Abstract
Background
Antinuclear antibody (ANA) tests are frequently used to screen children for chronic inflammatory diseases such as systemic lupus erythematosus (SLE). However, the diagnostic utility of this test is limited because of the large number of healthy children who have low-titer positive tests. We sought to determine the clinical utility of ANA tests in screening children for rheumatic disease and to determine whether there are specific signs or symptoms that enhance the clinical utility of ANA tests in children.
Methods
We undertook a retrospective analysis of 509 new patient referrals. Charts of patients referred because of results of ANA testing were selected for further analysis. Children with JRA, SLE, and other conditions were compared using demographic data, chief complaints at the time of presentation, and ANA titers.
Results
One hundred ten patients were referred because of an ANA test interpreted as positive. Ten patients were subsequently diagnosed with SLE. In addition, we identified one patient with mixed connective tissue disease, and an additional child with idiopathic Raynaud's phenomenon. Eighteen children of the children referred for a positive ANA test had juvenile rheumatoid arthritis (JRA). Another 80 children with positive ANA tests were identified, the majority of whom (n = 39, 49%) had musculoskeletal pain syndromes. Neither the presence nor the titer of ANA served to distinguish children with JRA from children with other musculoskeletal conditions. Children with JRA were readily identified on the basis of the history and physical examination. Children with SLE were therefore compared with children with positive ANA tests who did not have JRA, designated the "comparison group." Non-urticarial rash was more common in children with SLE than in children without chronic inflammatory disease (p = 0.007). Children with SLE were also older (mean ± sd = 14.2 ± 2.5 years) than the comparison group (11.0 ± 3.6 years; p = 0.001). ANA titer was also a significant discriminator between children with SLE and children without chronic inflammatory disease. The median ANA titer in children with SLE was 1: 1,080 compared with 1:160 for other children (p < 0.0001). ANA titers of ≥1,080 had a positive predictive value for SLE of 1.0 while titers of ≤1: 360 had a negative predictive value for lupus of 0.84.
Conclusion
Age and ANA titer assist in discriminating children with SLE from children with other conditions. ANA tests are of no diagnostic utility in either making or excluding the diagnosis of JRA.
Background
Systemic lupus erythematosus (SLE) is a chronic, multisystem disease characterized by inflammation in multiple organs, including kidney, heart, lung, and brain [1]. Although generally considered rare in children, as many as 15–17% of cases will present in childhood [2]. Because of its protean manifestations [3], SLE is frequently considered in the differential diagnosis of children presenting with otherwise common childhood symptoms, such as fatigue or arthralgia. Distinguishing children with SLE from children with mild or self-limited disease is complicated by the fact that the primary screening test for SLE, antinuclear antibody (ANA) assays, is commonly positive in healthy children [4,5]. Indeed, Malleson and colleagues [6] have shown that ANA tests may be positive in as many as 33% of healthy children. Thus, the practitioner may be thwarted in attempts to exclude the diagnosis of SLE in the face of low-titer positive tests.
We have recently reviewed our experience with juvenile rheumatoid arthritis (JRA), attempting to identify children with JRA based on the specific complaints with which they were referred for rheumatology consultation [7]. Children with JRA presented with symptoms of gait disturbance and joint swelling that facilitated their distinction from other children presenting with musculoskeletal complaints. Musculoskeletal pain was conspicuously absent as a presenting complaint in children with JRA.
These considerations notwithstanding, ANA tests continue to be used as a screening test for rheumatic disease in children. We therefore attempted to determine whether there are specific aspects of the history and physical examination that may assist the practitioner is determining whether an ANA test interpreted as "positive" by a clinical laboratory is diagnostically significant in a child.
Methods
Patients and patient records
Charts of all children seen for initial pediatric rheumatology consultation at the Children's Hospital of Oklahoma rheumatology clinic between April 1, 1998 and August 1, 2002 were reviewed. From these records, we selected all children 18 years of age or younger whose reason for referral included a positive ANA test. Records of children previously seen by another rheumatologist (e.g., for follow-up of a previously-diagnosed condition) were excluded. Patient records from these dates all document chief complaints articulated by the patient (or parent) as described by Weed [8,9]. Where necessary (e.g., where there were inadequate records or the patient was unclear of why they were sent to a specialist), identification of the problem for which the patient was referred was affirmed or clarified by contacting the referring physician at the time of consultation. In addition, charts were reviewed for demographic information that included: the patient's age, sex, duration of symptoms, and ultimate diagnosis. Results of complete blood counts (CBC) and differentials were also reviewed.
