Abstract
Farnesyl pyrophosphate (FPP) is a branch-point intermediate in the mevalonate pathway that is normally converted mainly to squalene by squalene synthase in the first committed step of sterol biosynthesis. Treatment with the squalene synthase inhibitor squalestatin 1 (SQ1) causes accumulation of FPP, its dephosphorylated metabolite farnesol, and several oxidized farnesol-derived metabolites. In addition, SQ1 treatment of primary cultured rat hepatocytes increases CYP2B expression through a mechanism that requires FPP synthesis and activation of the constitutive androstane receptor (CAR). Because direct farnesol treatment also increases CYP2B expression, it seems likely that SQ1-mediated CAR activation requires FPP dephosphorylation to farnesol. The lipid phosphatase, phosphatidic acid phosphatase domain containing 2 (PPAPDC2), was recently reported to catalyze FPP dephosphorylation. We therefore determined the effect of overexpressing or knocking down PPAPDC2 on SQ1-mediated CAR activation in primary cultured rat hepatocytes. Cotransfection of rat hepatocytes with a plasmid expressing rat or human PPAPDC2 enhanced SQ1-mediated activation of a CAR-responsive reporter by 1.7- or 2.4-fold over the SQ1-mediated activation that was produced when hepatocytes were cotransfected with empty expression plasmid. Similarly, transduction of rat hepatocytes with a recombinant adenovirus expressing PPAPDC2 enhanced SQ1-mediated CYP2B1 mRNA induction by 1.4-fold over the induction that was seen in hepatocytes transduced with control adenovirus. Cotransfection with a short hairpin RNA targeting PPAPDC2 reduced SQ1-mediated CAR activation by approximately 80% relative to the activation that occurred in hepatocytes transfected with nontargeting short hairpin RNA. These results indicate that PPAPDC2 plays an important role in SQ1-mediated CAR activation, most likely by catalyzing the conversion of FPP to farnesol.
Introduction
The mevalonate pathway comprises the first portion of the cholesterol biosynthetic pathway, and its rate-limiting enzyme, 3-hydroxy-3-methylglutarylcoenzyme A reductase (HMGCR), is the target of the statin class of cholesterol-lowering drugs. The mevalonate pathway is a highly conserved process whereby acetyl-CoA is converted to a series of progressively elongated isoprenoids that include the branch-point metabolite, farnesyl pyrophosphate (FPP; also known as farnesyl diphosphate). Although FPP is normally converted mainly to squalene by squalene synthase in the first committed step of sterol biosynthesis, it is also metabolized to several nonsterol isoprenoids, including ubiquinone (coenzyme Q), dolichol, dolichyl phosphate, heme O, heme A, and isopentenyl transfer RNA (Goldstein and Brown, 1990). In addition, FPP and its metabolite geranylgeranyl pyrophosphate (GGPP) are substrates for protein prenylation reactions, which are required for cellular signaling by small GTPases (Zhang and Casey, 1996). FPP and GGPP are also precursors to the isoprenols, farnesol and geranylgeraniol (Edwards and Ericsson, 1999). Under normal physiologic conditions, little FPP is dephosphorylated to farnesol; however, when squalene synthase is inhibited, FPP accumulates, leading to massive production of farnesol and its progressively oxidized metabolites (collectively known as farnesoids), farnesal, farnesoic acid, and a series of dicarboxylic acids that are excreted in urine (Bostedor et al., 1997; Vaidya et al., 1998).
We previously reported that treatment of primary cultured rat hepatocytes with the squalene synthase inhibitor, squalestatin 1 (SQ1), increases CYP2B1 expression by causing accumulation of an endogenous isoprenoid(s) that activates the constitutive androstane receptor (CAR; NR1I3) (Kocarek and Mercer-Haines, 2002; Jackson and Kocarek, 2008). Additional evidence implied that this effect required the conversion of FPP to farnesol, since direct treatment with farnesol caused CYP2B induction in cultured rat hepatocytes (Kocarek and Mercer-Haines, 2002) and rat liver (Horn et al., 2005). However, this possibility was not specifically addressed since the enzyme(s) responsible for converting FPP to farnesol was not known. Later, phosphatidic acid phosphatase domain containing 2 (PPAPDC2) was identified as a lipid phosphate phosphohydrolase that could convert presqualene diphosphate to presqualene monophosphate (Fukunaga et al., 2006). PPAPDC2 was subsequently shown to hydrolyze FPP and GGPP preferentially over several other phospholipid substrates in vitro and to deplete FPP levels when overexpressed in cells (Miriyala et al., 2010). These data suggested that PPAPDC2 could be an important component of the pathway for synthesis of isoprenols. Therefore, the objective of this investigation was to determine the effect of PPAPDC2 expression on SQ1-mediated activation of CAR in primary cultured rat hepatocytes.
