Abstract
B-type natriuretic peptide (BNP)–natriuretic peptide receptor A (NPRA) and gastrin-releasing peptide (GRP)–GRP receptor (GRPR) systems contribute to spinal processing of itch. However, pharmacological and anatomic evidence of these two spinal ligand-receptor systems are still not clear. The aim of this study was to determine the spinal functions of BNP-NPRA and GRP-GRPR systems for regulating scratching activities in mice by using pharmacological and immunohistochemical approaches. Our results showed that intrathecal administration of BNP (0.3–3 nmol) dose dependently elicited scratching responses, which could be blocked by the NPRA antagonist (Arg6,β-cyclohexyl-Ala8,D-Tic16,Arg17,Cys18)-atrial natriuretic factor(6-18) amide (A71915). However, A71915 had no effect on intrathecal GRP-induced scratching. In contrast, pretreatment with a GRPR antagonist (D-Tpi6,Leu13ψ(CH2-NH)-Leu14)bombesin(6-14) (RC-3095) inhibited BNP-induced scratching. Immunostaining revealed that NPRA proteins colocalize with GRP, but not GRPR, in the superficial area of dorsal horn, whereas BNP proteins do not colocalize with either GRP or GRPR in the dorsal horn. Intradermal administration of ligands including endothelin-1, U-46619, bovine adrenal medulla 8-22, and Ser-Leu-Ile-Gly-Arg-Leu-NH2 (SLIGRL) increased scratching bouts at different levels of magnitude. Pretreatment with intrathecal A71915 did not affect scratching responses elicited by all four pruritogens, whereas pretreatment with RC-3095 only inhibited SLIGRL-induced scratching. Interestingly, immunostaining showed that RC-3095, but not A71915, inhibited SLIGRL-elicited c-Fos activation in the spinal dorsal horn, which was in line with behavioral outcomes. These findings demonstrate that: 1) BNP-NPRA system may function upstream of the GRP-GRPR system to regulate itch in the mouse spinal cord, and 2) both NPRA and GRPR antagonists may have antipruritic efficacy against centrally, but not peripherally, elicited itch.
Introduction
Itch/pruritus is one of the key symptoms in patients suffering from a variety of systemic disorders, including infectious, uremic, hepatic, and hematologic diseases (Weisshaar and Dalgard, 2009; Yosipovitch and Bernhard, 2013; Hay et al., 2014). Given that itch is a significant clinical problem afflicting a large global population, there is a strong need for more research on the cause and treatment of itch. Generally, ligand-receptor signaling plays a fundamental role in the generation of itch. For instance, keratinocyte-derived histamine, which binds to the transmembrane H1 receptor, is a well known pruritogen and had been considered as a possible target for clinical therapies (Thurmond et al., 2015). However, antihistamines are not effective in alleviating itch derived from most dermatoses, systemic diseases, and opioid administration (Biro et al., 2005; Yosipovitch and Bernhard, 2013; van Zuuren et al., 2014). Therefore, more research is warranted to discover novel targets and develop effective antipruritics.
Recent rodent studies have identified several molecules and neural circuits in the spinal cord for regulating itch signaling (Akiyama and Carstens, 2013; Bautista et al., 2014; LaMotte et al., 2014). One of the central itch mediators is gastrin-releasing peptide (GRP), which acts as an enhancer of gastrin secretion from the gastric antrum through binding to its corresponding transmembrane GRP receptor (GRPR) (McDonald et al., 1979; Jensen et al., 2008). GRP has been used to elicit scratching responses in rodents (Bishop et al., 1986; Masui et al., 1993). With use of GRPR mutant mice, spinal GRP-GRPR signaling has been demonstrated to specifically regulate itch without altering pain thresholds (Sun and Chen, 2007). Additionally, GRP serum levels in patients with atopic dermatitis and GRPR expression in the spinal cord of monkeys with excessive scratching were shown to correlate with the severity of itch (Kagami et al., 2013; Nattkemper et al., 2013). More importantly, like μ opioid-related ligands, intrathecal administration of GRP elicited robust scratching responses in monkeys and these findings translate the functional role of GRP as an itch mediator from rodents to primates (Lee and Ko, 2015). However, GRPR antagonists are fully effective against GRP-elicited scratching, but they are ineffective or partially effective against scratching elicited by other pruritogens (Inan et al., 2011; Akiyama et al., 2013; Sukhtankar and Ko, 2013; Lee and Ko, 2015). Further research is warranted to determine the effectiveness of GRPR antagonists against itch scratching elicited peripherally by diverse pruritogens.