The diagnosis of SLE was made based on the 1982 ACR criteria [10]. The diagnosis of JRA was made based on standard criteria [11]. Non-inflammatory forms of musculoskeletal pain, overuse syndromes, and mechanical forms of musculoskeletal pain were diagnosed based on history, physical findings, and, where appropriate, laboratory and/or radiographic findings as described by Sherry and Malleson [12]. Viral arthritis was diagnosed in children with transient synovitis that resolved spontaneously within 2 months of onset and did not subsequently recur.
Laboratory values
ANA titers as obtained and recorded by the primary care physician were used as the basis of comparing children with SLE to children with other diagnoses.
Statistical analysis
Results were entered into a computer database and transferred to a commercially-available statistical software package (GraphPad Prism, San Diego, CA). Comparisons between groups (SLE vs. others) were undertaken using 2-tailed independent t-tests. Non-continuous variables and variables with non-parametric distribution were analyzed using the Mann-Whitney U test using the same statistical software. Proportions of different patients in specific subgroups were analyzed from contingency tables using Fisher's exact test. Statistical significance was assumed for p-values ≤0.05.
The positive or negative predictive values for specific ANA titers or specific ages was determined using conventional methods as follows:
![]()
![]()
Results
Records of 509 children were reviewed. Of these, 10 children were diagnosed as having SLE. In addition, one child each was diagnosed with mixed connective tissue disease and with Raynaud's phenomenon. The latter was followed for 18 months without any signs of systemic disease developing. For purposes of this paper, the children with Raynaud's phenomenon and mixed connective tissue disease were excluded from further analysis, as their numbers are too small for statistical comparison. In another 108 cases, a positive ANA test was indicated as a reason for referral. Ten of these cases were excluded from analysis because results of the ANA test and titer were not made available to the rheumatology consultant. Another 18 patients were diagnosed with JRA. We have reported the presenting signs and symptoms of the majority of these patients in another paper [7] but include analysis of the diagnostic utility of the ANA test in those patients here.
The clinical presentations of 10 patients with SLE were specifically compared to the 80 patients referred for positive ANA tests and subsequently shown not to have a chronic inflammatory disorder. For convenience, these children will now be referred to as the comparison group. Presenting complaints of children with SLE and the comparison group are shown in Table 1. Children with JRA all presented with joint swelling and/or gait disturbance as their chief complaints, as we have previously reported [7]. Diagnoses for the comparison group are shown in Table 2. Of these, the most common diagnoses were hypermobility syndrome and other mechanical musculoskeletal pain syndromes (n = 39; 49%), consistent with reports from other pediatric rheumatology centers [13,14]. Viral arthritis was the next most common diagnosis, seen in 10 patients (13%).
Table 1.
Presenting complaints of ANA-positive children
| Complaint | SLE (n = 10) | Comparison Group (n = 80) |
| Musculoskeletal pain | 3 (30%) | 51 (64%) |
| Fever | 3 (30%) | 7 (9%) |
| Rash (non-urticarial) | 4 (40%)* | 4 (5%) |
| Joint swelling | 2 (20%) | 6 (8%) |
| Fatigue | 2(20%) | 4 (5%) |
*P = 0.0136
Table 2.
Diagnoses of ANA-positive children who did not have chronic diflammatory disease (N = 80)
| Diagnosis | Number |
| Musculoskeletal Pain Syndromes | 39 |
| Hypermobility syndrome | 9 |
| Osgood-Schlatter's syndome | 4 |
| Patello-femoral syndrome | 4 |
| Other mechanical musculoskeletal disorders | 22 |
| Viral (transient) arthritis | 10 |
| Other | 31 |
Children with SLE presented with diverse complaints, as would be expected given the protean nature of the disease. Most striking was the finding that 4 children with SLE (40%) and 4 patients in the comparison group (5%) presented with rash (other than urticaria) as a reason for seeking medical care. This difference was statistically significant (p = 0.0136). All 4 of the SLE patients with rashes demonstrated prominent facial eruptions in a malar distribution, while 2 also had non-blanching, purpuric lesions on the hands and upper and lower extremities. Three patients in the comparison group also presented with urticaria; none of the SLE patients presented with urticarial rash.
Fever, fatigue, and joint swelling were also complaints seen in both groups. None of these was more significantly common in one group than in the other by Fisher's exact test.