Materials and Methods
Materials.
SQ1 was a gift from Bristol-Myers Squibb Co. (Stamford, CT) and pravastatin was from GlaxoSmithKline (Research Triangle Park, NC). Phenobarbital (PB) was purchased from Sigma-Aldrich (St. Louis, MO). Matrigel was purchased from Corning Life Sciences (Tewksbury, MA), PureCol from Advanced BioMatrix (San Diego, CA), and recombinant human insulin (Novolin R) from Novo Nordisk Pharmaceuticals, Inc. (Princeton, NJ). Williams’ E medium and Lipofectamine 2000 reagent were purchased from Invitrogen (Life Technologies, Grand Island, NY). SYBR Green PCR Master Mix was purchased from Applied Biosystems (Foster City, CA). Additional materials were obtained from the sources indicated below.
Primary Culture of Rat Hepatocytes.
Adult male Sprague-Dawley rats (200–250 g) were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and maintained in an Association for Assessment and Accreditation of Laboratory Animal Care–approved animal facility with free access to food and water for 1 week. Hepatocytes were isolated by two-step collagenase perfusion as described previously (Kocarek and Reddy, 1996). Freshly isolated hepatocytes were plated onto PureCol-coated plates at a density of 1.6 million hepatocytes per well for six-well plates (for RNA isolation) or 0.6 million hepatocytes per well for 12-well plates (for transfection experiments) and maintained at 37°C under a humidified atmosphere of 95% air/5% CO2 in Williams’ E medium supplemented with 0.25 U/ml insulin, 0.1 µM triamcinolone acetonide, 100 U/ml penicillin, and 100 µg/ml streptomycin. Culture medium was renewed every 24 hours and drug treatments were begun 48 hours after plating as described in the individual figure legends. Drugs were prepared as 1000× stock solutions in sterile water.
Overexpression and Knockdown of PPAPDC2 by Transient Transfection in Primary Cultured Rat Hepatocytes.
A CAR-responsive firefly luciferase reporter plasmid containing approximately 2.4 kb of the CYP2B1 5′-flanking region was previously described (Kocarek et al., 1998). An expression plasmid containing the rat PPAPDC2 cDNA and a cloning plasmid containing the human PPAPDC2 cDNA were purchased from GE Healthcare (Pittsburgh, PA). Human PPAPDC2 cDNA from the latter was transferred into an expression vector as described in the (Supplemental Methods). Four plasmids expressing 29-mer short hairpin RNAs (shRNAs) targeting rat PPAPDC2 and a nontargeting shRNA plasmid (TR30012) were purchased from Origene Technologies (Rockville, MD). The validation vector reporter assay that was used to evaluate the shRNAs for knockdown efficiency is described in the (Supplemental Methods).
For overexpression of rat or human PPAPDC2, hepatocytes were transiently transfected with Williams’ E medium containing 0.2 ml OptiMEM (Life Technologies) and a premixed complex of Lipofectamine 2000 (4 μl), the CYP2B1 reporter plasmid (1.2 μg), rat or human PPAPDC2 expression plasmid (50 ng), pRL-CMV luciferase reporter plasmid (1 ng), and pBlueScript II KS+ (350 ng; Stratagene Cloning Systems; Agilent Technologies, Santa Clara, CA) to adjust total DNA content to 1.6 μg. For knockdown, hepatocytes were transfected as described above, except that the complex contained 400 ng of nontargeting control or PPAPDC2-targeting shRNA instead of PPAPDC2 expression plasmid and pBlueScript II KS+. Five hours after transfection, medium was replaced with Williams’ E medium containing 0.8 mg/ml Matrigel. Drug treatments were begun the following day and repeated once after 24 hours. Hepatocytes were harvested 48 hours after initial treatment of measurement of firefly and Renilla luciferase activities using the Dual Luciferase Reporter Assay System and a GloMax luminometer (Promega Corporation, Madison, WI). Each transfection experiment included three wells per treatment group, and luciferase data were calculated as mean firefly/Renilla ratios and presented as described in the individual figure legends. Experiments were repeated in four independent rat hepatocyte preparations for overexpression and three for knockdown.