Natriuretic peptides are secreted by the ventricles of the heart, and they have been established as diagnostic and prognostic tools for cardiovascular diseases (Levin et al., 1998; Potter et al., 2009). B-type natriuretic peptide (BNP), originally isolated from porcine brain, binds to transmembrane natriuretic peptide receptor A (NPRA) (Mukoyama et al., 1991; Misono et al., 2011). A recent study reported that BNP-NPRA signaling is a key mechanism of itch transmission in the mouse spinal cord (Mishra and Hoon, 2013). In addition, an increased level of serum BNP was found to be associated with the degree of pruritus in hemodialysis patients (Shimizu et al., 2014). Anatomic and pharmacological studies have demonstrated that GRP-GRPR system acts downstream of the BNP-NPRA signaling in the pruriceptive circuit as an itch-selective regulation by BNP (Mishra and Hoon, 2013). However, a series of experiments conducted later indicated that BNP is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway (Liu et al., 2014). It is important to clarify the anatomic and pharmacological relationship between BNP-NPRA and GRP-GRPR systems in spinal regulation of itch. Moreover, no pharmacological evidence is currently available to demonstrate the effectiveness of NPRA antagonists against scratching evoked by different pruritogens. Therefore, it is pivotal to determine and compare the effectiveness of NPRA and GRPR antagonists in regulating itch neurotransmission, not only to improve our understanding of these signaling pathways but also to validate their therapeutic potential as antipruritics.
In the present study, we first determined the dose-response and time course of the NPRA antagonist (Arg6,β-cyclohexyl-Ala8,D-Tic16,Arg17,Cys18)-atrial natriuretic factor(6-18) amide (A71915) against intrathecal BNP-induced scratching in mice. To define the anatomic location of BNP and NPRA, we tested colocalization of BNP or NPRA with GRP and GRPR by using immunohistochemistry. More importantly, we compared the effectiveness of the NPRA antagonist A71915 and GRPR antagonist (D-Tpi6,Leu13ψ(CH2-NH)-Leu14)bombesin(6-14) (RC-3095) on scratching elicited by intradermal administration of pruritogens that included endothelin-1, U-46619, bovine adrenal medulla 8-22 (BAM8-22), and Ser-Leu-Ile-Gly-Arg-Leu-NH2 (SLIGRL), which are agonists activating different pruriceptors. Finally, we determined the effects of A71915 and RC-3095 on pruritogen-induced c-Fos expression in the spinal cord dorsal horn in support of the behavioral findings.
Materials and Methods
Animals.
Male CD-1 mice weighing 25–30 g were used (Harlan Laboratories, Indianapolis, IN) for all experiments. These naïve mice were housed five per cage under a 12-hour light/dark cycle and provided with water and food ad libitum. Each animal was used only once per experimental condition. All experimental procedures were approved by the Institutional Animal Care and Use Committee in Wake Forest University School of Medicine (Winston-Salem, NC), and they were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health (Bethesda, MD).
Drug Administration.
All drugs were dissolved in sterile water and diluted as needed. BNP (Advanced Targeting Systems, San Diego, CA), A71915 (Bachem Americas, Torrance, CA), GRP (R&D Systems, Minneapolis, MN), and RC-3095 (Sigma-Aldrich, St. Louis, MO) were intrathecally administered in the volume of 5 μl as previously described (Sukhtankar and Ko, 2013). The antagonist (A71915 or RC-3095) was administered 10 minutes before administration of pruritogens (i.e., intrathecal or intradermal pruritogens). Briefly, mice were secured by a firm grip on the pelvic girdle, and drugs were injected by lumbar puncture between L5 and L6 vertebrae using a 30-gauge needle fitted with Hamilton microsyringe. Endothelin-1 (R&D Systems), U-46619, BAM8-22, and SLIGRL-NH2 (abcam, Cambridge, MA) were intradermally administered in the nape of the neck. Fur at the injection site was clipped before experiment and drugs were administered in the volume of 100 μl using a 30-gauge needle fitted to a 1-ml syringe. To justify the drug distribution in the spinal cord, 2.5% Evans blue dye (Sigma-Aldrich) in sterile water was intrathecally administered in the volume of 5 μl by lumbar puncture between L5 and L6 vertebrae, and the brain and spinal cord were collected 10 minutes after injection.
Scratching Behavior.
Mice were habituated for 20 minutes in plastic cages with a small amount of bedding. Scratching response was quantified as the number of scratching bouts counted by individuals who were blind to the dosing conditions. One scratching bout was defined as lifting the hind limb, directing it toward the trunk area of body or nape regions following intrathecal or intradermal injection, respectively, scratching, and then placing the hind limb back on the floor. Scratching bouts were measured in 10-minute intervals for either 30 or 60 minutes as previously reported (Sukhtankar and Ko, 2013).
Rotarod Test.
