Abstract
There is wide variation in how patients respond to therapeutics. Factors that contribute to pharmacokinetic variations include disease, genetics, drugs, age, and diet. The purpose of this study was to determine the effect of calorie restriction on the expression of Abcb1a in the intestine and whether calorie restriction can alter the absorption of an Abcb1a substrate (i.e., digoxin) in mice. Ten-week-old C57BL/6 mice were given either an ad libitum diet or a 25% calorie-restricted diet for 3 weeks. To determine digoxin absorption, mice were administered [3H]-labeled digoxin by oral gavage. Blood and intestine with contents were collected at 1, 2, 4, and 12 hours after digoxin administration. Concentrations of [3H]-digoxin in plasma and tissues were determined by liquid scintillation. Calorie restriction decreased plasma digoxin concentrations (about 60%) at 1, 2, and 4 hours after administration. Additionally, digoxin concentrations in the small intestine of calorie-restricted mice were elevated at 4 and 12 hours after administration. Furthermore, calorie restriction increased Abcb1a transcripts in the duodenum (4.5-fold) and jejunum (12.5-fold). To confirm a role of Abcb1a in the altered digoxin pharmacokinetics induced by calorie restriction, the experiment was repeated in Abcb1a/b-null mice 4 hours after drug administration. No difference in intestine or plasma digoxin concentrations were observed between ad libitum–fed and calorie-restricted Abcb1a/b-null mice. Thus, these findings support the hypothesis that calorie restriction increases intestinal Abcb1a expression, leading to decreased absorption of digoxin in mice. Because Abcb1a transports a wide variety of therapeutics, these results may be of important clinical significance.
Introduction
The absorption of a number therapeutics can be affected by the expression and activity of drug transporters in the intestine. The first drug transporter described was P-glycoprotein (P-gp), also referred to as MDR1 or ABCB1 (Juliano and Ling, 1976; Ueda et al., 1986), which is encoded by the Abcb1a and Abcb1b genes in mice (Schinkel et al., 1994). P-gp is an ATP-dependent efflux pump that actively transports xenobiotics from cells (Gottesman and Pastan, 1993). P-gp transports a wide range of structurally different therapeutics including colchicine, tacrolimus, quinidine, chemotherapeutic drugs (such as etoposide, doxorubicin, and vinblastine), cardiac glycosides (such as digoxin), glucocorticoids (such as dexamethasone), and human immunodeficiency virus type 1 antiretroviral therapy drugs (Aller et al., 2009). Overexpression of Abcb1a and -1b can confer drug resistance by increasing efflux of these drugs from cells (Dhir et al., 1990; Raymond et al., 1990) and is a well-established cause of resistance to cancer chemotherapeutic drugs. Abcb1a is mainly expressed in the gastrointestinal tract, testis, and capillaries within the brain (Cui et al., 2009). In humans, the ABCB1 gene encodes P-gp, which has a similar tissue distribution pattern as in mice (Thiebaut et al., 1987, 1989; Cordon-Cardo et al., 1989). Thus, it is thought that P-gp functions to protect the body, limiting xenobiotic intestinal absorption and distribution to the central nervous system and germ cells. In enterocytes, P-gp is located on the apical surface where it transports xenobiotics back into the intestinal lumen, resulting in limited xenobiotic absorption (Croop et al., 1989; Takano et al., 2006). Because P-gp is known to transport a variety of therapeutics, altering its intestinal expression or activity may alter the bioavailability of a large range of therapeutics.
There is wide variation in the response of humans to therapeutics, both beneficially and adversely. A factor that is often proposed to contribute to this variation is diet (Won et al., 2010; Boullata and Hudson, 2012; Ötles and Senturk, 2014). Until recently, knowledge regarding food-drug interactions was primarily based on anecdotal accounts. However, recent studies have now proven examples of foods that can alter the pharmacokinetics of drugs. For example grapefruit juice inhibits cytochrome P450 (CYP) 3A4 (CYP3A4), which results in increased bioavailability of drugs such as saquinavir, cyclosporine, and felodipine (Seden et al., 2010). Phytochemicals in grapefruit juice (such as bergamottin and quercetin) and green tea catechins inhibit the cellular efflux of P-gp substrates in vitro (Zhou et al., 2004). Furthermore, the amount of protein in the diet can influence drug metabolism and glomerular filtration. For example, in human volunteers, a protein-restricted diet resulted in a 70% decrease in the clearance of oxipurinol and uric acid (Berlinger et al., 1985).