Overexpression of PPAPDC2 by Adenoviral Transduction in Primary Cultured Rat Hepatocytes.
The generation of a recombinant adenovirus for expression of rat PPAPDC2 (Ad5.CMV-PPAPDC2) is described in the (Supplemental Methods). Twenty-four hours after plating, primary cultured rat hepatocytes were transduced with Ad5.CMV-PPAPDC2 or control adenovirus without insert (Ad5375; O.D. 260 Inc., Boise, ID) at a multiplicity of infection of 5. After 5 hours of infection, the medium was replaced with Williams’ E medium containing 0.8 mg/ml Matrigel. Drug treatments were begun 24 hours after infection (two wells per treatment group) and were repeated once after 24 hours. Forty-eight hours after initial treatment, hepatocytes were harvested for RNA or protein extraction.
Quantitative Reverse-Transcription Polymerase Chain Reaction Analysis.
Total RNA was extracted using the Purelink RNA isolation kit (Ambion, Carlsbad, CA) and processed for cDNA synthesis and quantitative reverse-transcription polymerase chain reaction as described in the (Supplemental Methods). CYP2B1 mRNA levels were normalized to TATA box-binding protein (assay identifier Rn.PT.51.24118050; Integrated DNA Technologies, Coralville, IA). Relative changes in mRNA levels were calculated using the comparative threshold cycle method (user bulletin 2; Applied Biosystems). All assays were performed in duplicate and were repeated in four independent experiments.
Western Blot Analysis of PPAPDC2.
PPAPDC2 protein levels in primary cultured rat hepatocytes were measured by Western blot analysis 48 hours after adenoviral transduction using a rabbit polyclonal antibody (Origene Technologies). Additional details are presented in the (Supplemental Methods).
Statistical Analyses.
Statistical analyses were performed using GraphPad Prism (version 6.00 for Mac OS X; GraphPad Software Inc., San Diego, CA) to perform two-way analysis of variance with the Newman–Keuls correction for multiple comparisons. P < 0.05 was considered significantly different. All results are presented as means ± S.E.M.
Results and Discussion
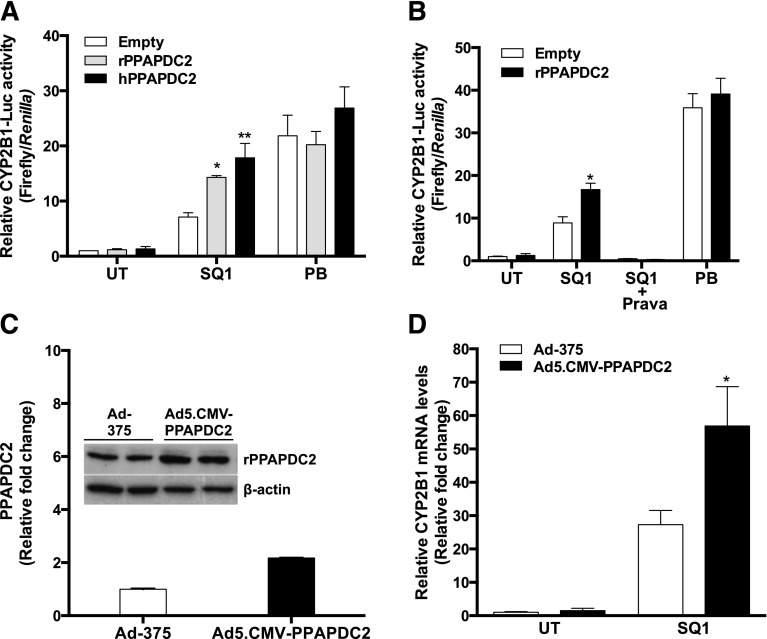
Based on the reported ability of PPAPDC2 to dephosphorylate FPP to farnesol (Miriyala et al., 2010), we hypothesized that overexpression of PPAPDC2 would enhance SQ1-mediated CAR activation in primary cultured rat hepatocytes. Therefore, rat hepatocyte cultures were cotransfected with an expression plasmid for PPAPDC2 (rat or human) or empty vector and a reporter plasmid containing the CAR-responsive region of CYP2B1 (Kocarek et al., 1998) and then treated with 0.1 μM SQ1 or 100 μM PB, the prototype CAR activator. SQ1 and PB treatment significantly increased CYP2B1 reporter activity by 7.1- and 22-fold, respectively, compared with the untreated control in hepatocytes transfected with empty vector (Fig. 1A). The SQ1-mediated induction of CYP2B1 reporter activity was significantly enhanced by overexpression of either rat or