The rotarod (IITC Life Science, Woodland Hills, CA), consisting of five textured drums of 1.25-cm diameter was used for the assessment of motor function. The total time that the mice were able to remain on the rotating drum was recorded. Mice were acclimatized to the rotarod at 5 rpm for 180 seconds for habituation and then allowed to remain on the rotarod at 10 and 15 rpm for 30 seconds as training. On the test day, mice were tested at 15, 20, 25, and 30 rpm for 180 seconds, and a 10-minute rest period was set between trials. Different groups of naïve animals were used in the rotarod test.
Immunohistochemistry.
Naïve mice were perfused transcardially with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Similar to other studies (Nojima et al., 2003; Nakano et al., 2008), the collected cervical (C3–C5) and lumbar (L4–L6) spinal cord and dorsal root ganglion (DRG, L4–6) were then postfixed for 2 hours and cryoprotected in a 25% sucrose solution at 4°C overnight. Frozen tissues embedded in optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA) were cut at 12 μm (spinal cord) or 10 μm (DRG) with a cryostat, and thaw-mounted on glass slides. The sections were treated with 0.1 M PBS containing 0.3% Triton X-100, and blocked with 5% normal donkey serum at room temperature for 2 hours. The sections were incubated with primary antibodies against BNP [goat polyclonal, 1:100; Santa Cruz Biotechnology, Dallas, TX (Zhang et al., 2010; Liu et al., 2014)], GRP [rabbit polyclonal, 1;4000; ImmunoStar, Hudson, WI (Sun and Chen, 2007; Solorzano et al., 2015)], NeuN (mouse monoclonal, 1:200; EMD Millipore, Billerica, MA), GRPR (rabbit polyclonal, 1:500; MBL International, Woburn, MA), NPRA (rat monoclonal, 25 μg/ml; LifeSpan BioSciences, Seattle, WA), or c-Fos (rabbit polyclonal, 1:50; Santa Cruz Biotechnology) at 4°C overnight. For preabsorption experiments, GRP antibody (1:4000; ImmunoStar) was preincubated with GRP (10 μg/ml; R&D Systems) or substance P (10 μg/ml; Sigma-Aldrich) in blocking buffer at 4°C overnight before it was applied to sections as previously reported (Solorzano et al., 2015). The following day, sections were washed and incubated in secondary antibodies conjugated with Alexa 488, Cy3 (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA), or Alexa 350 (1:200; ThermoFisher Scientific, Waltham, MA) at room temperature for 2 hours. Finally, the sections were washed, and then a cover glass with mounting medium containing DAPI and one with mounting medium without DAPI (Vector Laboratories, Burlingame, CA) were placed over spinal cord and DRG, respectively. Digital images were captured using a Nikon Eclipse Ni fluorescent microscope system (Nikon, Tokyo, Japan). For quantification of c-Fos in each mouse, the number of positive cells in the superficial laminae of the cervical (C3–C5) spinal cord was the average of four randomly selected sections from one segment of each mouse before c-Fos was viewed. All images of c-Fos labeling were taken at the same time with the same camera settings, and the persons performing the counts were blind to treatment groups. Brightness and contrast of fluorescent micrographs were minimally processed and colorized as needed, using Adobe Photoshop. For c-Fos images, adjustments made in Photoshop were applied uniformly in images from all treatment groups.
Statistical Analysis.
All data are presented as mean ± S.E.M. calculated from individual mouse. Some of behavioral data presenting the total number of scratching bouts (Fig. 1, B and D and Fig. 6, B, D, F, H) and histologic data presenting the number of c-Fos+ cells (Fig. 7C) were analyzed using one-way analysis of variance followed by Tukey’s test. Other behavioral data (Fig. 5, B and D) were analyzed using unpaired t test. The criterion for significance was set at P < 0.05.
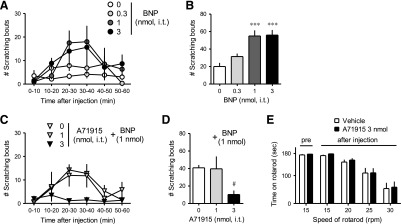
Fig. 1.
Effects of the NPRA antagonist A71915 on intrathecal BNP-induced scratching. A71915 was intrathecally administered 10 minutes prior to BNP. Scratching bouts were observed immediately after intrathecal BNP up to 1 hour. Time course in 10-minute intervals (A, C) and dose response of total BNP-induced scratching bouts for 1 hour (B, D) are shown. Top panels (A, B) present the effects of BNP alone. Bottom panels (C, D) present the effects of A71915 on BNP-induced scratching bouts. (E) Rotarod test was performed before and 10 minutes after administration of A71915. Each value represents mean ± S.E.M. (n = 6). ***P < 0.001 versus vehicle. #P < 0.05 versus vehicle/BNP.
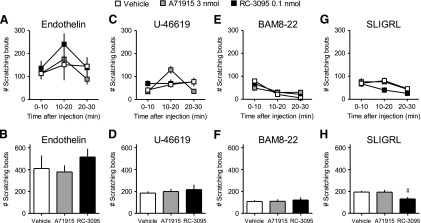
Fig. 6.
