Abstract
The extensive use of organophosphates (OPs) is an ongoing environmental health concern due to multiple reports of OP-related neurologic abnormalities. The mechanism of the acute toxicity of OPs has been attributed to inhibition of acetylcholinesterase (AChE), but there is growing evidence that this may not account for all the long-term neurotoxic effects of OPs. In previous experiments (using ex vivo and in vitro model systems) we observed that the insecticide OP chlorpyrifos impaired the movements of vesicles and mitochondria in axons. Here, using a time-lapse imaging technique, we evaluated the OP-nerve agent diisopropylfluorophosphate (DFP) across a wide range of concentrations (subnanomolar to micromolar) for effects on fast axonal transport of membrane-bound organelles (MBOs) that contain the amyloid precursor protein (APP) tagged with the fluorescent marker Dendra2 (APPDendra2). Both 1 and 24 hours of exposure to DFP and a positive control compound, colchicine, resulted in a decrease in the velocity of anterograde and retrograde movements of MBOs and an increase in the number of stationary MBOs. These effects occurred at picomolar (100 pM) to low nanomolar (0.1 nM) concentrations that were not associated with compromised cell viability or cytoskeletal damage. Moreover, the effects of DFP on axonal transport occurred at concentrations that did not inhibit AChE activity, and they were not blocked by cholinergic receptor antagonists. Given the fundamental importance of axonal transport to neuronal function, these observations may explain some of the long-term neurologic deficits that have been observed in humans who have been exposed to OPs.
Introduction
The chemicals known as organophosphates (OPs) are used for a variety of important agricultural, industrial, and domestic purposes worldwide. However, the prevalence of OPs in the environment has become a public health concern given their toxicity and the number of accidental and intentional poisonings by OPs (e.g., from suicide attempts) (Eddleston et al., 2008). Exposure to OP-based nerve agents from rogue governments and terrorist organizations is an additional risk that was exemplified by the Iraqi military attacks on Kurdish civilians in the 1980s (Macilwain, 1993), the Tokyo sarin attack in 1995 by domestic terrorists (Nagao et al., 1997), and the recent sarin attacks on civilians in Syria (Sellström et al., 2013).
The mechanism of the acute toxicity of OPs is well established and attributed to the irreversible inhibition of acetylcholinesterase (AChE), which leads to elevations of synaptic acetylcholine and a variety of peripheral, autonomic, and central nervous system symptoms such as muscle weakness and fasciculations, vomiting, and seizures, collectively described as the “cholinergic crisis,” which can be life threatening (Ecobichon, 2001; Pereira et al., 2014). A variety of long-term neurologic consequences of acute poisonings with OPs have also been documented and include electroencephalogram abnormalities, mood disorders (e.g., anxiety and depression), deficits in psychomotor speed and coordination, and a variety of cognitive deficits (Brown and Brix, 1998; Miyaki et al., 2005; Pereira et al., 2014). A number of epidemiologic studies also suggest that exposures to OPs at levels not associated with acute symptoms of toxicity can result in long-term neurobehavioral abnormalities, especially cognitive abnormalities (e.g., deficits in attention, working memory, executive function, visuospatial ability and visual memory [Pope et al., 2005; Ross et al., 2013]).
Although AChE inhibition is clearly an important mechanism of the toxicity of OPs, it may not account for all the long-term neurologic alterations associated with these chemicals. The deleterious effects of OPs that may be additive (or unrelated) to AChE inhibition include oxidative stress, impairments of mitochondrial function, neuroinflammation, and altered neurotrophin responses (Soltaninejad and Abdollahi, 2009; Banks and Lein, 2012; Terry, 2012). For several years our laboratory has been investigating the possibility that OPs impair axonal transport, a potentially significant issue given the fundamental importance of axonal transport to neuronal maintenance and function.
The original impetus for this work was a report by Reichert and Abou-Donia (1980) that relatively high doses of certain OPs (phenylphosphonothioate esters and tri-o-cresyl phosphate) known to be associated with OP-induced delayed neuropathies impaired fast anterograde axonal transport in a rat optic nerve preparation. Later studies in our laboratories indicated that both anterograde and retrograde transport of vesicles in the sciatic nerves (ex vivo) was impaired in rats repeatedly exposed to chlorpyrifos (CPF) (14 total exposures), an OP not associated with OP-induced delayed neuropathies except at doses well above the LD50 (Richardson, 1995). Importantly, the doses used in our CPF study were below the threshold for acute toxicity; further, the deficits in axonal transport were detected for up to14 days after the last CPF injection, indicating that the impairments were persistent (Terry et al., 2003, 2007).
In a series of subsequent experiments using time-lapse imaging techniques, we also observed impairments in the movement of mitochondria in axons in primary neuronal culture (Middlemore-Risher et al., 2011) associated with both CPF and its metabolite CPF-oxon. The changes occurred at concentrations of CPF and CPF-oxon that did not inhibit AChE activity, they were not blocked by cholinergic receptor antagonists, and they did not appear to be associated with direct (OP-related) effects on mitochondrial viability or function (i.e., mitochondrial membrane potential or ATP production). Most recently, we observed (using a magnetic resonance imaging technique) that repeated exposures to doses of CPF that were below the threshold for acute toxicity led to prolonged impairments of axonal transport in the brains of living rodents (Hernandez et al., 2015).
The purpose of the experiments described here was to evaluate an OP of a different structural class, diisopropylfluorophosphate (DFP), for effects on axonal transport using a new in vitro model system (see Discussion for further details). DFP is an alkyl phosphorofluoridate originally synthesized by the German chemist Gerhard Schrader and later evaluated as a potential chemical warfare agent by the Germans, British, and Americans (Pope et al., 2005). It possesses a great deal of structural homology with other highly toxic nerve agents such as sarin and soman, but is less potent (Hobbiger, 1972) and dangerous for laboratory personnel. As a positive control for axonal transport deficits, the tropolone alkaloid colchicine was also evaluated in these studies.
Materials and Methods
Drugs
Atropine, colchicine (COL), mecamylamine, deuterium oxide (D2O), and DFP were obtained from Sigma-Aldrich (St. Louis, MO), stored as recommended by the source vendor, and stock solutions were prepared in deionized water. COL and DFP were prepared to use at the following final concentrations (in nM): 0.01, 0.1, 1.0, 10.0, 100.0, 1000.0, and 10000.0). Atropine and mecamylamine were prepared to use at 50.0 and 10.0 µM, respectively. All drug stock solutions were prepared at 100-fold higher concentrations in deionized water (≤5 [v/v] %, pH 7.0) within 15 minutes of the start of each 1- or 24-hour exposure period.
Embryonic Cortical Cultures
The cerebral cortices from E17-18 Sprague-Dawley rat embryos were extracted and cultured as described elsewhere (Gao et al., 2014) under aseptic conditions. Timed pregnant rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and housed and maintained on a 12-hour light/dark cycle in a temperature-controlled room (25°C) with free access to food and water for at least 3 days before initiating cultures. All procedures used during this study were reviewed and approved by the Georgia Regents University Institutional Animal Care and Use Committee and are consistent with the Association for Assessment and Accreditation of Laboratory Animal guidelines. Appropriate measures were taken to minimize pain or discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NRC, 2011).