human PPAPDC2 compared with the SQ1-treated, empty vector–transfected group (1.7-fold and 2.4-fold enhancement, respectively). Therefore, rat and human PPAPDC2, which share 89% amino acid sequence similarity, were functionally comparable in their abilities to enhance SQ1-inducible CAR activation. PPAPDC2 overexpression did not affect reporter activity in either untreated or PB-treated hepatocytes, indicating that the enhancing effect of PPAPDC2 on CAR activity only occurred when squalene synthase was inhibited. Cotreatment of rat hepatocytes with the HMGCR inhibitor pravastatin (30 μM), which blocks production of mevalonate (and therefore FPP), abolished the effect of SQ1, without or with PPAPDC2 overexpression, on CAR activity (Fig. 1B), demonstrating the dependence of the PPAPDC2-enhanced CAR activation on the ongoing production of mevalonate and its isoprenoid metabolites.
Fig. 1.
Effect of PPAPDC2 overexpression on SQ1-mediated CAR activation in primary cultured rat hepatocytes. (A) Twenty-four hour–old primary cultures of rat hepatocytes were transiently transfected with a CAR-responsive reporter plasmid (CYP2B1-Luc) and either an empty expression plasmid (Empty) or an expression plasmid for rat (rPPAPDC2) or human PPAPDC2 (hPPAPDC2). Then, 24 hours after transfection, cultures were incubated in medium alone (UT) or containing 0.1 µM SQ1 or 100 µM PB. Each bar represents the mean ± S.E.M. of the mean normalized (firefly/Renilla) luciferase measurements from four independent experiments (n = 4). All values are normalized to the Empty/UT group. *P < 0.05; **P < 0.01 (significantly different from Empty/SQ1 group). (B) Rat hepatocytes were cotransfected with CYP2B1-Luc and either empty vector (Empty) or rPPAPDC2 and treated with medium alone (UT), 0.1 μM SQ1, 0.1 μM SQ1 and 30 μM pravastatin (Prava), or 100 μM PB. Forty-eight hours after drug treatment, transfected cultures were harvested for measurement of luciferase activity. Each bar represents the mean ± S.E.M. of mean normalized (firefly/Renilla) luciferase measurements from three independent experiments (n = 3). All values are normalized to the Empty/UT group. *P < 0.05 (significantly different from the Empty/SQ1 group). (C) Rat hepatocytes were transduced with empty adenoviral vector (Ad-375) or adenoviral vector expressing rPPAPDC2 (Ad5.CMV-PPAPDC2) and harvested after 48 hours for measurement of either rat PPAPDC2 or β-actin protein. The bar graph shows band intensity quantification of PPAPDC2 levels normalized to β-actin. (D) Rat hepatocytes were transduced with empty adenoviral vector (Ad-375) or adenoviral vector expressing rPPAPDC2 (Ad5.CMV-PPAPDC2), treated for 48 hours with medium alone (UT) or 0.1 μM SQ1, and harvested for measurement of CYP2B1 mRNA levels. Each bar represents the mean CYP2B1 levels ± S.E.M from four independent experiments normalized to the Ad-375/UT group (n = 4). *P < 0.05 (significantly different from Ad-375/SQ1 group).