Musculoskeletal pain was a symptom for which 3 children with SLE (30%) were referred, in contrast to the comparison group, where musculoskeletal pain was the most common chief complaint (n = 51, 64%). This difference was not statistically significant. Thus, children with SLE differ from children with JRA, who seldom present with musculoskeletal pain as a primary complaint, as we have previously reported [7].
Patient age was significantly associated with the likelihood that a patient with a positive ANA test had lupus. Patients with SLE were significantly older (mean ± sd = 14.2 ± 2.5 years) than the comparison group (11.0 ± 3.6 years; p = 0.007). However, the positive predicative value for SLE of a positive ANA test in patients 13 years of age or older was still low (0.17). In contrast, the positive predictive value of a positive ANA test in children under the age of 13 years was extraordinarily low (0.09).
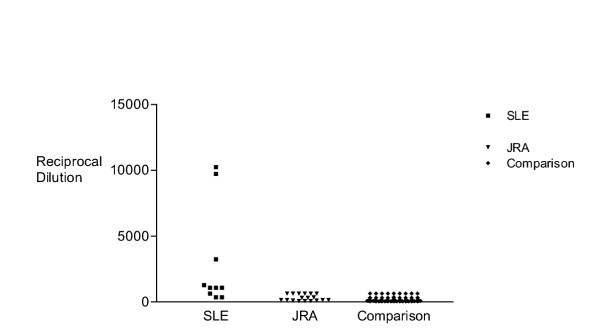
ANA titers were the best indicator of SLE (Figure 1). While there was some overlap in ANA titers between the patients with SLE (1:360–1:10,240) and the comparison group (1:40–1:640), the median titer between the two groups (1:1,080 vs. 1:160) was significantly different (p =< 0.0001). All 7 patients with ANA titers ≥1:1,080 had SLE. Thus, the positive predictive value of an ANA titer of ≥1:1,080 for SLE was =1.0. However, there were 9 patients in the comparison group with ANA titers of 1:640 in whom history, physical examination, clinical course, and other supporting laboratory studies excluded SLE. Thus, the positive predictive value for SLE of an ANA titer of ≥1:640 was 0.42. Low titers were also diagnostically useful. The negative predictive value of a titer of ≤1:360 was 0.84, and of titers ≤1:180 was 1.0.
Figure 1.
Scatter plot comparing ANA titers (reciprocal dilution) in children with SLE, JRA, and other disorders. The median ANA titer was significantly higher in patients with SLE (1:1,080) compared with children with JRA (1:240) and children in the comparison group (1:160).
Seven of the 10 patients referred for a positive ANA test who were shown to have SLE also had an abnormal CBC. These included 6 with anemia (hemoglobin <11 g/dL), 4 with leucopenia (white blood cell count <4,000/cu mm) and one with thrombocytopenia (platelet count <150,000/cu mm).
Among the patients referred for a positive ANA test, 18, as noted above, were diagnosed with JRA. ANA titers in JRA patients ranged between 1:80–1:640 that is, they completely overlapped the range seen in otherwise healthy children. Furthermore, the median ANA titer in children with JRA (1:240) was significantly lower than the ANA titer seen children with SLE (p = 0.0004). Furthermore, in the 18 patients with JRA, the ANA test had no diagnostic utility. That is, the diagnosis of JRA was readily made on the history of joint swelling and/or gait disturbance and the physical finding of synovial proliferation [15] at the time of evaluation in the rheumatology clinic.
Discussion
SLE is a complex, perplexing illness with protean manifestations. While the majority of cases occur in adulthood, as many as 15–17% of patients present at age 18 years or younger [2] Thus, pediatricians are likely to encounter this disease during their careers. Furthermore, because of the myriad of clinical presentations with which childhood SLE presents [16,17] it is frequently in the differential diagnosis of children presenting with challenging or difficult illnesses. Pediatricians are hampered in making this diagnosis by the frequency with which positive ANA tests are encountered in otherwise healthy children. Nonetheless, ANA tests continue to be used as a screening procedure to include or exclude rheumatic disease in children with musculoskeletal complaints. Thus, we sought to determine whether there are predictable presenting complaints that would allow the practitioner to distinguish children with SLE or JRA from the large number of children with benign or self-limited illnesses who have positive ANA tests.