Comparison of effects of the GRPR antagonist RC-3095 and NPRA antagonist A71915 on intradermal pruritogen-induced scratching. Each antagonist was intrathecally administered 10 minutes prior to intradermal pruritogen. Scratching bouts were observed immediately after intradermal administration of endothelin (A, B), U-46619 (C, D), BAM8-22 (E, F), or SLIGRL (G, H) up to 30 minutes. Time course in 10-minute intervals (tops: A, C, E, G) and total scratching bouts for 30 minutes (bottoms: B, D, F, H) are shown. Each value represents mean ± S.E.M. (n = 6). #P < 0.05 versus vehicle/SLIGRL.
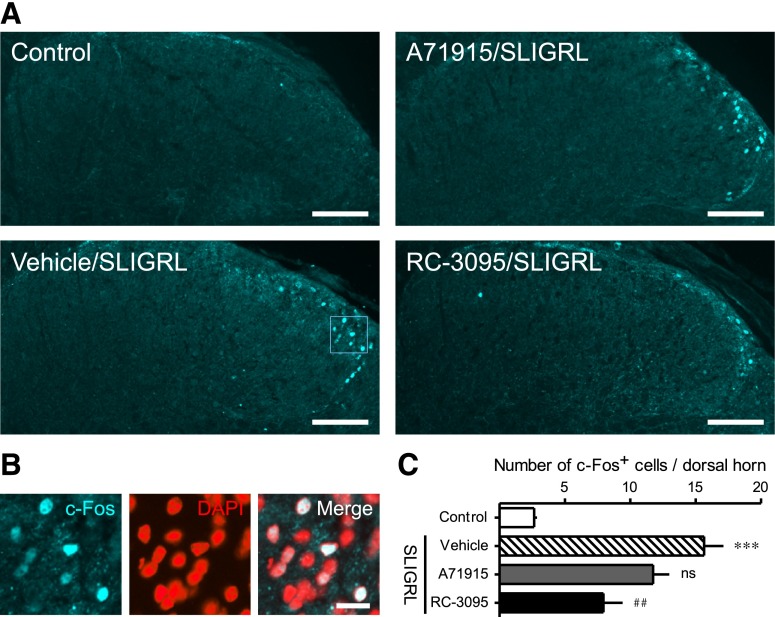
Fig. 7.
Effects of the GRPR antagonist RC-3095 and NPRA antagonist A71915 on intradermal SLIGRL-induced c-Fos activation. Each antagonist was intrathecally administered 10 minutes prior to SLIGRL. Expression of c-Fos protein in cervical dorsal horn was evaluated 2 hours after intradermal administration of SLIGRL by immunohistochemistry. Representative micrograph (A) and mean number of c-Fos+ cells (C) in each group are shown. (B) Higher magnification of bordered square in vehicle/SLIGRL indicates c-Fos overlaps with DAPI. Scale bars, 100 μm (A) and 20 μm (B). Each value represents mean ± S.E.M. (n = 5). ***P < 0.001 versus vehicle control, ##P<0.01 versus vehicle/SLIGRL. ns, Not significant versus vehicle/SLIGRL.
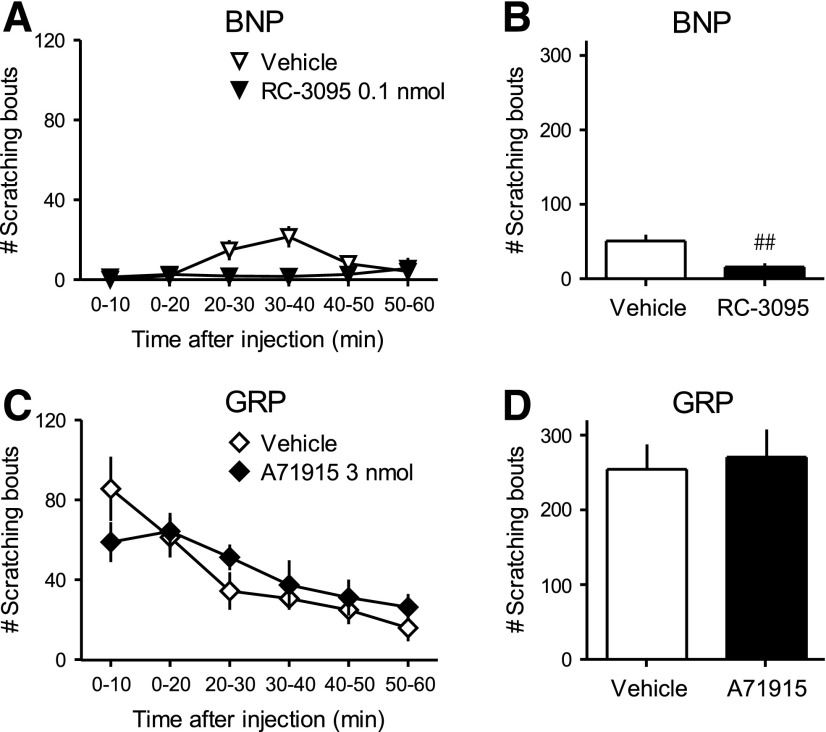
Fig. 5.