Briefly, cortices were extracted and trypsinized (Trypsin-EDTA no. 25200; Life Technologies, Carlsbad, CA), then dissociated cellular material was seeded at a density of 5 × 105 cell/ml media onto poly-l-lysine (Sigma-Aldrich) coated glass coverslips (25 mm) and 96-well plates [for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell-proliferation assays]. For the AChE activity assays, cells were seeded at a density 1.1 × 106 cell/ml media on 10-cm Petri dishes. Cultures were maintained at 37°C in a 5.0% CO2 humidified atmosphere in Neurobasal (cat. no. 21103) supplemented with B27 (2.0 (v/v) %; no. 17504), Glutamax (0.5 M, cat. no. 35050), and penicillin–streptomycin (100 U/ml; cat. no. 15140-122). All culture media materials were purchased from Life Technologies (Grand Island, NY).
Cell Transfection and Treatments
All culture transfections were conducted at 37°C after 5 to 7 days in vitro with the amyloid precursor protein (APP) tagged with the fluorescent marker Dendra2 (APPDendra2), APPDendra2-cDNA (Magrane et al., 2012), and Lipofectamine 2000 (Life Technologies). All time-lapse imaging studies (as discussed later) were conducted 24 to 36 hours after transfection. Doses of COL or DFP employed for 1 hour before exposures were (in nM): 0.001, 0.01, 0.1, 1.0, 10.0, 100.0, 1000.0, or 10,000.0 and for 24 hours before exposures were (in nM): 0.01, 0.1, 1.0, 10.0, or 100.0. Cultures were treated along the same timeline with deionized water 5.0 (v/v) % as a control and are indicated as vehicle. Before all live imaging experiments, the culture medium was exchanged with phenol-free Neurobasal (no. 12348-017; Life Technologies).
Live Imaging and Measurements of Axonal Transport
Transfected cells were located in primary cortical cultures using an inverted epifluorescent microscope (Deltavision, Deconvolution Olympus IX71; Olympus, Bothell, WA) connected to a charge-coupled device camera (Photometrics CoolSNAP HQ2; Roper Scientific, Tucson, AZ). Axons successfully transfected with APPDendra2 were identified by their fluorescence and morphologic features (i.e., long neurites, constant thin diameter, no branching, perpendicular emergence from the cell body). Throughout the duration of the imaging session, cultures were maintained at 37°C under 5% CO2 conditions within an environmental (heat and mixed gas controller) chamber. Once the APPDendra2 transfected neurons were localized (under 60× magnification, 1.42 numerical aperture), the axons were video recorded and frames captured every 5 seconds for 5 minutes (SoftWoRx; Applied Precision, Issaquah, WA) to track the movements of dynamic particles and identify stationary particles. Using the National Institutes of Health ImageJ software (http://rsb.info.nih.gov/ij/) with input/output and kymograph plug-ins, images were compressed into audio video interleaved animation files.
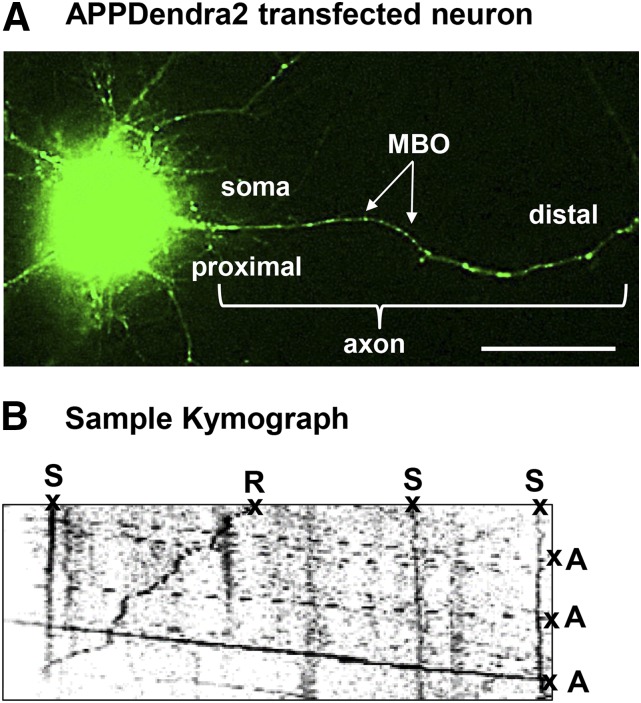
Briefly, kymographs (a graphic representation of a sample’s position versus time) were generated to analyze the nature of APPDendra2 particle transport and directionality (i.e., anterograde, retrograde, or stationary). Particle transport in the anterograde direction was identified by its movement away from the cell body, and transport in the retrograde direction was identified by its movement toward the cell body. Stationary particles were identified by the absence of all movement. The distance traveled by each particle was measured in micrometers (µm). Individual particle velocities were measured in µm per second during periods of sustained dynamic movement (i.e., ≥5 consecutive frames). Data were plotted as the ratio of the number of particles (retrograde, anterograde, or stationary) to the total number of analyzed particles. Figure 1 provides an example of an APPDendra2 transfected neuron and a representative kymograph.
Fig. 1.
Methods for assessing the effects of diisopropylfluorophosphate (DFP) on axonal transport in vitro. (A) Representative image demonstrating successful transfection of APPDendra2 in rat primary cortical neurons. Green fluorescent MBOs in the axon are indicated by the arrows. (B) Kymograph recorded at 5-second intervals for 5 minutes generated from APPDendra2-labeled MBOs after treatment with DFP. MBOs are categorized in one of three ways: anterograde (A), retrograde (R), or stationary (S). Velocity information was obtained from the slope of the lines. Scale bar = 20 µm.
Mecamylamine and Atropine Coincubation Experiments
The method described earlier for assessing axonal transport was also used to determine the effects of coincubation of DFP with the muscarinic antagonist atropine or the nicotinic antagonist mecamylamine. Specifically, cortical neurons were coincubated (for 24 hours) with either atropine (50 μM) or mecamylamine (10 μM) and a representative concentration of DFP shown to impair axonal transport (10 nM) in the first set of experiments (see Results). The representative concentrations of atropine or mecamylamine were based on previous in vitro studies (Middlemore-Risher et al., 2011).
Evaluation of Cultured Cell Viability and Toxicity
The concentration and time-dependent effects of COL or DFP on cell viability (total number of viable cells) were assessed using a 96-well plate format VybrantMTT Cell Proliferation Assay kit (Life Technologies) according to the manufacturer’s instructions. Cell viability measurements are reported as the percentage of viable cells in comparison with the vehicle-treated cultures (i.e., control). To measure cellular toxicity associated with COL or DFP, a Lactate Dehydrogenase Activity Assay Kit (lot B9B270726V; Sigma-Aldrich) was also used according to the manufacturer’s instructions. Data for both cell viability and toxicity assays are expressed as the percentage of output value (i.e., absorbance values) for COL or DFP-exposed compared with the output value of vehicle- or untreated cell cultures (i.e., control).
Immunocytochemistry.
Cultures (on 25-mm coverslips) were also processed to evaluate gross changes in cellular morphology and structure after both 1 (10.0 µM) or 24 hours (100.0 nM) of exposure to COL or DFP compared with vehicle-treated controls. Immediately after COL or DFP treatment, coverslips were washed 3 times in phosphate-buffered saline (PBS) sequentially decreasing in temperature from 37°C to 4°C, gradually fixed for 30 minutes in formalin (Thermo Fisher Scientific, Waltham, MA) (from 2.5% [v/v] to 5% [v/v] then a final 10% [v/v]) and thoroughly washed with PBS. To assess whole-cell and axon morphology, antibodies targeting microtubule-associated protein (MAP2A/2B, cat. no. MAB364; Millipore, Temecula, CA) and doublecortin (DCX; cat. no. 4604; Cell Signaling Technology, Danvers, MA) were used as somatodendritic and axonal markers, respectively.