There is a lack of knowledge regarding the effect of decreased calorie consumption on the pharmacokinetics of drugs. However, Renaud et al. (2014) previously reported that in mice a calorie-restricted diet had a profound effect on gene expression in liver. More specifically, Renaud et al. (2014) found that calorie restriction substantially increased hepatic Abcb1a transcripts. These results raised the question of whether calorie restriction alters the expression of Abcb1a in the intestine, where this transporter is known to have a major impact on xenobiotic absorption. In this study, we reveal that calorie restriction causes decreased absorption of digoxin—likely through a mechanism of calorie restriction–induced expression of P-gp. These results support the hypothesis that diet is an important regulator of drug absorption.
Materials and Methods
Chemicals.
Digoxin (0.25 mg/ml) was purchased from Westward Pharm Corp (Eatontown, NJ), RNA-Bee RNA Isolation Reagent was purchased from Tel-Test Inc. (Friendswood, TX), the High Capacity Reverse Transcriptase kit and SYBR Green were purchased from Applied Biosystems (Foster City, CA), [3H]-digoxin and Ultima Gold counting fluid were purchased from PerkinElmer (Shelton, CT), and bovine serum albumin was purchased from Amresco (Solon, OH). Unless otherwise stated, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animals.
C57BL/6 male mice, eight weeks of age, were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Abcb1a/b-null mice were purchased from Taconic Inc. (Hudson, NY) and were back-crossed into the C57BL/6 background (>99% congenicity). Mice were bred and housed in a light-, temperature-, and humidity-controlled environment in an Association for Assessment and Accreditation of Laboratory Animal Care (Frederick, MD) accredited animal housing facility at the University of Kansas Medical Center. All studies were approved by the University of Kansas Medical Center’s Institutional Animal Care and Use Committee.
Calorie Restriction.
After 2-week acclimatization, mice were housed individually and given Laboratory Rodent Chow 8604 (Harlan, Madison, WI), either ad libitum or with 75% of the feed consumed by ad libitum feeding (calorie restriction). The average ad libitum daily consumption of feed was 4 g per mouse (determined using the average daily intake from 10 mice). Thus, mice in the calorie restriction group were given approximately 2.7–3.0 g of feed per day. Mice remained on these diets for 3 weeks, after which they were either used for RNA studies or digoxin absorption studies.
Tissue Collection for RNA Studies.
In the morning (8:00–10:00 AM) mice were euthanized with pentobarbital and their small intestines were collected. Fecal matter was flushed from the small intestines using a saline solution. Small intestines were divided into three equal parts (duodenum, jejunum, and ileum), immediately frozen in liquid nitrogen, and then stored at −80°C until further analysis.
RNA Extraction.
RNA was isolated from frozen intestine using RNA Isolation Reagent RNA-Bee following the manufacturer’s protocol (Tel-Test Inc.). Using a NanoDrop1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE), RNA concentrations were quantified at a wavelength of 260 nm.
Messenger RNA Quantification [Reverse Transcription Quantitative Polymerase Chain Reaction (qPCR)].
To perform real-time polymerase chain reaction, we first reverse transcribed RNA to cDNA using an Applied Biosystems High Capacity Reverse Transcriptase kit. In brief, equal volumes of 2X reverse transcriptase, 50 ng/µl RNA, and random primers were mixed and placed in a Mastercycler (Eppendorf, Hauppauge, NY) under the following conditions: 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 minutes. With the resulting cDNA, qPCR was performed as detailed subsequently. The primers for qPCR of Abcb1a (GenBank Accession No. NM_011076) were designed using Primer-BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast) (forward, GCGACTCCGATACATGGTTT; reverse, ACCCTGTAGCCCCTTTCACT) and were synthesized by Integrated DNA Technologies (Coralville, IA). For qPCR, the following were contained per reaction in a 384-well plate (Applied Biosystems): 2.5 µl of 3 µM forward and reverse primer mix, 5 µl of Applied Biosystems SYBR Green PCR master mix, 0.5 µl RNAse-free H2O, and 2 µl of 2 ng/µl cDNA. Fluorescence was quantified with an Applied Biosystems 7300 Real Time PCR System under the following conditions: 50°C for 2 minutes and 95°C for 10 minutes (95°C for 15 seconds, 60°C for 1 minute) × 40 cycles. To ensure primer specificity, melt curves were performed for every reaction. To determine the relative mRNA expression, the comparative ΔΔCt method was applied using 18S as a reference transcript. Values from calorie-restricted mice were normalized to values from mice fed ad libitum.
Digoxin Absorption Experiments.