Cross-examination of effects of the GRPR antagonist RC-3095 and NPRA antagonist A71915 on intrathecal BNP- and GRP-induced scratching. Each antagonist was intrathecally administered 10 minutes prior to BNP (1 nmol) or GRP (0.1 nmol). Scratching bouts were observed immediately after intrathecal BNP or GRP up to 1 hour. Time course in 10-minute intervals (A, C) and total scratching bouts for 1 hour (B, D) are shown. Top panels (A, B) and bottom panels (C, D) present the effects of RC-3095 and A71915, respectively. Each value represents mean ± S.E.M. (n = 6). ##P < 0.01 versus vehicle/BNP.
Results
Figure 1 shows the effects of the NPRA antagonist A71915 (1–3 nmol) on intrathecal BNP (1 nmol)-induced scratching in mice. Compared with the vehicle treatment, BNP (0.3–3 nmol) elicited scratching bouts in dose-dependent and time-dependent manners [F(3, 20) = 11.99; P < 0.05] (Fig. 1, A and B). Pretreatment with A71915 (1–3 nmol) dose-dependently antagonized BNP (1 nmol)-induced scratching [F(2, 15) = 3.878; P < 0.05] (Fig. 1, C and D). Before intrathecal administration, all mice were able to balance on the rotarod at 15 rpm for approximately 180 seconds. Intrathecal A71915 (3 nmol) did not affect motor function, demonstrated by the similar amount of time mice stayed on the rotarod at 15, 20, 25, and 30 rpm in comparison with vehicle treatment (Fig. 1E).
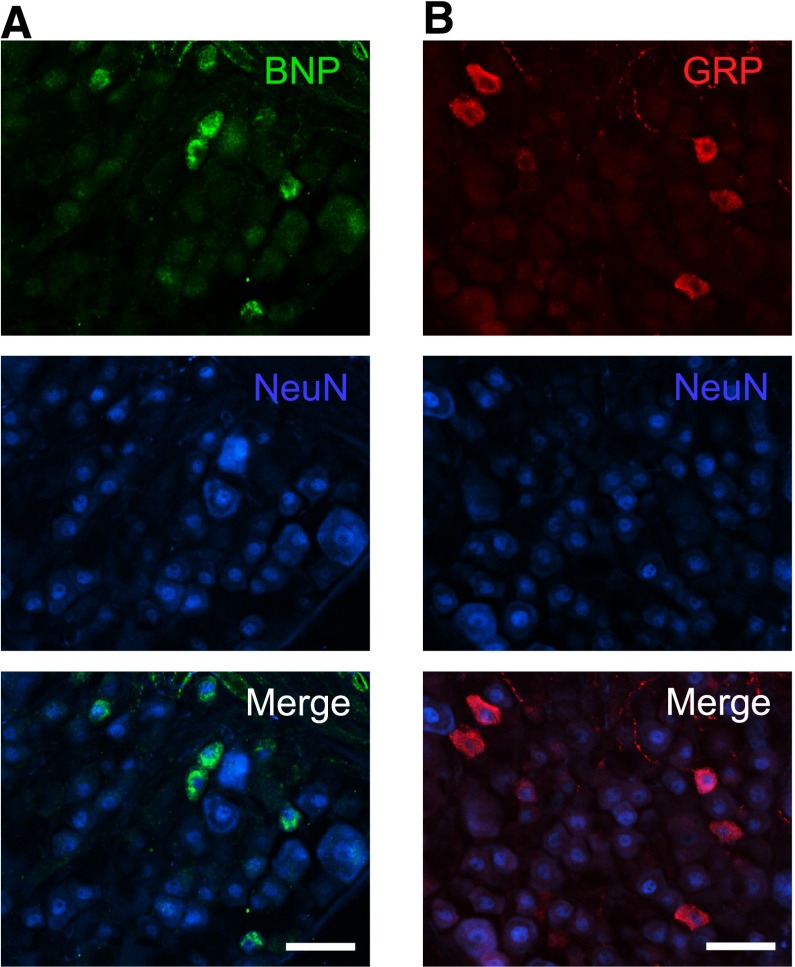
Figure 2 shows the expression of BNP and GRP in the mouse DRG. Immunostaining using antibodies against BNP or GRP revealed the expression of BNP and GRP proteins in the DRG, and both BNP and GRP were coexpressed with neuronal nuclei (NeuN) (Fig. 2, A and B).