Briefly, coverslips were blocked in goat (MAP2A/2B) or donkey (DCX) serum-based blocking buffer, in percentage (v/v): 10 serum, 1.0 bovine serum albumin, 0.1 Triton X-100, 0.1 cold fish gelatin, and 0.05 Tween-20 for 30 minutes at 25°C, then incubated in anti-MAP2A/2B (1:250) or DCX (1:400) overnight at 4°C. After the PBS washes, the ABC method was employed to amplify and detect the primary antibody signal, followed by streptavidin-conjugated AlexaFluor594 (Life Technologies/Molecular Probes, Eugene, OR). Coverslips were incubated for 2 hours at 25°C in Acti-stain 555 (Cytoskeleton, Denver, CO), a fluorophore-conjugated phalloidin, to assess cytoskeletal structure. All coverslips were mounted in 4ʹ,6-diamidino-2-phenylindole-mounting media (Vectashield; Vector Laboratories, Burlingame, CA) to counterstain nuclei. Using confocal microscopy, all fluorescent staining was localized and identified under low magnification (≤40×) followed by image acquisition at 63× magnification (Zeiss 780 upright; Zeiss, Thornwood, NY). Image processing (i.e., conversion between formats, background noise reduction, pseudo-coloring, etc.) was completed using ZEN (Zeiss), GIMP 2.8 (http://gimp.org), and Adobe Photoshop (Adobe Systems, San Jose, CA) softwares.
Preparation of Cell Lysates for AChE Activity Assays
After transfections and/or drug exposures, cultures were washed with PBS, transferred to a microtube after scraping from coverslips into PBS (pH 8.0) containing Triton X-100 (final concentration = 1.0 (v/v) %) (PBS-TX100), and stored at −80°C. Samples were solubilized in PBS-TX100 at 4°C for 2 hours with constant agitation. To remove cellular debris, samples were centrifuged at 13,400g for 1 minute at 4°C to clarify samples and collect the supernatant. The total protein for each supernatant collected was measured using a detergent-compatible protein assay (Pierce Micro BCA Protein Assay Kit; ThermoFisher Scientific Inc. Rockford, IL) according to the manufacturer’s instructions.
Measurement of Acetylcholinesterase Activity
Cell lysates (yielding a minimum of 8.0 μg/μl total protein) were assayed in duplicate to measure acetylcholinesterase (AChE) activity in samples after exposure to DFP. AChE activity was assessed using the Ellman method with modifications to accommodate a 96-well microplate format at 25°C (Prendergast et al., 2007; Middlemore-Risher et al., 2011). The specific concentrations used for each chemical (Sigma-Aldrich) in the reaction mixture prepared in 1.0 mM sodium phosphate buffer (pH 7.0 ± 0.05) were as follows (in mM): 0.48 acetylthiocholine, 7000 tetraisopropyl pyrophosphoramide (iso-OMPA, a butyrylcholinesterase inhibitor), and 0.52 mM 5,5′-dithiobis(2-nitrobenzoic acid). The formation of reaction product was monitored by measuring absorbance values at 412 nm every 2 minutes for 16 minutes (Mx Synergy Microplate Spectrophotometer; BioTek Instruments, Winooski, VT). The rate of AChE activity was then calculated for each time point of measurement using the formula (Change in absorbance/min)/(1.36 × 104), then normalized to the intraexperiment vehicle-treated control by exposure time (i.e., 1 or 24 hours).
Statistical Analyses
All statistical analyses were performed using SigmaPlot (Systat Software, San Jose, CA) Analysis of variance was used to compare the concentration-dependent effects of drug treatments to vehicle-treated controls, and the method of Holm-Sidak was used to examine post hoc differences when indicated. Statistical significance was assessed using an alpha level of 0.05. Values depicted in the figures reflect the mean ± S.E.M. The number of independent experiments conducted for each drug evaluation and the number of replicates per drug concentration are indicated in the figure legends.
Results
APPDendra2 Transfection and Live Imaging.
In the current study, we adapted and optimized previous methods for transfecting APPDendra into rat primary motor neuron cultures (Magrane et al., 2012) for APPDendra2 to rat primary cortical neuron cultures for live imaging. Twenty-four hours after transfection, the cultured neurons exhibited clear expression of APPDendra2 in the soma and axons (both of which were identified morphologically) (Fig. 1A). For imaging, individual APPDendra2-labeled membrane-bound organelles (MBOs) in axons were identified under lower magnification as distinct (green fluorescent) structures with a circular or tubular appearance (see the arrows in Fig. 1A). At higher magnification (63×), definitive proximal and distal axonal regions of each axon were identified, and the corresponding kymographs (see Fig. 1B for a sample) demonstrate that many MBOs were highly mobile, moving in both the anterograde and retrograde directions, while others remained stationary.
Effects of Colchicine and DFP on Cell Viability, Neuronal Morphology, and Cytoskeletal Integrity.
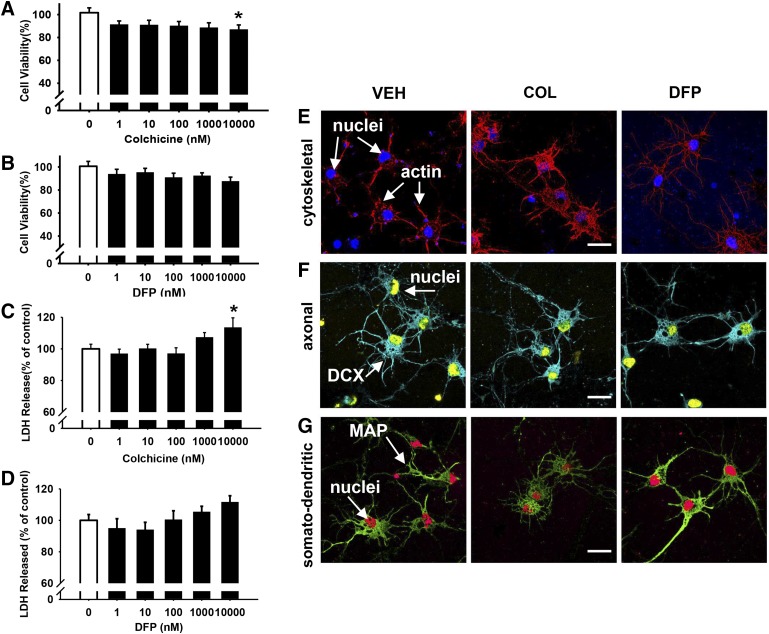
The effects of COL and DFP on cell viability (across the same range of concentrations that were later evaluated in the axonal transport studies) were initially assessed using an MTT assay. As shown in Fig. 2A, 1 hour of incubation with COL significantly decreased cell survival (by about 10%) only at the highest concentration that was evaluated (10 μM), while there were no significant effects of DFP at any of the concentrations that were evaluated (Fig. 2B). As a secondary method for evaluating the effects of COL and DFP on neuronal cell viability, an assay to measure lactate dehydrogenase (LDH) levels in the media was used.
Fig. 2.
One hour of exposure to colchicine (COL) but not diisopropylfluorophosphate (DFP) is associated with concentration-dependent impairments in cell viability. After an acute (1 hour) exposure to COL (A, C) or DFP (B, D), cell viability was measured by both MTT colorimetric and LDH release assays, presented as the percentage of cell survival or release, respectively, compared with vehicle-treated controls. Each bar represents the mean ± S.E.M. from two to three independent experiments. *P < 0.05 compared with vehicle control conditions. Representative immunocytochemistry images (pseudo-colored for contrast and to facilitate identifying stains in the figure and indicated structures) comparing the cellular morphology and structure after acute 10 μM COL or DFP exposure. (E) Fluorophore-conjugated actin (Acti-stain 555, red) and nuclei (blue). (F) Antidoublecortin (DCX, axon, blue) and nuclei (yellow). (G) Antimicrotubule-associated protein 2 (MAP2, somatodendritic, green) and nuclei (red). Scale bar = 10 µm.