The protocol followed for these experiments was adapted from an earlier study (Mayer et al., 1996). Each mouse was given 0.2 mg/kg digoxin by oral gavage, labeled with [3H]-digoxin (1 µCi/30g b.wt.). Wild-type mice were euthanized 1, 2, 4, or 12 hours after digoxin administration, and Abcb1a/b-null mice were euthanized 4 hours after digoxin administration. Blood was collected by orbital bleeding into heparinized tubes and centrifuged for 10 minutes at 2000g to isolate plasma. Plasma (100 µl) was transferred to 4 ml of Ultima Gold high counting efficiency scintillation cocktail. The entire intestine from stomach to rectum (including fecal matter) was also collected, weighed, and homogenized in 4% (w/v) bovine serum albumin. Thus, all intestine samples included the fecal matter. Henceforth, we will refer to the intestine + contents samples as intestine. Two-hundred µl of intestine homogenate was added to 4 ml Ultima Gold high counting efficiency scintillation cocktail. Radioactivity was quantified by liquid scintillation counting using a Packard Tri-Carb 2100TR Liquid Scintillation Analyzer (Packard Instrument Company, Meriden, CT). Concentrations of digoxin were calculated from a standard curve.
Statistics.
Statistical differences between ad libitum and calorie restriction were determined using an unpaired, two-tailed Student’s t test. For the RNA expression data (Fig. 1), the individual values were log transformed to obtain the normal distribution before performing the t test. Comparisons resulting in P < 0.05 were considered statistically significant. All data are presented as the mean + S.E.M.
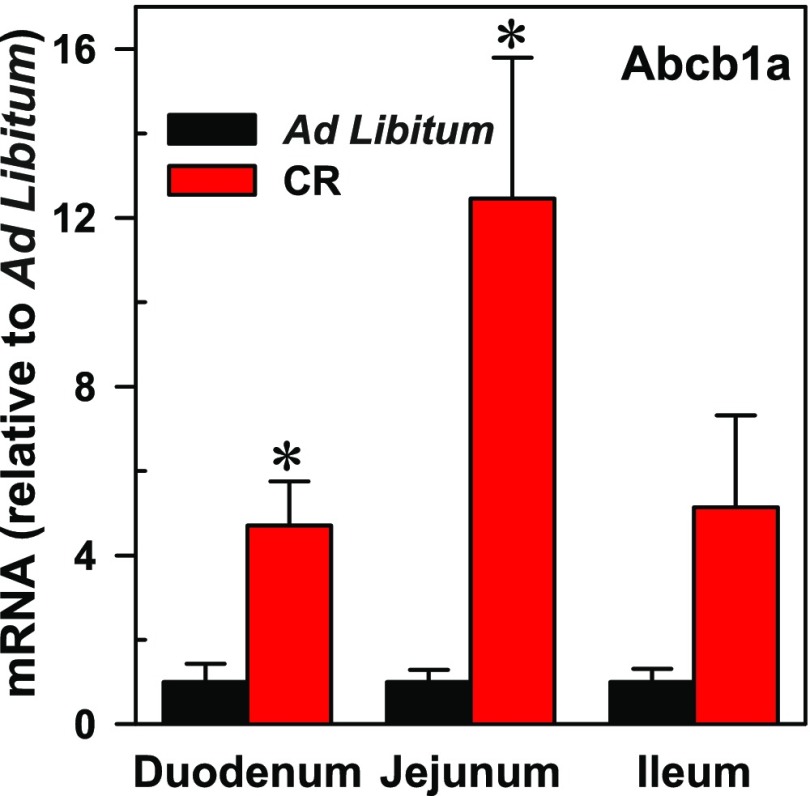
Fig. 1.
Mean mRNA of Abcb1a (± S.E.M.) in mice on an ad libitum diet or calorie restriction (CR). Asterisks (*) denote data values statistically significant from control (ad libitum) (P < 0.05 as determined by a Student’s t test, n = 3).
Results
Calorie Restriction Increases Abcb1a mRNA Expression in the Small Intestine.
The effect of calorie restriction on mRNA expression of Abcb1a in the small intestines of mice was assessed by reverse transcription qPCR (Fig. 1). Calorie restriction significantly (P < 0.05) increased expression of Abcb1a mRNA in the duodenum (4.5-fold) and jejunum (12.5-fold) compared with mice fed the ad libitum diet (control). Expression of Abcb1a was also increased in the ileum; however, it was not statistically different from the controls.
Calorie Restriction Alters Absorption of Digoxin in Mice.