Other studies have verified the lack of specificity of ANA tests in children. However, each of these previous studies used a different approach from ours. Both Cabral and colleagues [4] and Dean and colleagues [5] examined children seen in pediatric rheumatology centers. From their total patient populations, they selected children with positive ANA tests and found that as many as 27% had no evidence of a chronic inflammatory disease, even when followed for periods as long as 7.5 years. Malleson and colleagues [6] examined records of all children with positive ANA tests in a large children's hospital and similarly documented the lack of specificity of the test. Our study is unique in having started from the practitioners' point of view. That is, our study focused on children who were referred because of a positive ANA test and asked whether there were clinical clues that might have assisted the referring physician in making or excluding the diagnosis of SLE prior to referral for subspecialty evaluation. This is a critical piece of information, since expensive subspecialty care in many medical environments is subjected to stringent oversight. Thus, data that assist primary care physicians in identifying children with the most need for a subspecialty referral are urgently needed.
We now report that, unlike children with JRA, children with SLE do not immediately distinguish themselves based on clinical features. While the diagnosis of SLE may be straightforward when children present with classic signs and symptoms of the illness (hematuria, fever, weight loss, malar rash, and alopecia), our data demonstrated no single complaint that predicted SLE at presentation. Musculoskeletal pain, fatigue, and fever were not seen significantly more frequently in children with SLE than in children with other illnesses. The sole exception was the presence of non-urticarial rash, which was more common in patients with SLE than in the comparison group. This finding contrasts with our study of children with JRA, in which affected children were readily distinguished from other children with musculoskeletal complaints based on their clinical presentation [7].
Patient age and ANA titer were the best measures distinguishing children with SLE from ANA-positive children with other illnesses or self-limited conditions. While ANA titers as high as 1:640 can be seen in children who do not have SLE, in our study, titers of ≥1: 1,080 were highly predictive of SLE. Similarly, titers of ≤1:360, particularly if obtained in children under the age of 13 years, safely excluded SLE.
Although positive ANA tests are sometimes seen in children with JRA [18] and juvenile dermatomyositis [19] positive ANA tests are not distinguishing features of either of these diseases. As we have previously reported and demonstrate again here, the history and physical examination are the critical elements in making the diagnosis of JRA. Indeed, in this study, ANA tests demonstrated no ability to discriminate children with JRA from children presenting with other musculoskeletal complaints. Similarly, the diagnosis of juvenile dermatomyositis is based on the presence of a characteristic rash and clinical evidence of chronic muscle inflammation [20]. Both of these can be established without an ANA test. It is also important to point out that children with acute lymphocytic leukemia, a relatively common cause of musculoskeletal pain in children [21], may also have positive ANA tests [22].
Because of their limited diagnostic specificity and high prevalence of false positives, we believe that ANA tests should be used to address only one clinical question in pediatrics: does this child have SLE? Titers ≥1:1,080 should be considered strongly supportive of that diagnosis, while titers ≤1:360 make that diagnosis unlikely, particularly before adolescence. As ever, laboratory findings must be placed in an appropriate clinical context, and no single ANA titer should be considered diagnostic of SLE. History, physical examination, and other supportive laboratory tests (urinalysis, erythrocyte sedimentation rate or C-reactive protein, serum complement levels, etc. [3]) are required to establish or exclude a diagnosis of SLE [10].
We conclude that children with SLE are often clinically indistinguishable from other children with positive ANA tests in the spectrum of complaints with which they present for initial medical care. However, judicious use of the history (presence of morning stiffness), physical examination (presence of rash), and thoughtful interpretation of ANA titers will significantly assist in distinguishing children with this life-threatening illness from children with more benign conditions. ANA tests have little diagnostic utility in clarifying the diagnosis of JRA, and should not be used as a screening tool if JRA is the diagnosis under consideration.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Ms. McGhee was supported by a summer research fellowship from the American College of Rheumatology Research and Education Foundation. Ms. Kickingbird was supported by a summer fellowship from the University of Oklahoma Native American Center of Excellence. Dr. Jarvis is supported by grants from the Arthritis Foundation and the American College of Rheumatology Research and Education Foundation.
The authors wish to thank Drs. Mark Ferguson and Andrea Sestak for their thoughtful reviews and suggestions with this manuscript.
Contributor Information
Julie L McGhee, Email: julie-mcghee@ouhsc.edu.
Lauren M Kickingbird, Email: lauren-kickingbird@ouhsc.edu.
James N Jarvis, Email: james-jarvis@ouhsc.edu.