Compared with the control group treated with vehicle only, 1 hour of incubation with COL did not significantly increase LDH release except at the highest concentration evaluated (10 μM) where it increased by about 15% (Fig. 2C). Conversely, 1 hour of exposure to DFP was not associated with significant increases in LDH release at any of the concentrations that were evaluated (Fig. 2D). Visual (qualitative) analyses of the immunocytochemistry images (actin, DCX, and MAP; see Fig. 2, E–G) revealed no overt alterations in cellular morphology or structure after 1 hour of exposure to 10 µM of COL or DFP.
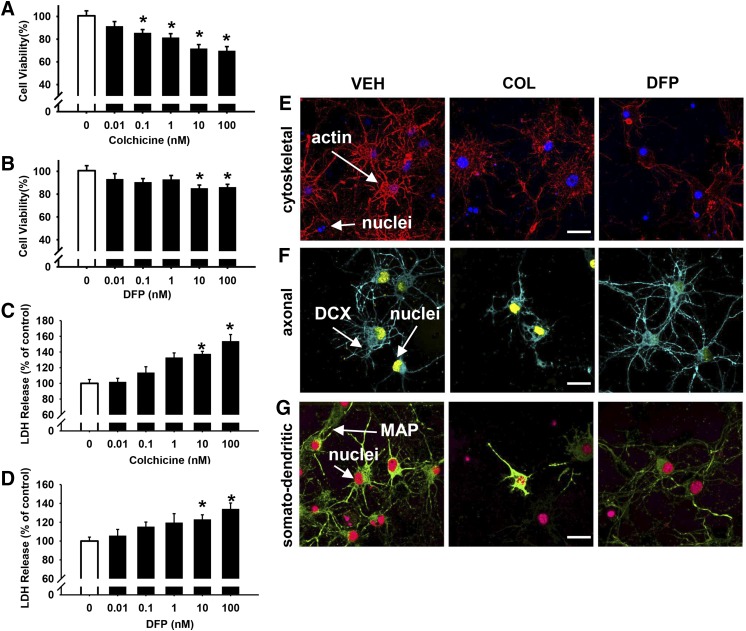
Exposure to concentrations of COL of 0.1 nM and above for 24 hours were associated with modest impairments in cell viability in the MTT assay (e.g., ∼30% decrease in viability at 100 nM; see Fig. 3A). Likewise, LDH levels were increased at the 10 and 100 nM concentrations of COL (see Fig. 3C). In the DFP (24-hour exposure) experiments, the two highest concentrations (10 and 100 nM) were also associated with modest impairments in cell viability (by approximately 15% to 20%) as determined in the MTT assay (Fig. 3B). Similar to COL, LDH levels were also increased at the 10 and 100 nM concentrations of DFP (see Fig. 3D). Visual analyses of the immunocytochemistry images (actin, DCX, and MAP; Fig. 3, E–G) revealed some alterations in the cellular morphology and structure after 24 hours of exposure to 10 µM COL or DFP.
Fig. 3.
Twenty-four hours of exposure to colchicine (COL) and diisopropylfluorophosphate (DFP) are associated with concentration-dependent impairments in cell viability. After 24 hours of exposure to COL (A, C) or DFP (B, D), cell viability was measured by both MTT colorimetric and LDH release assays, presented as the percentage of cell survival or release, respectively, compared with vehicle-treated controls. Each bar represents the mean ± S.E.M. (n = 2 to 3 independent experiments). *P < 0.05 compared vehicle control conditions. Representative immunocytochemistry images (pseudo-colored) for contrast and to facilitate identifying stains in the figure and indicated structures) comparing the cellular morphology and structure after 24 hours of exposure to 10 μM COL or DFP. (E) Fluorophore-conjugated actin (Acti-stain 555, red) and nuclei (blue). (F) Antidoublecortin (DCX, axon, blue) and nuclei (yellow). (G) Antimicrotubule-associated protein 2 (MAP2, somatodendritic, green) and nuclei (red). Scale bar = 10 µm.
Colchicine Impairs APP Axonal Transport.
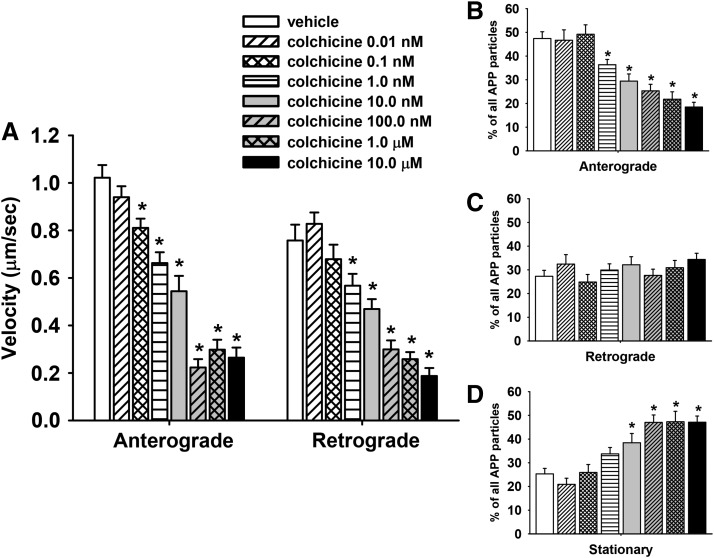
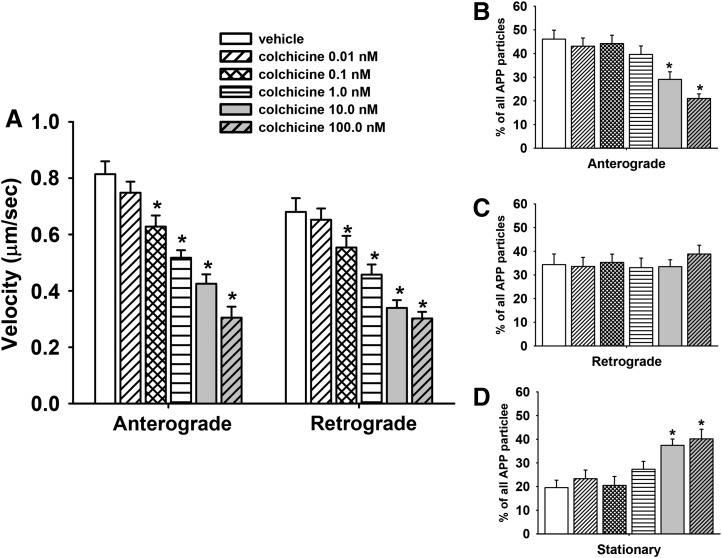
To validate the methods used for measuring fast axonal transport in primary cortical neurons, we evaluated COL, a compound known to impair tubulin polymerization and to impair fast axonal transport in multiple model systems (see further details in Discussion). Kymograph analysis revealed that, after 1 hour of exposure to COL, there was a significant (P ≤ 0.05) decrease in the velocity of APP transport in both the anterograde and retrograde direction associated with most of the doses (0.1 nM–10μM) that were evaluated (Fig. 4A). Notably, compared with the vehicle-control group, COL at concentrations as low as 0.1 nM significantly (P < 0.05) decreased MBO velocity (by ∼21%) in the anterograde direction.
Fig. 4.