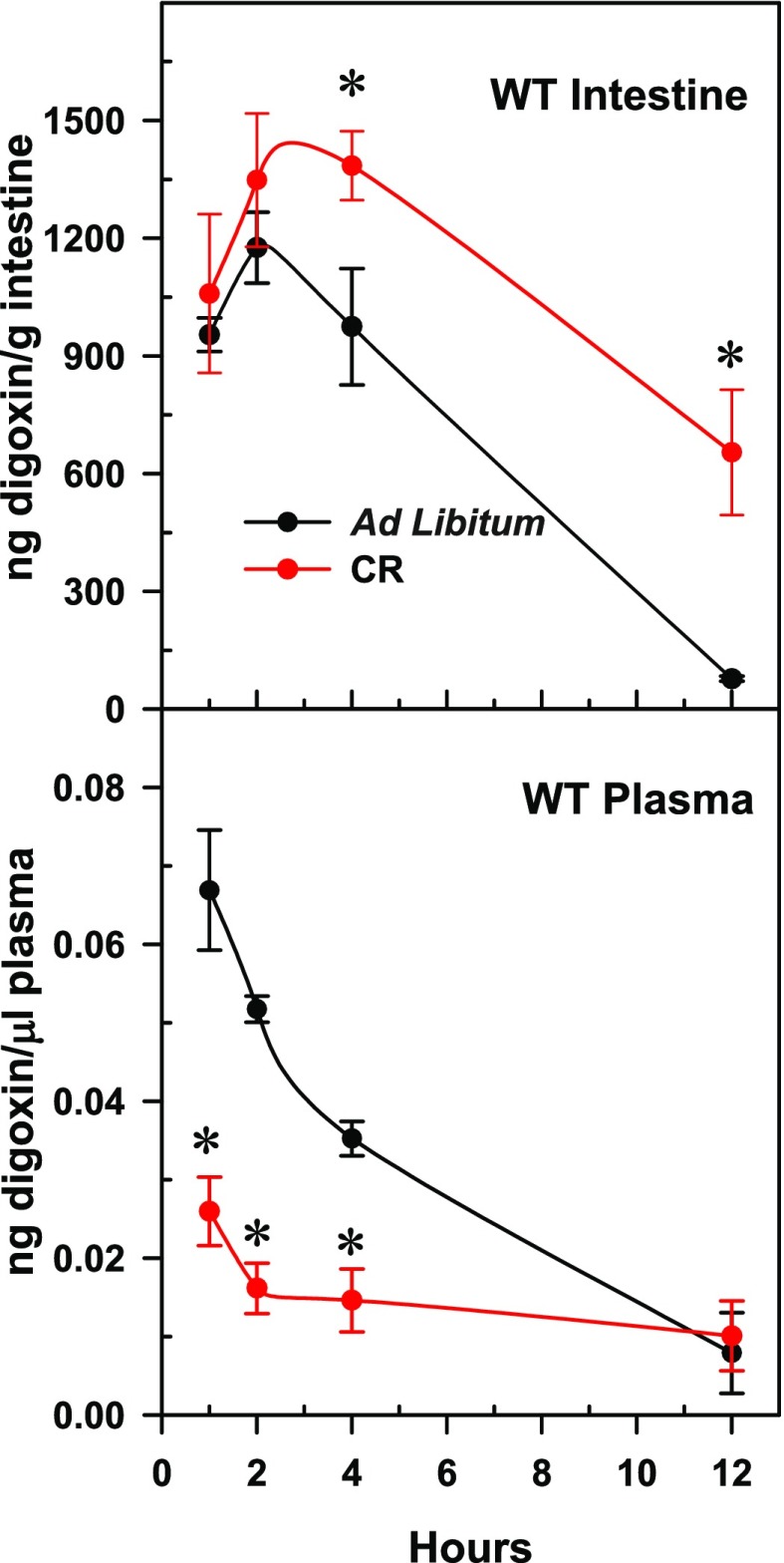
To determine whether calorie restriction alters absorption of drugs transported by Abcb1a in the intestine, mice were given [3H]-labeled digoxin and euthanized 1, 2, 4, and 12 hours after administration. The time courses of the intestine-digoxin and plasma-digoxin concentrations are presented Fig. 2. The intestine of calorie-restricted mice contained 1.5-fold more digoxin at 4 and 12 hours than the intestine of ad libitum fed mice. In contrast to the intestine, the plasma concentrations of digoxin were 61%, 69%, and 57% lower in the calorie-restricted mice than in the control mice at 1, 2, and 4 hours, respectively.
Fig. 2.
Mean concentrations of digoxin (± S.E.M.) in intestine and plasma over time in mice fed an ad libitum diet or calorie restriction. Asterisks (*) denote data values statistically significant from the same time point ad libitum control (P < 0.05 as determined by Student’s t test, n = 4 or 5).
