporter fluorescence may indicate the presence of a relatively greater quantity of a low-affinity ligand, or a presence of a relatively low quantity of a highly active phytochemical, or in some cases a partial agonist. Estimations of ligand affinity might be inferred from our system, provided the level of expression and distribution of the reporter system is known and relatively constant. Further development of the reporter system is required for this to be used as a quantitative assay that is comparable to standard in-vitro assay systems that allow for estimations of receptor affinity and bio-activity.
Likewise, the reporter system is subject to potential for false-positive results. Generally, plants may produce auto-fluorescent chemicals, and the presence of such novel compounds in a mutant, for example, may be difficult to visually distinguish from weak activation of the reporter system. However, the potential for such an end product is likely very low and such false-positives would be easily identified in follow-up bio-assays used to confirm activity of an identified positive mutant.
Although this work focuses specifically on the ER, an important aspect of the technology is that it is applicable to the search for plant-based ligands for any human nuclear receptor and/or transcription factor. This includes nuclear receptors inferred from genomic sequences that are of unknown function, and for which no known ligands currently exist. Importantly, the many similarities between nuclear regulations in eukaryotic cells, suggest that plant cells may be a fertile source of such ligands. This is potentially important because the development of genomic approaches to characterize and diagnose tumors based on their abnormal cell biology should make small molecule regulators of nuclear receptors and transcription factors increasingly important in cancer therapy. The technologies described are designed to provide a functional plant genomics approach to seeking natural products that have appropriate actions.
Acknowledgments
This work was supported by National Center for Complementary & Alternative Medicine (NIH grant R42 AT006639 awarded to John Littleton, Naprogenix Inc.).
Footnotes
Ethical Statement Authors declare that they have no conflict of interest and no human or animal samples were used during this research experiment. John Littleton and Dennis Trent Rogers are employees of Naprogenix Inc that is the sole licensee of the technology from the University of Kentucky Research Foundation.
References
- Ahn KS, Sethi G, Sung B, Goel A, Ralhan R, Aggarwal BB. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–4415. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli E, Minassi A, Appendino G, Moro L. 8-Prenylnaringenin, inhibits estrogen receptor-alpha mediated cell growth and induces apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2007;107:140–148. doi: 10.1016/j.jsbmb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Connell BJ, Saleh TM. Differential Neuroprotection of Selective Estrogen Receptor Agonists against Autonomic Dysfunction and Ischemic Cell Death in Permanent versus Resperfusion Injury. Advances in Pharmacol Sci. 2011;2011:1–9. doi: 10.1155/2011/976951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL. Prescription for an ailing pharmaceutical industry. Nat Biotechnol. 2002;20:331. doi: 10.1038/nbt0402-331. [DOI] [PubMed] [Google Scholar]
- De Wilde A, Lieberherr M, Colin C, Pointillart A. A low dose of daidzein acts as an ERbeta-selective agonist in trabecular osteoblasts of young female piglets. J Cell Physiol. 2004;2:253–262. doi: 10.1002/jcp.20008. [DOI] [PubMed] [Google Scholar]
- Gyling M, Leclercq G. Estrogen and antiestrogen interaction with estrogen receptor of MCF-7 cells—Relationship between processing and estrogenicity. J Steroid Biochemistry. 1988;29(1):1–8. doi: 10.1016/0022-4731(88)90368-8. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Arao Y, Otsuka H, Nomoto S, Horiguchi H, Kato S, Kayama F. Terpenoids found in the umbelliferae family act as agonists/antagonists for ER(alpha) and ERbeta: differential transcription activity between ferutinine-liganded ER(alpha) and ERbeta. Biochem Biophys Res Commun. 2002;291:354–360. doi: 10.1006/bbrc.2002.6446. [DOI] [PubMed] [Google Scholar]
- Levenson AS, Gehm BD, Pearce ST, Horiguchi J, Simons LA, Ward JE, Jameson JL, Jordan VC. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha. Int J Cancer. 2003;104:587–596. doi: 10.1002/ijc.10992. [DOI] [PubMed] [Google Scholar]
- Louvion JF, Havaux-Copf B, Picard D. Fusion of GAL4-VP16 to a steroid-binding domain provides a tool for gratuitous induction of galactose-responsive genes in yeast. Gene. 1993;131:129–134. doi: 10.1016/0378-1119(93)90681-r. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Recchia AG, Bonofiglio D, Catalano S, Vivacqua A, Carpino A, Rago V, Rossi R, Ando S. The red wine phenolicspiceatannol and myricetin act as agonists for estrogen receptor alpha in human breast cancer cells. J Mol Endocrinol. 2005;35:269–281. doi: 10.1677/jme.1.01783. [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283:49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek RJ. Estrogenic flavonoids: structural requirements for biological activity. Proc Soc Exp Biol Med. 1995;208:44–50. doi: 10.3181/00379727-208-43830. [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Pearce ST, Jordan VC. The biological role of estrogen receptors alpha and beta in cancer. Crit Rev Oncol Hematol. 2004;50:3–22. doi: 10.1016/j.critrevonc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O’Neal JM, Cornwell T, Pastor I, Fridlender B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- Siemering KR, Golbik R, Sever R, Haseloff J. Mutations that suppress the thermosensitivity of green fluorescent protein. Curr Biol. 1996;6:1653–1663. doi: 10.1016/s0960-9822(02)70789-6. [DOI] [PubMed] [Google Scholar]
- Tavva VS, Dinkins RD, Palli SR, Collins GB. Development of a methoxyfenozide-responsive gene switch for applications in plants. The Plant J. 2006;45:457–469. doi: 10.1111/j.1365-313X.2005.02628.x. [DOI] [PubMed] [Google Scholar]
- Usui T. Pharmaceutical Prospects of Phytoestrogens. Endocrine J. 2006;53:7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- Xu H, Kraus WL, Shuler ML. Development of a stable dual cell-line GFP expression system to study estrogenic endocrine disruptors. Biotechnol Bioeng. 2008;101:1276–1287. doi: 10.1002/bit.21991. [DOI] [PubMed] [Google Scholar]