References
- Arkachaisri T, Lehman TJ. Systemic lupus erythematosus and related disorders of childhood. Curr Op Rheumatol. 1999;11:384–392. doi: 10.1097/00002281-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Petty RE, Cassidy JT. Systemic lupus erythematosus. In: Cassidy JT, Petty RE, editor. A Textbook of Pediatric Rheumatology. Philadelphia: WB Saunders Co; 2001. p. 397. [Google Scholar]
- Iqbal S, Sher MR, Good RA, Cawkwell GD. Diversity of presenting manifestations of systemic lupus erythematosus. J Pediatr. 1999;135:500–505. doi: 10.1016/s0022-3476(99)70174-5. [DOI] [PubMed] [Google Scholar]
- Cabral DA, Petty RE, Fung M, Malleson P. Persistent antinuclear antibodies in children without identifiable rheumatic or autoimmune disease. Pediatrics. 1992;89:441–444. [PubMed] [Google Scholar]
- Deane PMG, Liard G, Siegel DM, Baum J. The outcome of children referred to a pediatric rheumatology clinic with a positive antinuclear antibody test but without an autoimmune disease. Pediatrics. 1995;95:892–895. [PubMed] [Google Scholar]
- Malleson PN, Sailer M, Mackinnon MJ. Usefulness of antinuclear antibody testing to screen for rheumatic diseases. Arch Dis Child . 1997;77:299–304. doi: 10.1136/adc.77.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee JL, Burks FN, Sheckels JL, Jarvis JN. Identifying children with chronic arthritis based on chief complaints. Absence of musculoskeletal pain as a predictor of chronic arthritis in children. Pediatrics. 2002;110:354–359. doi: 10.1542/peds.110.2.354. [DOI] [PubMed] [Google Scholar]
- Weed LL. Quality control and the medical record. Arch Int Med. 1971;127:101–105. doi: 10.1001/archinte.127.1.101. [DOI] [PubMed] [Google Scholar]
- Weed LL. Medical records that guide and teach. N Eng J Med. 1968;278:593–657. doi: 10.1056/NEJM196803142781105. [DOI] [PubMed] [Google Scholar]
- Tan EM. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Cassidy JT, Levinson JE, Brewer EJ. The development of classification criteria for children with juvenile rheumatoid arthritis. Bull Rheum Dis. 1989;38:1–7. [PubMed] [Google Scholar]
- Sherry DD, Malleson PN. Nonrheumatic musculoskeletal pain. In: Cassidy JT, Petty RE, editor. Textbook of Pediatric Rheumatology. Philadelphia: WB Saunders Co; 2001. pp. 362–380. [Google Scholar]
- Rosenberg AM. Analysis of a pediatric rheumatology clinic population. J Rheumatol. 1990;17:827–830. [PubMed] [Google Scholar]
- Bowyer S, Roettcher P, members of the pediatric rheumatology database research group Pediatric rheumatology clinic populations in the United States: results from a 3 year survey. J Rheumatol. 1996;23:1968–1974. [PubMed] [Google Scholar]
- Jarvis JN. Mechanisms of pathogenesis and inflammation in the pediatric rheumatic diseases. Curr Opinion Rheumatol. 1998;10:459–467. doi: 10.1097/00002281-199809000-00011. [DOI] [PubMed] [Google Scholar]
- Lehman T, McCurdy DK, Bernstein BH, King KK, Hanson V. Systemic lupus erythematosus in the first decade of life. Pediatrics. 1989;83:235–239. [PubMed] [Google Scholar]
- Lehman TJ. A practical guide to systemic lupus erythematosus. Pediatr Clin N Amer. 1995;42:1223–1238. doi: 10.1016/s0031-3955(16)40060-x. [DOI] [PubMed] [Google Scholar]
- Jarvis JN. Juvenile rheumatoid arthritis: a guide for pediatricians. Pediatr Ann. 2002;31:437–446. doi: 10.3928/0090-4481-20020701-08. [DOI] [PubMed] [Google Scholar]
- Feldman BM, Reichlin M, Laxer RM, Targoff IN, Stein LD, Silverman ED. Clinical significance of specific autoantibodies in juvenile dermatomyositis. J Rheumatol. 1996;23:1794–1797. [PubMed] [Google Scholar]
- Rennenbohm R. Juvenile dermatomyositis. Pediatr Ann. 2002;31:426–433. doi: 10.3928/0090-4481-20020701-07. [DOI] [PubMed] [Google Scholar]
- Cabral D, Tucker L. Malignancies in children who initially present with rheumatic complaints. J Pediatr. 1999;134:53–57. doi: 10.1016/s0022-3476(99)70372-0. [DOI] [PubMed] [Google Scholar]
- Saulsbury FT, Sabio H. Acute leukemia presenting as arthritis in children. Clin Pediat. 1985;24:625–628. doi: 10.1177/000992288502401103. [DOI] [PubMed] [Google Scholar]