To measure the effect of PPAPDC2 overexpression on an endogenous marker of CAR activation, we used a recombinant adenovirus to achieve high-efficiency transfer of rat PPAPDC2 (Ad5.CMV-PPAPDC2) into the primary cultured rat hepatocytes. Transduction with PPAPDC2 at a multiplicity of infection of 5 resulted in an approximately 2.2-fold increase in PPAPDC2 immunoreactive protein content relative to that measured in empty adenoviral control vector–transduced cells after 48 hours (Fig. 1C). SQ1 treatment increased CYP2B1 mRNA content by approximately 27-fold in hepatocytes transduced with control vector and approximately 38-fold in PPAPDC2-transduced cells (Fig. 1C), for a significant 1.4-fold enhancement of SQ1-mediated CYP2B1 mRNA induction.
Overall, we interpret the PPAPDC2 overexpression data as follows. Under untreated conditions, most of the FPP is converted to squalene and relatively little is converted to farnesol, likely reflecting the relative affinities of the pathways. In addition, the farnesol that is produced is readily metabolized, so farnesol does not accumulate sufficiently to cause CAR activation. Increasing PPAPDC2 expression in untreated hepatocytes probably does not cause much diversion of FPP away from the squalene synthase pathway; therefore, the increase in farnesol production that occurs is still inadequate to cause effective CAR activation. When squalene synthase is inhibited, FPP accumulates, resulting in marked dephosphorylation to farnesol and substantial CAR activation. Under these conditions, PPAPDC2 overexpression is able to enhance the process enough to cause a further increase in CAR activation.
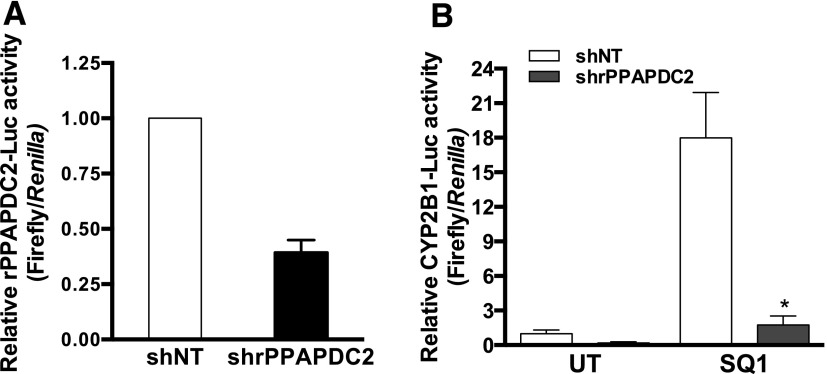
The above data show that supplementing the rat hepatocyte’s capacity to convert FPP to farnesol increased the ability of a drug that causes FPP accumulation to activate CAR, suggesting that FPP dephosphorylation to farnesol is a necessary step in the mechanism by which SQ1 treatment activates CAR. However, these data do not address whether PPAPDC2 is a major enzyme that normally performs this function in hepatocytes. We therefore evaluated the effect of knocking down PPAPDC2 on SQ1-mediated CAR activation by performing cotransfections with shRNA expression plasmids (nontargeting and PPAPDC2-targeting) and the CYP2B1 reporter plasmid. Transfection with the shRNA that produced the greatest knockdown of PPAPDC2 in a validation vector reporter assay (approximately 62% reduction; Fig. 2A) significantly attenuated SQ1-inducible CYP2B1 reporter activation (approximately 80% reduction; Fig. 2B), indicating that PPAPDC2 plays an essential role in SQ1-mediated CAR activation in primary cultured rat hepatocytes.
Fig. 2.