Calorie Restriction Does Not Alter Digoxin Absorption in Abcb1a/b-Null Mice.
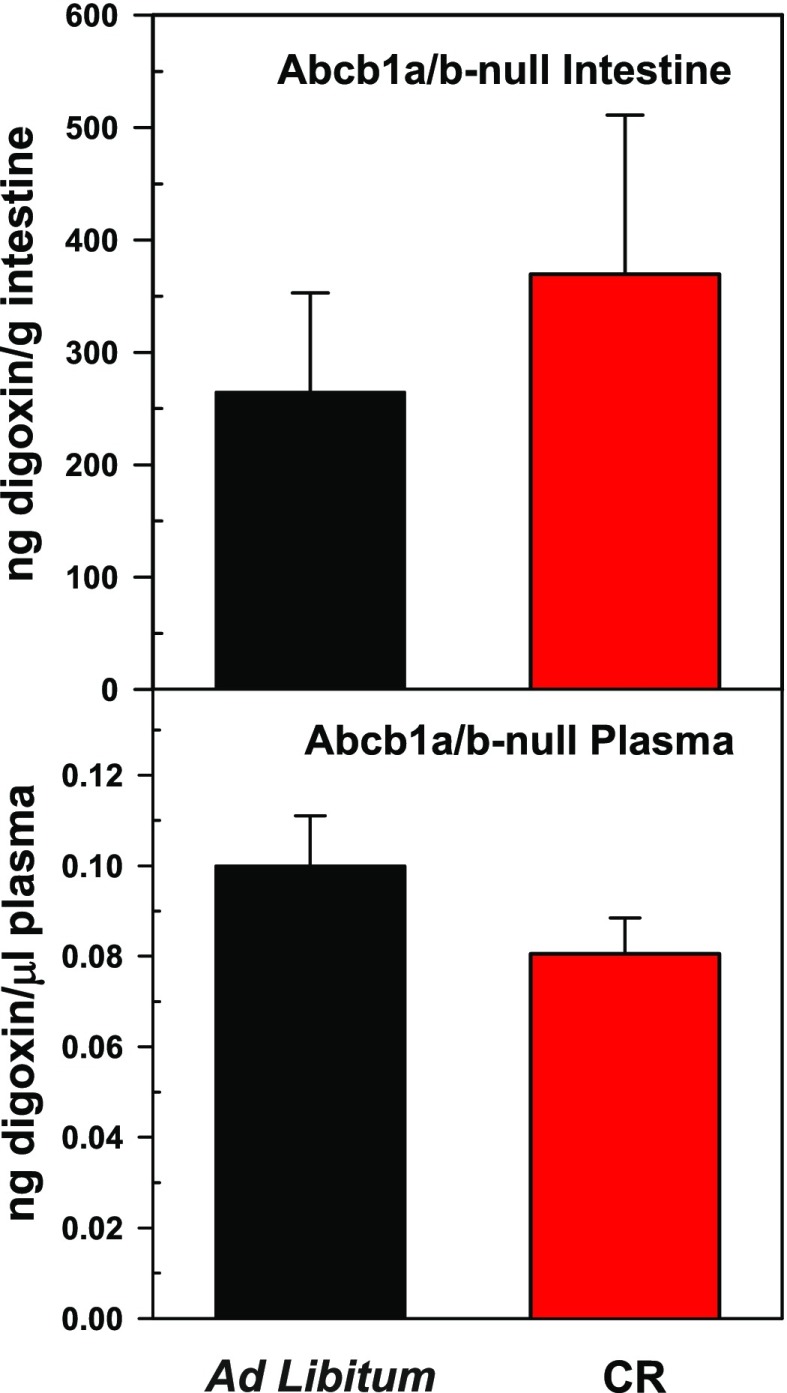
In mice fed ad libitum, compared with wild-type mice, Abcb1a/b-null mice had lower digoxin concentrations in their intestines (974 versus 265 ng digoxin/gram intestine) and higher digoxin concentrations in plasma (0.035 versus 0.100 mean ng digoxin/μl plasma) 4 hours after digoxin administration (Figs. 2 and 3). This result was as predicted because Abcb1a-null mice do not have the capacity to efflux digoxin via P-gp, thus resulting in increased digoxin absorption.
Fig. 3.
Mean concentrations of digoxin (± S.E.M.) 4 hours after administration in intestine and plasma of Abcb1a/b-null mice fed an ad libitum diet or calorie restriction (CR) (n = 5).
No statistical differences in the concentrations of digoxin in intestine or plasma were observed between ad libitum fed and calorie-restricted Abcb1a/b-null mice (Fig. 3). Thus, these results support the findings that decreased digoxin absorption in calorie-restricted wild-type mice is likely due to increased intestinal P-gp expression.