Fig. 2.
Expression of BNP and GRP in the mouse DRG. Expression and localization of BNP (A), GRP (B), and NeuN in DRG were examined by immunohistochemistry in naïve mice. Representative micrographs from four mice are shown. Scale bars, 50 μm (A, B).
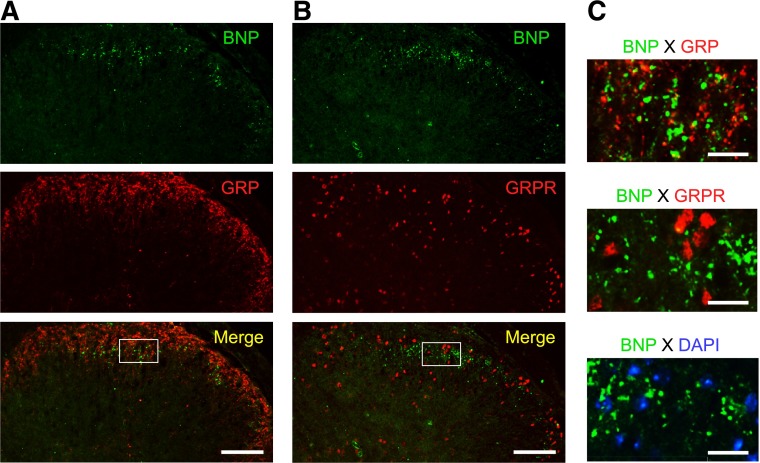
Figure 3 illustrates the expression of BNP in lumbar dorsal horn of mice. Double immunostaining using antibodies against BNP or GRP revealed the expression of BNP and GRP proteins in the superficial area of dorsal horn. BNP proteins did not colocalize with GRP expression (Fig. 3, A and C). In addition, BNP was not coexpressed with GRPR or nuclei (DAPI) in the sensory laminae of dorsal horn (Fig. 3, B and C). In preabsorption experiments, GRP immunoreactivity completely disappeared by preincubation with GRP. On the other hand, positive staining of GRP was observed after preincubation with substance P (Supplemental Fig. S1).
Fig. 3.
Expression of BNP in lumbar dorsal horn of mice. Expression and localization of BNP, GRP and GRPR in lumbar dorsal horn were examined by immunohistochemistry in naïve mice. Representative micrographs from 4 mice in lower magnification (A, B) and higher magnification (C) of bordered square are shown. Scale bars,100 μm (A, B) and 20 μm (C).
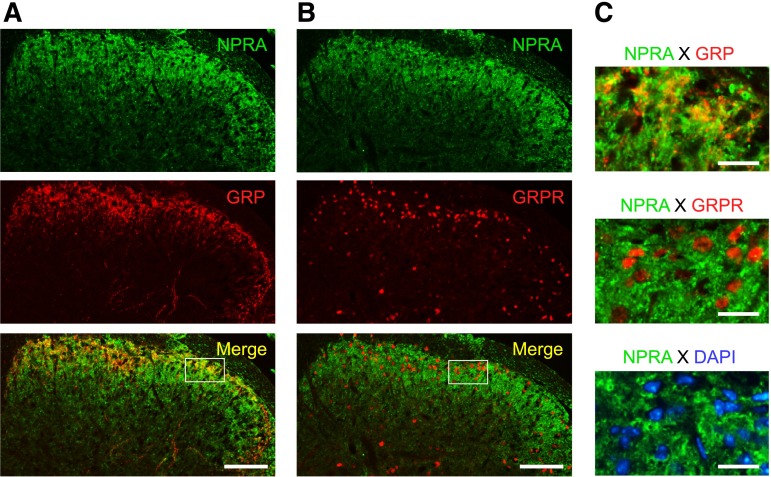
Figure 4 illustrates the colocalization of NPRA and GRP in lumbar dorsal horn of naïve mice. Double immunostaining using antibodies against NPRA or GRP revealed the expression of NPRA and GRP proteins in the superficial area of dorsal horn. NPRA expression in superficial area colocalized with GRP expression (Fig. 4, A and C). On the other hand, NPRA did not overlap with GRPR and nuclei in the sensory laminae of dorsal horn (Fig. 4, B and C).
Fig. 4.
Colocalization of NPRA and GRP in lumbar dorsal horn of mice. Expression and localization of NPRA, GRP, and GRPR in lumbar dorsal horn were examined by immunohistochemistry in naïve mice. Representative micrographs from four mice in lower magnification (A, B) and higher magnification (C) of bordered square are shown. Scale bars, 100 μm (A, B) and 20 μm (C).