Colchicine impairs axonal transport in primary cortical neurons after 1 hour of exposure. (A) Velocities of APP containing MBOs in the anterograde and retrograde direction; n (moving events) = 116. (B–D) Percentage of mobile (anterograde or retrograde) and stationary APP particles in segments of control and colchicine-treated axons; n (axonal segments) = 38. Each bar represents the mean ± S.E.M. from four independent experiments and four to five replicates per concentration. *P < 0.05 compared with vehicle control conditions.
Representative time lapse images of single axons exposed to vehicle or COL 10.0 nM for 1 hour are provided in (Supplemental Movies 1 and 2, respectively. The reduction in velocity was accompanied by a concentration-dependent decrease in the overall percentage of MBOs moving in the anterograde direction (Fig. 4B) and an increase in the percentage of stationary MBOs (Fig. 4D). Interestingly, COL did not significantly affect the overall percentage of APP particles moving in retrograde direction (Fig. 4C).
Subsequent experiments were conducted to determine whether the deficits in transport associated with COL occurred after a longer exposure period (24 hours). The same general trend of effects were observed: concentration-dependent decreases in the velocity of anterograde and retrograde transport of MBOs (Fig. 5A), a decrease in the overall percentage of particles moving in the anterograde (but not retrograde) direction (Fig. 5, B and C, respectively), and an increase in the overall percentage of stationary particles (Fig. 5D). Here it is important to note that axonal transport measurements were conducted only in neurons that appeared healthy with moving MBOs, an important consideration for the higher concentrations of COL (and DFP) at the 24-hour time point where some evidence of compromised cell viability was detected (as noted earlier).
Fig. 5.
Colchicine impairs axonal transport in primary cortical neurons after 24 hours of exposure. (A) Velocities of APP containing MBOs in the anterograde and retrograde direction; n (moving events) = 91. (B–D) Percentage of mobile (anterograde or retrograde) and stationary APP particles in segments of control and colchicine-treated axons; n (axonal segments) = 33. Each bar represents the mean ± S.E.M. from three independent experiments and four to five replicates per concentration. *P < 0.05 compared with vehicle control conditions.
DFP Impairs APP Axonal Transport.
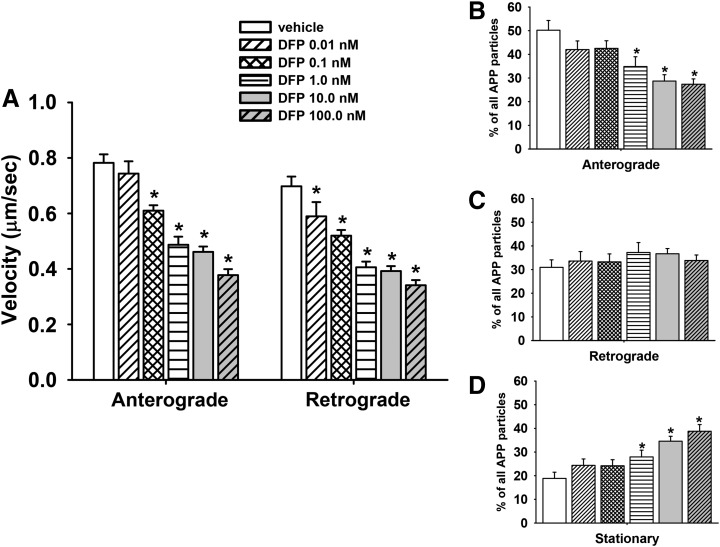
Kymograph analysis indicated that 1 hour of exposure to DFP (similar to COL) resulted in a concentration-dependent decrease in the velocity of APP transport in both the anterograde and retrograde directions. Concentrations of DFP as low as 0.1 nM resulted in a ∼20% decrease MBO velocity in the anterograde direction and ∼30% decrease in velocity in the retrograde direction (Fig. 6A). A representative time-lapse image of a single axon exposed to DFP 10.0 nM for 1 hour is provided in (Supplemental Movie 3).
Fig. 6.
Diisopropylfluorophosphate (DFP) impairs axonal transport in primary cortical neurons after 1 hour of exposure. (A) Velocities of APP containing MBOs in the anterograde and retrograde direction; n (moving events) = 235. (B–D) Percentage of mobile (anterograde or retrograde) and stationary APP particles in segments of control and colchicine-treated axons; n (axonal segments) = 52. Each bar represents the mean ± S.E.M. from four independent experiments and four to five replicates per concentration. *P < 0.05 compared with vehicle control conditions.
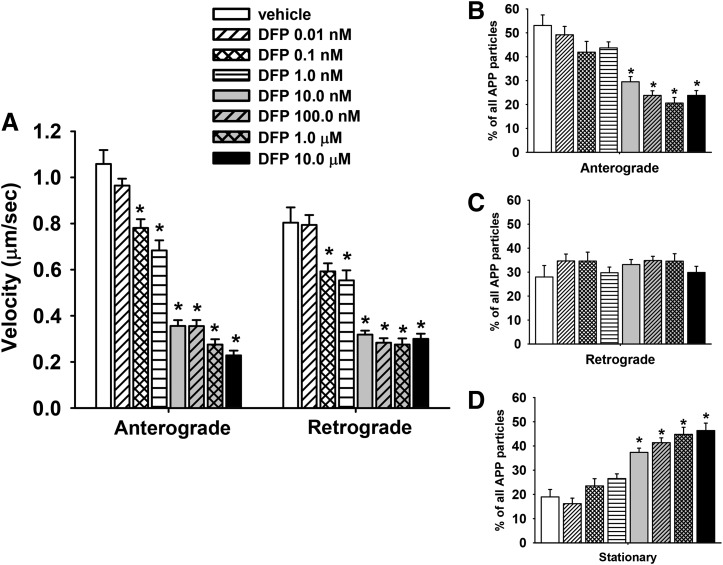
Like COL, DFP exposure resulted in a decrease in the overall percentage of particles moving in the anterograde (but not retrograde) direction (Fig. 6, B and C, respectively), and an increase in the overall percentage of stationary particles (Fig. 6D). Subsequent experiments were also conducted to determine whether the deficits in transport associated with DFP at 1 hour of exposure occurred after a longer exposure period (24 hours). Again, the same general trend of effects were observed: concentration-dependent decreases in the velocity of anterograde and retrograde transport of MBOs (Fig. 7A), a decrease in the overall percentage of particles moving in the anterograde (but not retrograde) direction (Fig. 7, B and C, respectively), and an increase in the overall percentage of stationary particles (Fig. 7D).
Fig. 7.
Diisopropylfluorophosphate (DFP) impairs axonal transport in primary cortical neurons after 24 hours of exposure. (A) Velocities of APP containing MBOs in the anterograde and retrograde direction; n (moving events) = 90. (B–D) Percentage of mobile (anterograde or retrograde) and stationary APP particles in segments of control and colchicine-treated axons; n (axonal segments) = 36. Each bar represents the mean ± S.E.M. from three independent experiments and four to five replicates per concentration. *P < 0.05 compared with vehicle control conditions.
Muscarinic and Nicotinic Antagonists Do Not Affect DFP-Related Impairments in Axonal Transport.