Discussion
In clinical practice, a challenging problem in drug therapy is individual variations in patient response. Environmental, genetic, and pathophysiologic factors are known contributors to variations in drug responses and adverse effects; however, little attention has been given to the impact of food and diet on drug disposition. Thus, the results in this study help further our knowledge of how diet impacts drug absorption. Specifically, the current work revealed that calorie restriction can increase the expression of intestinal Abcb1a and decrease drug absorption. Because Abcb1a transports a wide variety of therapeutics, these results in mice beg the question of whether this phenomenon might also occur in humans.
The gastrointestinal tract functions to digest and absorb nutrients from the diet. However, this organ is also exposed to ingested xenobiotics, and thus also functions as a defense barrier, expressing many metabolic enzymes and efflux transporters. Modulation of efflux transporter expression or function in the intestinal tract can lead to altered systemic and local xenobiotic concentrations (Murakami and Takano, 2008; Huang et al., 2010). The best characterized efflux transporter is P-gp. It is located apically on enterocytes and transports substrates back into the intestinal lumen, resulting in lowered systemic drug concentrations. Increased intestinal P-gp function or expression can markedly affect drug pharmacokinetics, leading to decreased therapeutic effect. In humans, intestinal MDR1 mRNA is inversely correlated with oral tacrolimus concentrations (Masuda et al., 2004), cyclosporine pharmacokinetics (Masuda and Inui, 2006), and talinolol pharmacokinetics (Bernsdorf et al., 2006). Additionally, xenobiotics that alter the function of P-gp can cause drug-drug interactions; for example, St. John’s Wort induces intestinal P-gp, leading to decreased talinolol area under the curve in human subjects (Schwarz et al., 2007). There are many known therapeutics that inhibit P-gp, leading to drug-drug interactions including cyclosporine A, ketoconazole, quinidine, ritonavir, verapamil, and reserpine, to name a few (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm). However, little is known about how nutrients in the diet or the amount of calories consumed affect P-gp activity or expression.
Factors in the diet currently suspected to affect P-gp activity include certain flavonoids. In vitro studies have shown that the flavonoids morin, biochanin A, silymarin, and phloretin all have the ability to increase intracellular daunomycin (a P-gp substrate) concentrations in human P-gp positive cells but not in P-gp negative cells. Furthermore, the increase in daunomycin accumulation by these flavonoids is dependent on both flavonoid concentration and P-gp expression, suggesting that these flavonoids inhibit P-gp activity (Zhang and Morris, 2003). Thus, one would anticipate that these flavonoids would increase the absorption of drugs; however, in vivo, biochanin A failed to alter the pharmacokinetics of orally administered P-gp substrates (Zhang et al., 2010). The authors conclude that the disconnect between the in vitro and in vivo data may be due to poor bioavailability and rapid clearance of biochanin A in vivo. It remains to be determined whether flavonoids have the ability to inhibit P-gp in vivo.
Grapefruit juice and orange juice are known to inhibit CYP3A4 and are also suspected to alter P-gp activity (reviewed in Won et al., 2010). Grapefruit juice is well known to increase plasma concentrations of drugs by decreasing CYP3A4, and possibly also by inhibiting P-gp and organic anion-transporting polypeptide activity. Thus, considering that many P-gp substrates are also CYP3A substrates, it is difficult to distinguish the contribution of each factor in grapefruit juice–induced altered drug pharmacokinetics.
The molecular mechanism of how calorie restriction causes an induction of P-gp remains unknown at the present time. In vitro studies using cells derived from intestinal tissues have indicated the pregnane-X receptor as a key player in drug-induced P-gp expression (Maier et al., 2007; Kim et al., 2015). For example, rifampin induces P-gp via casein kinase 2-mediated phosphorylation of heat shock protein 90β, and subsequent stabilization of the pregnane-X receptor (Kim et al., 2015). Thus, increased activation of the pregnane-X receptor might be a good candidate to investigate as a possible mechanism of calorie restriction–induced P-gp expression.
The present study used the P-gp substrate digoxin as an indicator of P-gp activity. This drug is commonly used to evaluate P-gp function in mice because the pharmacokinetic attributes of digoxin are highly P-gp dependent. Additionally, another advantage of using digoxin is that it is not significantly metabolized in mice (Schinkel et al., 1995, 1997; Mayer et al., 1997; Kawahara et al., 1999). Although it is possible that digoxin is transported by other transporters (Taub et al., 2011), our observation was that calorie-restricted Abcb1a/b-null mice did not have decreased plasma concentration or increased digoxin content in intestine compared with ad libitum Abcb1a/b-null mice. This suggests that the altered digoxin concentrations observed in calorie-restricted wild-type mice is most likely due to altered P-gp expression. Additionally, digoxin is almost exclusively excreted by the gut mucosa (Mayer et al., 1996), thus it is not likely that the decrease in plasma digoxin concentrations is due to altered P-gp expression in kidney or liver.