Figure 5 illustrates the effects of GRPR antagonist RC-3095 and NPRA antagonist A71915 on intrathecal BNP- and GRP-induced scratching in mice, respectively. Pretreatment with RC-3095 (0.1 nmol) inhibited BNP (1 nmol)-induced scratching (P < 0.05) (Fig. 5, A and B). In contrast, pretreatment with A71915 (3 nmol) had no effect on GRP-induced scratching (Fig. 5, C and D). It is noteworthy that GRP (0.1 nmol) elicited scratching at a greater magnitude compared with BNP (1 nmol).
Figure 6 compares the effects of NPRA antagonist A71915 and GRPR antagonist RC-3095 on intradermal pruritogen-induced scratching in mice. Endothelin-1 (0.1 nmol, Fig. 6, A and B), U-46619 (10 nmol, Fig. 6, C and D), BAM8-22 (50 nmol, Fig. 6, E and F), or SLIGRL (100 nmol, Fig. 6, G and H) elicited scratching bouts at different degrees of magnitude. A71915 (3 nmol) did not affect scratching induced by all four pruritogens. In contrast, RC-3095 (0.1 nmol) inhibited the SLIGRL-induced scratching [F(2, 15) = 4.576; P < 0.05] (Fig. 6, G and H) but had no effect on other pruritogens.
Figure 7 demonstrates the effects of GRPR antagonist RC-3095 and NPRA antagonist A71915 on intradermal SLIGRL-induced c-Fos activation in cervical dorsal horn at 2 hours after administration. SLIGRL (100 nmol) elicited c-Fos activation and approximately 16 c-Fos+ cells per section were observed in the nuclei of sensory laminae of dorsal horn (Fig. 7, B and C). Treatment with RC-3095 (0.1 nmol), but not A71915 (3 nmol), inhibited SLIGRL-induced c-Fos activation [F(3, 16) = 20.76; P < 0.05] (Fig. 7, A and C).
Discussion
The present study provides four novel findings advancing our understanding of how the itch sensation is regulated in the mouse spinal cord. First, intrathecal BNP dose dependently elicited scratching responses that were antagonized by the NPRA antagonist A71915, providing pharmacological evidence of spinal BNP-NPRA system for regulating itch. Second, NPRA proteins colocalized with GRP, but not GRPR, in the superficial area of dorsal horn, whereas BNP proteins did not colocalize with either GRP or GRPR in the dorsal horn; and a GRPR antagonist RC-3095 inhibited intrathecal BNP-induced scratching. These data support the notion that BNP-NPRA system may function upstream of the GRP-GRPR signaling in the pruriceptive circuit. Third, intrathecal A71915 did not attenuate scratching induced by intradermal administration of four different pruritogens. On the other hand, RC-3095 partially attenuated scratching induced by only SLIGRL but not by other pruritogens. At functionally receptor-selective doses, GRPR and NPRA antagonists seem limited in alleviating peripherally elicited itch. Fourth, RC-3095, but not A71915, inhibited intradermal SLIGRL-elicited c-Fos activation in the spinal dorsal horn. This finding complements behavioral effects of RC-3095 and A71915 on SLIGRL-induced scratching.
Prior to the present study, there was no dose-response study of BNP-elicited scratching. We found that intrathecal administration of BNP (0.3–1 nmol) dose dependently elicited scratching responses in mice (Fig. 1). However, the onset and magnitude of BNP-induced scratching are slower and smaller than GRP (Sukhtankar and Ko, 2013). Pretreatment with an NPRA antagonist A71915 dose dependently attenuated intrathecal BNP-induced scratching (Fig. 1). This finding not only identifies a functionally selective antipruritic dose of the NPRA antagonist without compromising motor function but also conceptually supports previous findings showing that treatment with BNP-saporin inhibited BNP-induced scratching (Mishra and Hoon, 2013). In addition, immunostaining revealed the expression of NPRA protein in the superficial area of dorsal horn (Fig. 4), which is similar to findings by Liu et al. (2014). Moreover, the expression of BNP proteins in the DRG and the superficial area of dorsal horn (Figs. 2 and 3) suggests that BNP may be produced by primary sensory neurons, which is anatomically similar to previous findings (Zhang et al., 2010; Mishra and Hoon, 2013). These lines of functional and anatomic evidence indicate that BNP-NPRA system is involved in the spinal processing of itch.