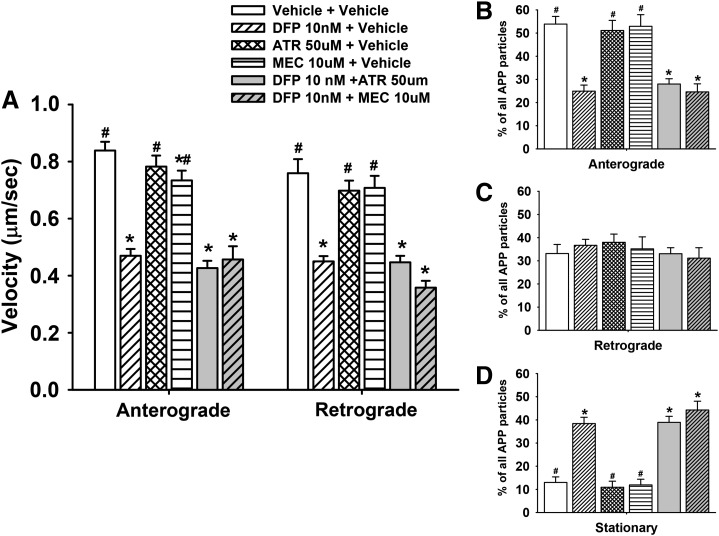
Additional experiments were also conducted (at the 24-hour exposure period) to determine whether either the muscarinic acetylcholine receptor antagonist atropine or the nicotinic acetylcholine receptor antagonist mecamylamine would affect the DFP-related impairments of axonal transport. The results of these experiments are illustrated in Fig. 8, and they clearly show that the DFP-related deficits in anterograde and retrograde axonal transport persisted in the presence of either atropine or mecamylamine (Fig. 8A). Likewise, neither atropine nor mecamylamine changed the DFP-related effects on the number of moving (Fig. 8, B and C) or stationery particles (Fig. 8D). Moreover, neither atropine nor mecamylamine (when administered alone) affected anterograde or retrograde axonal transport (Fig. 8A) or the number of moving or stationary particles (Fig. 8, B–D).
Fig. 8.
Muscarinic and nicotinic antagonists do not affect diisopropylfluorophosphate (DFP)-related impairments in axonal transport in primary cortical neurons after 24 hours of exposure. (A) Velocities of APP containing MBOs in the anterograde and retrograde direction; n (moving events) = 90. (B–D) Percentage of mobile (anterograde or retrograde) and stationary APP particles in segments of control and colchicine-treated axons; n (axonal segments) = 36. Each bar represents the mean ± S.E.M. from two independent experiments and four to five replicates per concentration. ATR, atropine; MEC, mecamylamine. *P < 0.05 compared with vehicle control conditions. #P < 0.05 compared with DFP 10 nM + vehicle conditions.
Effects of DFP on AChE Activity.
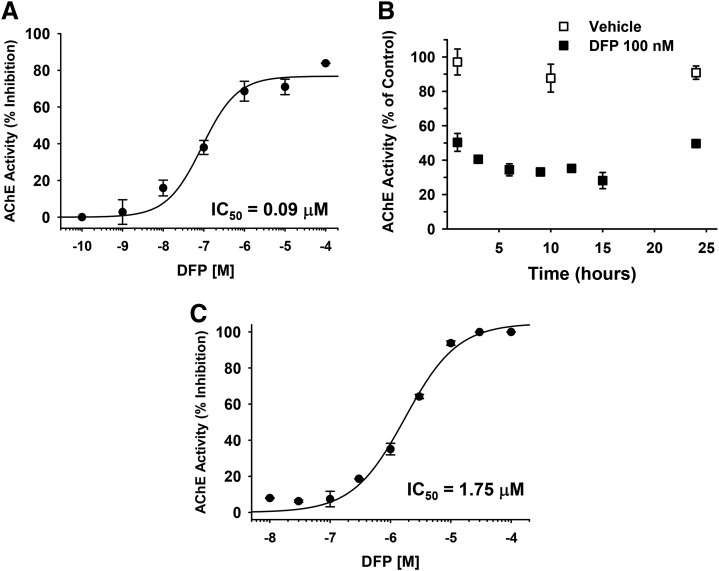
The effects of DFP on AChE activity in vitro are shown in Fig. 9. In Fig. 9A, the effects of 1 hour of exposure to DFP on neuronal cell lysates across the range of concentrations evaluated in the axonal transport studies indicate that the IC50 was approximately 90 nM and that the threshold for inhibition was likely to be somewhere just below 10 nM. In Fig. 9B we evaluated a concentration of DFP that was close to the IC50 (100 nM) across a time course that ranged from 1 to 24 hours. Although there was some variation in activity, the stability of DFP (as indicated by its ability to decrease AChE activity) was retained for up to 24 hours.
Fig. 9.
Effects of diisopropylfluorophosphate (DFP) on acetylcholinesterase (AChE) activity in vitro. (A) The effects of 1 hour of exposure to DFP on AChE activity in neuronal cell lysates across the range of concentrations evaluated in the axonal transport studies. (B) Effects of DFP (100 nM) on AChE activity in neuronal cell lysates across a time course that ranged from 1–24 hour. (C) Effects of DFP across a wide range of concentrations on purified eel AChE. Each symbol represents the mean ± S.E.M. from two independent experiments and four replicates per concentration.
For comparison purposes, we also evaluated a wide range of concentrations of DFP in a well-established assay in our laboratory using purified eel AChE. Here (Fig. 9C) the IC50 for DFP was considerably higher (∼1.75 μM) when compared with neuronal cell lysates. Likewise, the threshold for inhibition was considerably higher (i.e., between 0.1 and 0.3 μM).
This difference in the effects of DFP in the two assays may be a function the relatively low levels of AChE in embryonic cortex. For example, Thomas (1985) reported that the percentage of AChE-positive neurons in E15 rat cortex (cells cultured for 2 weeks) was approximately 2%. As noted earlier, our cultures were prepared from E17-18 rat cortex and (cells cultured for 5 to 7 days before transfection and subsequent OP studies at days 8 to 9).
Discussion
The major findings of this study can be summarized as follows: 1) Using a novel in vitro model system and time-lapse imaging techniques, we observed that 1 and 24 hours of exposure to the nerve agent DFP resulted in a concentration-dependent decrease in the anterograde and retrograde transport of MBOs in cortical axons. 2) The neuronal changes occurred at concentrations of DFP that did not inhibit AChE activity and were below the threshold for neurotoxicity (as would be suggested by comprised cell viability or evidence of cytoskeletal damage), and they were not blocked by cholinergic receptor antagonists. The later observation further supports the argument that the DFP effects on axonal transport are not directly related to AChE inhibition and consequent elevations in synaptic levels of acetylcholine. Moreover, DFP was found to be stable in the buffers used in the culture conditions as indicated in nuclear magnetic resonance studies (see (Supplemental Methods and Results, and Supplemental Figure 1) as well as the AChE activity assays where a DFP concentration close to the IC50 retained its inhibitory activity up to 24 hours.
The new method used in this study to measure axonal transport employs time-lapse microscopy techniques to measure the trafficking of MBOs containing a transfected fluorophore-tagged amyloid precursor protein (APP) cDNA construct (Magrane et al., 2012). This particular cDNA construct, referred to as APPDendra2, is derived from the APP695 isoform, which has several desirable properties for in vitro studies such as those described in this report. For example, it is known to be expressed in the human brain, it is preferentially expressed in neurons (as opposed to astrocytes) (Rohan de Silva et al., 1997), and it is highly expressed in cholinergic forebrain neurons (Harkany et al., 2002). In addition, APP is well documented to travel in neurons by fast axonal transport (Koo et al., 1990; Sisodia et al., 1993) in a process that is dependent on conventional kinesin (Amaratunga et al., 1993; Ferreira et al., 1993; Buxbaum et al., 1998). To visualize the transport of APP in neuronal axons in culture, we transfected rat cortical neurons with a plasmid encoding APP695 tagged with Dendra2. Dendra2 provides a unique combination of advantageous properties, including a monomeric state suitable for protein labeling and efficient chromophore maturation at 37°C in mammalian cells. These properties make Dendra2 an ideal tool for tracking the movements of proteins labeled with Dendra2 in real time.