In rats, protein-calorie malnutrition suppresses the hepatic expression of P-gp, causing reduced canalicular excretion of the P-gp substrate daunomycin (Lee et al., 2003). These results are opposite what we observed in intestinal tissue in the present study and in hepatic tissue in the study by Renaud et al. (2014). However, these two studies are difficult to compare due to species and diet differences. The protein-calorie–restricted diet in the study by Lee et al. (2003) was iso-caloric to the control diet but contained only 20% of the protein (qualitative malnutrition). However, in the present study and in a previous study by Renaud et al. (2014), the calorie-restricted mice received 25% less of the entire diet compared with the amount consumed by mice fed ad libitum (quantitative malnutrition). Nevertheless, it is evident that the quantity and the composition of the diet are certainly capable of modulating P-gp expression in both rats and mice.
A calorie-restricted diet can arise out of choice (weight loss strategy) or from complications of an illness or drug therapy. Appetite loss leading to a calorie-restricted diet often occurs in cancer and AIDS patients, which is particularly relevant to this study because P-gp transports many chemotherapeutics as well as human immunodeficiency virus antiviral therapies. Thus, the results from this study showing that calorie restriction can alter the absorption of a P-gp substrate have implications for pharmacokinetic research and clinical practice.
In summary, this paper indicates that a calorie-restricted diet can affect the absorption of P-gp substrates in mice, and is probably due to induced expression of intestinal P-pg. This study further underscores the importance of evaluating the influence of diet on drug disposition. Determining how diet can influence drug pharmacokinetics is essential to improving our understanding of interindividual differences in response to therapeutic agents.
Acknowledgments
We thank Dr. Julia Cui for help with collecting tissues.
Abbreviations
- CYP
cytochrome P450
- P-gp
P-glycoprotein
- qPCR
quantitative polymerase chain reaction
Authorship Contributions
Participated in research design: Renaud, Klaassen, Csanaky.
Conducted experiments: Renaud, Csanaky.
Performed data analysis: Renaud, Csanaky.
Wrote or contributed to the writing of the manuscript: Renaud, Klaassen, Csanaky.
Footnotes
This work was supported by the National Institutes of Health [Grants ES09649 & ES025708].
These studies were presented as a lecture at the 54th Annual Meeting of the Society of Toxicology; 2015 Mar 23; San Diego, CA. The abstract was published in Toxicol Sci, 144, S1:12, 2015 (I.L.C., H.J.R., C.D.K.).
References
- Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, et al. (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlinger WG, Park GD, Spector R. (1985) The effect of dietary protein on the clearance of allopurinol and oxypurinol. N Engl J Med 313:771–776. [DOI] [PubMed] [Google Scholar]
- Bernsdorf A, Giessmann T, Modess C, Wegner D, Igelbrink S, Hecker U, Haenisch S, Cascorbi I, Terhaag B, Siegmund W. (2006) Simvastatin does not influence the intestinal P-glycoprotein and MPR2, and the disposition of talinolol after chronic medication in healthy subjects genotyped for the ABCB1, ABCC2 and SLCO1B1 polymorphisms. Br J Clin Pharmacol 61:440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullata JI, Hudson LM. (2012) Drug-nutrient interactions: a broad view with implications for practice. J Acad Nutr Diet 112:506–517. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. (1989) Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA 86:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop JM, Raymond M, Haber D, Devault A, Arceci RJ, Gros P, Housman DE. (1989) The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol 9:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YJ, Cheng X, Weaver YM, Klaassen CD. (2009) Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos 37:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir R, Buschman E, Gros P. (1990) Structural and functional characterization of the mouse multidrug resistance gene family. Bull Cancer 77:1125–1129. [PubMed] [Google Scholar]
- Gottesman MM, Pastan I. (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427. [DOI] [PubMed] [Google Scholar]
- Huang SM, Zhao H, Lee JI, Reynolds K, Zhang L, Temple R, Lesko LJ. (2010) Therapeutic protein-drug interactions and implications for drug development. Clin Pharmacol Ther 87:497–503. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Ling V. (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455:152–162. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Sakata A, Miyashita T, Tamai I, Tsuji A. (1999) Physiologically based pharmacokinetics of digoxin in mdr1a knockout mice. J Pharm Sci 88:1281–1287. [DOI] [PubMed] [Google Scholar]