Effect of PPAPDC2 knockdown on SQ1-mediated CAR activation in primary cultured rat hepatocytes. (A) Validation of rPPAPDC2 knockdown. Twenty-four hours after plating, primary cultured rat hepatocytes were transiently transfected with a validation reporter vector for rat PPAPDC2 (rPPAPDC2-Luc) and either a nontargeting control shRNA (shNT) or a shRNA targeting rat PPAPDC2 (shrPPAPDC2). After 48 hours, hepatocytes were harvested for measurement of luciferase activity. Each bar represents the mean ± S.E.M. of mean normalized (firefly/Renilla) luciferase measurements from three independent experiments (n = 3). (B) Rat hepatocytes were transiently transfected with the CYP2B1-Luc reporter and either the shNT or shrPPAPDC2 plasmid. Then, 24 hours after transfection, cultures were incubated for 48 hours in medium alone (UT) or containing 0.1 μM SQ1 and harvested for measurement of luciferase activity. Each bar represents the mean ± S.E.M. of mean normalized (firefly/Renilla) luciferase measurements from three independent experiments (n = 3). All values are normalized to the shNT/UT group. *P < 0.05 (significantly different than the shNT/SQ1 group).
Our data demonstrate that PPAPDC2 plays an essential role in the mechanism by which SQ1 treatment causes CAR activation in primary cultured rat hepatocytes, most likely by catalyzing the conversion of FPP to farnesol. Either farnesol itself or a metabolite is then an endogenous CAR activator. Miriyala et al. (2010) established that PPAPDC2 catalyzes FPP dephosphorylation and that its overexpression can modulate FPP-dependent cellular processes. Overexpression of PPAPDC2 in yeast or mammalian cells decreased FPP levels and caused cytotoxicity. In human embryonic kidney 293 cells, overexpression of PPAPDC2, but not a catalytically defective mutant, caused cytostasis and accumulation of multinucleate cells. In mouse aortic vascular smooth muscle cells, PPAPDC2 overexpression affected processes associated with Rho family GTPase function, which is dependent on protein prenylation. Our data are the first to link PPAPDC2 to a signaling mechanism in normal hepatocytes.
Nonsterol isoprenoids play important roles in a variety of biologic processes, and the control of their cellular levels is therefore an important therapeutic approach for several pathologies (Buhaescu and Izzedine, 2007; Oldfield, 2010; Li et al., 2012). For example, the nitrogen-containing bisphosphonate class of drugs (e.g., alendronate, ibandronate) are first-line treatments for osteoporosis that produce their therapeutic effects by inhibiting FPP synthase and thus production of FPP and its metabolites, including GGPP. Therefore, the bisphosphonates reduce geranylgeranylation of small GTPase proteins in the osteoclast, which leads to osteoclast apoptosis (Drake et al., 2015). The isoprenols farnesol and geranylgeraniol themselves have been implicated in several biologic processes; for example, farnesol is a nonsterol regulator of HMGCR (Correll et al., 1994; Meigs and Simoni, 1997), and these alcohols can be converted back to FPP and GGPP through sequential monophosphorylation reactions (Thai et al., 1999). Therefore, the mechanisms controlling the interconversion of FPP and farnesol are likely important determinants of isoprenoid-regulated cellular processes. Our findings provide further insight into the role of PPAPDC2 as a modulator of hepatic physiology. Specifically, PPAPDC2 provides a metabolic link between the cholesterol biosynthetic pathway and the xenobiotic sensor CAR. Therefore, dietary, physiologic, pathophysiological, or pharmacological conditions that cause enhanced PPAPDC2-mediated conversion of FPP to farnesol could alter CAR-regulated processes, such as xenobiotic metabolism and transport.
Supplementary Material
Acknowledgments
The authors thank Drs. Elizabeth Rondini and Hailin Fang for technical guidance during these experiments.
Abbreviations
- CAR
constitutive androstane receptor (NR1I3)
- FPP
farnesyl pyrophosphate
- GGPP
geranylgeranyl pyrophosphate
- HMGCR
3-hydroxy-3-methylglutaryl coenzyme A reductase
- PB
phenobarbital
- PPAPDC2
phosphatidic acid phosphatase domain containing 2
- PPRE
peroxisome proliferator response element
- shRNA
short hairpin RNA
- SQ1
squalestatin 1
Authorship Contributions
Participated in research design: Pant, Kocarek.
Conducted experiments: Pant.
Performed data analysis: Pant.
Wrote or contributed to the writing of the manuscript: Pant, Kocarek.