The velocity of APP movements in cortical axons observed in this study (under control conditions for the 1-hour drug exposure experiments) was similar to that described by Magrane et al. (2012) for motor neurons (0.5–0.7 µm/s in anterograde and 0.4–0.5µm/s in retrograde direction). In our current study and the previous Magrane study, axonal transport assessments were made 24 hours after transfection with Lipofectamine 2000 (Magrane et al., 2012). Compared with the 24-hour transfection, however, the APP particle velocity was decreased by about 20% after the 48-hour transfection (∼0.4 µm/s), a time period necessary to accommodate 24-hour drug exposure experiments.
The initial experiments with COL were conducted to confirm our ability to use this tracking method for detecting fast axonal transport deficits associated with drug exposure. COL is a tropolone alkaloid that binds tightly to tubulin thus impairing tubulin polymerization and the assembly of microtubules. The consequent disruption of microtubule dynamics impairs the ability of motor proteins to transport cargo in axons (Hastie, 1991; Uppuluri et al., 1993; Han et al., 1998). As described in Results, the use of colchicine as a positive control was validated. Specifically, exposure to COL for 1 or 24 hours was associated with a concentration-dependent impairment in the velocity of APPDendra2-labeled MBOs both in the anterograde and retrograde direction, and these effects were accompanied by an increase in the percentage of stationary MBOs. Although there was little evidence that COL exposure for 1 hour was associated with compromises in cell viability, morphology, or cytoskeletal integrity, there was some evidence of compromised cell viability after 24 hours. The results of the DFP experiments followed a similar pattern as was observed with COL across all of the experiments, except that DFP was not as toxic to the neurons.
The molecular mechanisms underlying the COL-related impairments in axonal transport are assumed to involve impairments in tubulin polymerization and consequent microtubule disruption (as described earlier), but the mechanism of the DFP effects are unclear. However (like COL), some OPs were observed to impair tubulin polymerization in previous studies. For example, using a spectrophotometric method, Prendergast et al. (2007) demonstrated that chlorpyrifos-oxon inhibited the polymerization of tubulin, and (using organotypic slice cultures of rodent brain and histologic methods) caused a marked decrease in the concentration of microtubule associated protein-2. Moreover, using atomic force microscopy, Lockridge and colleagues observed that chlorpyrifos-oxon disrupted tubulin polymerization, and further (using mass spectrometry) that chlorpyrifos-oxon covalently binds to tubulin, an effect that may explain the disruptions in tubulin polymerization (Grigoryan and Lockridge, 2009; Jiang et al., 2010).
An alternative (or perhaps complementary) hypothesis is that OPs like DFP might (in some manner) alter the function of motor proteins such as kinesin and dynein to impair axonal transport (Terry, 2012). The hypothesis that OPs negatively affect kinesin-driven axonal transport is supported by our previous studies as well those of as other laboratories. Specifically, using in vitro microtubule motility assays, we observed an increase in the number of locomoting microtubules that detached from kinesin-coated glass when kinesin was preincubated with the OPs chlorpyrifos, chlorpyrifos-oxon, or DFP (Gearhart et al., 2007). These data suggested that OPs might covalently modify kinesin, thereby weakening the kinesin-microtubule interactions that are necessary for anterograde axonal transport. This hypothesis was supported by another study where (using the biotin-tagged OP agent FP-biotin) OP binding to tyrosine in the human kinesin 3C motor domain was demonstrated (Grigoryan et al., 2009). Our observation that retrograde (as well as anterograde) axonal transport was impaired by DFP suggests that the retrograde motor protein dynein might also be (in some manner) affected by OPs. To our knowledge no studies have (to date) addressed this possibility.
It is difficult to make causal connections between these observations with in vitro models and the wide variety of long-term neurologic symptoms that have been associated with OP exposure in humans, but OP-related effects on axonal transport may represent one attractive hypothesis. Axonal transport is an essential process in neurons that is responsible for the movement of a variety of important macromolecules (e.g., mitochondria, receptor proteins, growth factors) to and from a neuron’s cell body (reviewed by Duncan and Goldstein [2006]); further, impairments in axonal transport have been implicated in the pathology of a wide variety of neurologic illnesses, such as amyotrophic lateral sclerosis, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, Pick’s disease, and progressive supranuclear palsy (see Stokin and Goldstein [2006] for review). It is noteworthy that many of these illnesses are characterized by similar neurobehavioral deficits that have been observed in people who have been exposed to OP-based pesticides.
Interestingly, among the potential contributing factors to Gulf War illness, which is characterized by multiple neurologic and neurobehavioral symptoms, exposures to OP-based insecticides and nerve agent OPs (after the destruction of an Iraqi munitions storage complex at Khamisiyah, Iraq, in March 1991) have been implicated (RAC, 2014). It is also important to note there is a small but growing body of literature to suggest that OP exposure may even represent a potential risk factor for Alzheimer’s disease as well as some of the other neurodegenerative disorders mentioned previously (Hancock et al., 2008; Hayden et al., 2010; Zaganas et al., 2013).
The potential effects of OPs on molecules that are directly involved in axonal transport (e.g., kinesin, tubulin) may be added to the growing list of noncholinesterase targets for OPs, which now includes a variety of esterase and nonesterase enzymes, neurotransmitter receptors, and elements of cell signaling pathways including carboxylesterase, acylpeptide hydrolase, adenylyl cyclase, neuropathy target esterase, muscarinic receptors, cannabinoid receptors, albumin, transferrin, and ATP synthase (Duysen et al., 2001; Casida and Quistad, 2005; LoPachin and DeCaprio, 2005; Terry, 2012). Importantly, it has been suggested that interactions of OPs with such noncholinesterase targets may contribute to the more delayed and persistent effects observed after chronic exposure to OPs (Lotti and Moretto, 2005; Costa, 2006).
In conclusion, the results of this in vitro study suggest that one underlying mechanism of the wide variety OP-based neurologic deficits that have been reported might involve alterations in axonal transport. Future studies designed to determine the molecular basis of this effect of OPs may lead to the development of therapeutic strategies for the neurologic deficits associated with OP exposure.
Supplementary Material
Acknowledgments
The authors thank Ashley Davis for administrative assistance in preparing this article.
Abbreviations
- AChE
acetylcholinesterase
- APP
amyloid precursor protein
- APPDendra2
amyloid precursor protein tagged with Dendra2
- COL
colchicine
- CPF
chlorpyrifos
- DCX
doublecortin
- DFP
diisopropylfluorophosphate
- LDH
lactate dehydrogenase
- MAP
microtubule-associated protein
- MBO
membrane-bound organelle
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OP
organophosphate
- PBS
phosphate-buffered saline
Authorship Contributions
Participated in research design: Hernandez, Singh, Terry, Thomas, Wulff.
Conducted experiments: Beck, Gao, Hernandez, Kaidery, Naughton, Singh.
Contributed new reagents or analytic tools: Magrane.
Performed data analysis: Gao, Hernandez, Singh, Terry.
Wrote or contributed to the writing of the manuscript: Gao, Hernandez, Terry, Wulff.