- Kim SW, Hasanuzzaman M, Cho M, Heo YR, Ryu MJ, Ha NY, Park HJ, Park HY, Shin JG. (2015) Casein kinase 2 (CK2)–mediated phosphorylation of Hsp90β as a novel mechanism of rifampin-induced MDR1 expression. J Biol Chem 290:17029–17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Song IS, Kim SG, Lee MG, Chung SJ, Shim CK. (2003) The suppressed expression and functional activity of hepatic P-glycoprotein in rats with protein-calorie malnutrition. J Pharm Sci 92:1323–1330. [DOI] [PubMed] [Google Scholar]
- Maier A, Zimmermann C, Beglinger C, Drewe J, Gutmann H. (2007) Effects of budesonide on P-glycoprotein expression in intestinal cell lines. Br J Pharmacol 150:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Inui K. (2006) An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther 112:184–198. [DOI] [PubMed] [Google Scholar]
- Masuda S, Uemoto S, Goto M, Fujimoto Y, Tanaka K, Inui K. (2004) Tacrolimus therapy according to mucosal MDR1 levels in small-bowel transplant recipients. Clin Pharmacol Ther 75:352–361. [DOI] [PubMed] [Google Scholar]
- Mayer U, Wagenaar E, Beijnen JH, Smit JW, Meijer DK, van Asperen J, Borst P, Schinkel AH. (1996) Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdr1a P-glycoprotein. Br J Pharmacol 119:1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, Schinkel AH. (1997) Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. J Clin Invest 100:2430–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Takano M. (2008) Intestinal efflux transporters and drug absorption. Expert Opin Drug Metab Toxicol 4:923–939. [DOI] [PubMed] [Google Scholar]
- Ötles S, Senturk A. (2014) Food and drug interactions: a general review. Acta Sci Pol Technol Aliment 13:89–102. [PubMed] [Google Scholar]
- Raymond M, Rose E, Housman DE, Gros P. (1990) Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Mol Cell Biol 10:1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud HJ, Cui JY, Lu H, Klaassen CD. (2014) Effect of diet on expression of genes involved in lipid metabolism, oxidative stress, and inflammation in mouse liver—insights into mechanisms of hepatic steatosis. PLoS One 9:e88584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, Voordouw AC, Spits H, van Tellingen O, et al. (1997) Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci USA 94:4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. (1995) Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz UI, Hanso H, Oertel R, Miehlke S, Kuhlisch E, Glaeser H, Hitzl M, Dresser GK, Kim RB, Kirch W. (2007) Induction of intestinal P-glycoprotein by St John’s Wort reduces the oral bioavailability of talinolol. Clin Pharmacol Ther 81:669–678. [DOI] [PubMed] [Google Scholar]
- Seden K, Dickinson L, Khoo S, Back D. (2010) Grapefruit-drug interactions. Drugs 70:2373–2407. [DOI] [PubMed] [Google Scholar]
- Takano M, Yumoto R, Murakami T. (2006) Expression and function of efflux drug transporters in the intestine. Pharmacol Ther 109:137–161. [DOI] [PubMed] [Google Scholar]
- Taub ME, Mease K, Sane RS, Watson CA, Chen L, Ellens H, Hirakawa B, Reyner EL, Jani M, Lee CA. (2011) Digoxin is not a substrate for organic anion-transporting polypeptide transporters OATP1A2, OATP1B1, OATP1B3, and OATP2B1 but is a substrate for a sodium-dependent transporter expressed in HEK293 cells. Drug Metab Dispos 39:2093–2102. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84:7735–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. (1989) Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem 37:159–164. [DOI] [PubMed] [Google Scholar]
- Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, Riordan JR. (1986) The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun 141:956–962. [DOI] [PubMed] [Google Scholar]
- Won CS, Oberlies NH, Paine MF. (2010) Influence of dietary substances on intestinal drug metabolism and transport. Curr Drug Metab 11:778–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Morris ME. (2003) Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther 304:1258–1267. [DOI] [PubMed] [Google Scholar]
- Zhang S, Sagawa K, Arnold RD, Tseng E, Wang X, Morris ME. (2010) Interactions between the flavonoid biochanin A and P-glycoprotein substrates in rats: in vitro and in vivo. J Pharm Sci 99:430–441. [DOI] [PubMed] [Google Scholar]
- Zhou S, Lim LY, Chowbay B. (2004) Herbal modulation of P-glycoprotein. Drug Metab Rev 36:57–104. [DOI] [PubMed] [Google Scholar]