Footnotes
This research was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01HL050710] and the National Institutes of Health National Institute of Environmental Health Sciences [Center Grant P30ES020957]. A.P. was supported, in part, through a Thomas C. Rumble fellowship.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Bostedor RG, Karkas JD, Arison BH, Bansal VS, Vaidya S, Germershausen JI, Kurtz MM, Bergstrom JD. (1997) Farnesol-derived dicarboxylic acids in the urine of animals treated with zaragozic acid A or with farnesol. J Biol Chem 272:9197–9203. [DOI] [PubMed] [Google Scholar]
- Buhaescu I, Izzedine H. (2007) Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem 40:575–584. [DOI] [PubMed] [Google Scholar]
- Correll CC, Ng L, Edwards PA. (1994) Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem 269:17390–17393. [PubMed] [Google Scholar]
- Drake MT, Clarke BL, Lewiecki EM. (2015) The pathophysiology and treatment of osteoporosis. Clin Ther 37:1837–1850. [DOI] [PubMed] [Google Scholar]
- Edwards PA, Ericsson J. (1999) Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem 68:157–185. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Arita M, Takahashi M, Morris AJ, Pfeffer M, Levy BD. (2006) Identification and functional characterization of a presqualene diphosphate phosphatase. J Biol Chem 281:9490–9497. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. (1990) Regulation of the mevalonate pathway. Nature 343:425–430. [DOI] [PubMed] [Google Scholar]
- Horn TL, Long L, Cwik MJ, Morrissey RL, Kapetanovic IM, McCormick DL. (2005) Modulation of hepatic and renal drug metabolizing enzyme activities in rats by subchronic administration of farnesol. Chem Biol Interact 152:79–99. [DOI] [PubMed] [Google Scholar]
- Jackson NM, Kocarek TA. (2008) Suppression of CYP2B induction by alendronate-mediated farnesyl diphosphate synthase inhibition in primary cultured rat hepatocytes. Drug Metab Dispos 36:2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocarek TA, Kraniak JM, Reddy AB. (1998) Regulation of rat hepatic cytochrome P450 expression by sterol biosynthesis inhibition: inhibitors of squalene synthase are potent inducers of CYP2B expression in primary cultured rat hepatocytes and rat liver. Mol Pharmacol 54:474–484. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Mercer-Haines NA. (2002) Squalestatin 1-inducible expression of rat CYP2B: evidence that an endogenous isoprenoid is an activator of the constitutive androstane receptor. Mol Pharmacol 62:1177–1186. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Reddy AB. (1996) Regulation of cytochrome P450 expression by inhibitors of hydroxymethylglutaryl-coenzyme A reductase in primary cultured rat hepatocytes and in rat liver. Drug Metab Dispos 24:1197–1204. [PubMed] [Google Scholar]
- Li L, Zhang W, Cheng S, Cao D, Parent M. (2012) Isoprenoids and related pharmacological interventions: potential application in Alzheimer’s disease. Mol Neurobiol 46:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs TE, Simoni RD. (1997) Farnesol as a regulator of HMG-CoA reductase degradation: characterization and role of farnesyl pyrophosphatase. Arch Biochem Biophys 345:1–9. [DOI] [PubMed] [Google Scholar]
- Miriyala S, Subramanian T, Panchatcharam M, Ren H, McDermott MI, Sunkara M, Drennan T, Smyth SS, Spielmann HP, Morris AJ. (2010) Functional characterization of the atypical integral membrane lipid phosphatase PDP1/PPAPDC2 identifies a pathway for interconversion of isoprenols and isoprenoid phosphates in mammalian cells. J Biol Chem 285:13918–13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E. (2010) Targeting isoprenoid biosynthesis for drug discovery: bench to bedside. Acc Chem Res 43:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai L, Rush JS, Maul JE, Devarenne T, Rodgers DL, Chappell J, Waechter CJ. (1999) Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions. Proc Natl Acad Sci USA 96:13080–13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya S, Bostedor R, Kurtz MM, Bergstrom JD, Bansal VS. (1998) Massive production of farnesol-derived dicarboxylic acids in mice treated with the squalene synthase inhibitor zaragozic acid A. Arch Biochem Biophys 355:84–92. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 65:241–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.