Footnotes
This work was supported by the National Institutes of Health Institute of Environmental Health Sciences [Grant ES012241], the Congressionally Directed Medical Research Programs, specifically, the Gulf War Illness Research Program [Grant W81XWH-12-1-0536, the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurologic Disorders and Stroke [Grant U54NS079202].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Amaratunga A, Morin PJ, Kosik KS, Fine RE. (1993) Inhibition of kinesin synthesis and rapid anterograde axonal transport in vivo by an antisense oligonucleotide. J Biol Chem 268:17427–17430. [PubMed] [Google Scholar]
- Banks CN, Lein PJ. (2012) A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 33:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Brix KA. (1998) Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J Appl Toxicol 18:393–408. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Thinakaran G, Koliatsos V, O’Callahan J, Slunt HH, Price DL, Sisodia SS. (1998) Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J Neurosci 18:9629–9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. (2005) Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact 157-158:277–283. [DOI] [PubMed] [Google Scholar]
- Costa LG. (2006) Current issues in organophosphate toxicology. Clin Chim Acta 366:1–13. [DOI] [PubMed] [Google Scholar]
- Duncan JE, Goldstein LS. (2006) The genetics of axonal transport and axonal transport disorders. PLoS Genet 2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, Lockridge O. (2001) Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther 299:528–535. [PubMed] [Google Scholar]
- Ecobichon DJ. (2001) Pesticide use in developing countries. Toxicology 160:27–33. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Buckley NA, Eyer P, Dawson AH. (2008) Management of acute organophosphorus pesticide poisoning. Lancet 371:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Caceres A, Kosik KS. (1993) Intraneuronal compartments of the amyloid precursor protein. J Neurosci 13:3112–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Adam B-L, Terry AV., Jr (2014) Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer’s disease. Bioorg Med Chem Lett 24:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr (2007) Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol 218:20–29. [DOI] [PubMed] [Google Scholar]
- Grigoryan H, Li B, Anderson EK, Xue W, Nachon F, Lockridge O, Schopfer LM. (2009) Covalent binding of the organophosphorus agent FP-biotin to tyrosine in eight proteins that have no active site serine. Chem Biol Interact 180:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Lockridge O. (2009) Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: implications for neurotoxicity. Toxicol Appl Pharmacol 240:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Malak H, Chaudhary AG, Chordia MD, Kingston DG, Bane S. (1998) Distances between the paclitaxel, colchicine, and exchangeable GTP binding sites on tubulin. Biochemistry 37:6636–6644. [DOI] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. (2008) Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Varga C, Grosche J, Mulder J, Luiten PG, Hortobágyi T, Penke B, Härtig W. (2002) Distinct subsets of nucleus basalis neurons exhibit similar sensitivity to excitotoxicity. Neuroreport 13:767–772. [DOI] [PubMed] [Google Scholar]
- Hastie SB. (1991) Interactions of colchicine with tubulin. Pharmacol Ther 51:377–401. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Norton MC, Darcey D, Ostbye T, Zandi PP, Breitner JC, Welsh-Bohmer KA, Cache County Study Investigators (2010) Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology 74:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Beck WD, Naughton SX, Poddar I, Adam BL, Yanasak N, Middleton C, Terry AV., Jr (2015) Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. Neurotoxicology 47:17–26. [DOI] [PubMed] [Google Scholar]
- Hobbiger F. (1972) Chemotherapy in pesticide poisoning, in Toxicology, Biodegradation and Efficacy of Livestock Pesticides (Kahn MA, Haufe WO. eds) pp 252–281, Swets & Zeitlinger, Amsterdam. [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. (2010) Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci 115:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. (1990) Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA 87:1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopachin RM, Decaprio AP. (2005) Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicol Sci 86:214–225. [DOI] [PubMed] [Google Scholar]
- Lotti M, Moretto A. (2005) Organophosphate-induced delayed polyneuropathy. Toxicol Rev 24:37–49. [DOI] [PubMed] [Google Scholar]
- Macilwain C. (1993) Study proves Iraq used nerve gas. Nature 363:3. [DOI] [PubMed] [Google Scholar]
- Magrané J, Sahawneh MA, Przedborski S, Estévez AG, Manfredi G. (2012) Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. J Neurosci 32:229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Adam BL, Lambert NA, Terry AV., Jr (2011) Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. J Pharmacol Exp Ther 339:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto Y, Kikuchi Y, Kumagai N, et al. (2005) Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health 47:299–304. [DOI] [PubMed] [Google Scholar]
- Nagao M, Takatori T, Matsuda Y, Nakajima M, Iwase H, Iwadate K. (1997) Definitive evidence for the acute sarin poisoning diagnosis in the Tokyo subway. Toxicol Appl Pharmacol 144:198–203. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) (2011) Guide for the Care and Use of Laboratory Animals. 8th ed National Academies Press, Washington, DC, http://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf. [Google Scholar]
- Pereira EF, Aracava Y, DeTolla LJ, Jr, Beecham EJ, Basinger GW, Jr, Wakayama EJ, Albuquerque EX. (2014) Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. J Pharmacol Exp Ther 350:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C, Karanth S, Liu J. (2005) Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol 19:433–446. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr (2007) Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience 146:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Advisory Committee on Gulf War Veterans’ Illnesses (RAC) (2014) Gulf War Illness and the Health of Gulf War Veterans: Research Update and Recommendations, 2009–2013. U.S. Department of Veterans Affairs, Washington, DC. http://www.va.gov/RAC-GWVI/RACReport2014Final.pdf. [Google Scholar]
- Reichert BL, Abou-Donia MB. (1980) Inhibition of fast axoplasmic transport by delayed neurotoxic organophosphorus esters: a possible mode of action. Mol Pharmacol 17:56–60. [PubMed] [Google Scholar]
- Richardson RJ. (1995) Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature. J Toxicol Environ Health 44:135–165. [DOI] [PubMed] [Google Scholar]
- Rohan de Silva HA, Jen A, Wickenden C, Jen LS, Wilkinson SL, Patel AJ. (1997) Cell-specific expression of beta-amyloid precursor protein isoform mRNAs and proteins in neurons and astrocytes. Brain Res Mol Brain Res 47:147–156. [DOI] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O. (2013) Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol 43:21–44. [DOI] [PubMed] [Google Scholar]
- Sellström A, Cairns S, Barbeschi M. (2013) United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic, United Nations, New York: http://www.un.org/disarmament/content/slideshow/Secretary_General_Report_of_CW_Investigation.pdf. [Google Scholar]
- Sisodia SS, Koo EH, Hoffman PN, Perry G, Price DL. (1993) Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci 13:3136–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltaninejad K, Abdollahi M. (2009) Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit 15:RA75–RA90. [PubMed] [Google Scholar]
- Stecher PG. (1968) The Merck Index: An Encyclopedia of Chemicals and Drugs, Merck, Rahway, N.J. [Google Scholar]
- Stokin GB, Goldstein LS. (2006) Axonal transport and Alzheimer’s disease. Annu Rev Biochem 75:607–627. [DOI] [PubMed] [Google Scholar]
- Terry AV., Jr (2012) Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther 134:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Beck WD, Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. (2007) Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther 322:1117–1128. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. (2003) Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J Pharmacol Exp Ther 305:375–384. [DOI] [PubMed] [Google Scholar]
- Thomas WE. (1985) Morphology of acetylcholinesterase-containing neurons in primary cultures of dissociated rat cerebral cortex. Brain Res 361:392–395. [DOI] [PubMed] [Google Scholar]
- Uppuluri S, Knipling L, Sackett DL, Wolff J. (1993) Localization of the colchicine-binding site of tubulin. Proc Natl Acad Sci USA 90:11598–11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaganas I, Kapetanaki S, Mastorodemos V, Kanavouras K, Colosio C, Wilks MF, Tsatsakis AM. (2013) Linking pesticide exposure and dementia: what is the evidence? Toxicology 307:3–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.