Similar to previous studies (Masui et al., 1993; Sukhtankar and Ko, 2013), intrathecal GRP rapidly elicited scratching (Fig. 5). The expression of GRPR protein was observed in the sensory laminae of dorsal horn (Figs. 3 and 4), consistent with previous reports (Sun and Chen, 2007; Fleming et al., 2012). These findings may suggest that intrathecally-delivered GRP acts directly on GRPR, resulting in rapid scratching behavior. Using a functionally receptor-selective dose (0.1 nmol) of the GRPR antagonist RC-3095 (Sukhtankar and Ko, 2013), we found that RC-3095 blocked BNP-induced scratching (Fig. 5). However, A71915 was ineffective in blocking GRP-induced scratching. Here we found direct colocalization of NPRA and GRP in the superficial area of dorsal horn (Fig. 4), as indirectly supported by previous findings using GRP-driven green fluorescent protein–expressing mice (Mishra and Hoon, 2013). Although GRP antibody may have cross-reacted with substance P in the preabsorption control experiment (Supplemental Fig. S1), we have demonstrated GRP-specific immunoreactivity in the dorsal horn in accordance with a recent study (Solorzano et al., 2015). Furthermore, we present the first evidence demonstrating that NPRA does not colocalize with GRPR in the dorsal horn (Fig. 4) and that BNP does not colocalize with either GRP or GRPR in the dorsal horn (Fig. 3). According to their protein expression patterns in the dorsal horn, BNP might be mainly secreted from primary afferent terminals. On the other hand, GRP might be provided by both primary afferent terminals and spinal interneurons. This notion is conceptually supported by previous studies (Sun and Chen, 2007; Takanami et al., 2014; Solorzano et al., 2015). These lines of evidence suggest that BNP-NPRA system might modulate GRP-expressing neurons without direct effects on GRPR-expressing neurons in the dorsal horn. On the basis of anatomic and functional evidence from previous finings (Mishra and Hoon, 2013) and the present study, we hypothesized that BNP-NPRA system functions upstream of GRP-GRPR system through the GRP release subsequent to NPRA activation. The evidence presenting each independent expression of GRP and GRPR in sensory laminae of dorsal horn also supports this hypothesis (Solorzano et al., 2015). Collectively, these findings indicate that BNP-induced scratching requires GRP release, thereby causing a delayed onset of BNP-induced scratching in comparison with GRP.
Several pruritogens have been identified and used in rodent studies to peripherally elicit itch scratching. We used endothelin-1 (Trentin et al., 2006), U-46619 [thromboxane A2 analog (Andoh et al., 2007)], BAM8-22 [activator of Mas-related G protein-coupled receptors (Sikand et al., 2011)], and SLIGRL [a proteinase-activated receptor-2 agonist (Shimada et al., 2006)], to elicit scratching through activation of their respective pruriceptors in primary afferents; we then determined the involvement of BNP-NPRA and GRP-GRPR systems in spinal processing of histamine-independent itch. Following lumbar intrathecal administration, a ligand is generally considered to distribute within the whole mouse spinal cord including cervical region (Nojima et al., 2003; Sun and Chen, 2007; Akiyama et al., 2013; Mishra and Hoon, 2013), as we demonstrated an even distribution throughout the spinal cord by using Evans blue dye (Supplemental Figure S2). Hence, using the same procedure as reported previously [e.g., Pereira et al. (2011); Akiyama et al. (2013); Akiyama et al. (2014)], we measured whether intrathecal pretreatment with either NPRA or GRPR antagonist can attenuate scratching responses elicited by intradermal pruritogens in the neck. At the dose sufficient to block spinal BNP-induced scratching, A71915 did not attenuate scratching responses elicited by these four pruritogens (Fig. 6). This is the first pharmacological evidence showing the minimal role of spinal BNP-NPRA system in regulating peripherally elicited scratching in mice. On the other hand, RC-3095 partially inhibited only SLIGRL-induced scratching, but not others. Indeed, the different effectiveness of RC-3095 against SLIGRL- versus BAM8-22-induced scratching observed herein is similar to that found by Akiyama et al. (2013). Nonetheless, other reports show that peripherally elicited scratching by not only SLIGRL but also other pruritogens were greatly reduced in BNP- or GRPR-knockout mice (Sun and Chen, 2007; Mishra and Hoon, 2013). Although these studies illustrate the cellular mechanisms of itch processing, knockout mice may often have unexpected compensatory influence on the homeostatic system (Lariviere et al., 2001). In addition, by using targeted toxins (i.e., saporin), neuronal ablation eliminates not only targeted receptors but also other molecules coexpressed on the same neurons. Findings from these approaches may not agree with the functional evidence of pharmacological blockade (Sasaki et al., 2013). Thus, we concluded that pharmacological antagonism of spinal NPRA or GRPR is not sufficient to relieve peripherally elicited itch under these conditions.
Conventionally, behavioral effect is supported by anatomic evidence or biologic action. The c-Fos is one of the early response genes and it is considered to be the suitable marker molecule of neuronal activation (Hunt et al., 1987; Morgan and Curran, 1989). Especially, c-Fos expression has often been evaluated to justify the activation of pain- or itch-processing neurons in the central nervous system (Presley et al., 1990; Yao et al., 1992). Several reports indicate that the c-Fos expression in dorsal horn neurons correspond with peripherally administered pruritogens, including SLIGRL (Nakano et al., 2008; Imamachi et al., 2009). In the present study, c-Fos activation was observed in the superficial area of cervical dorsal horn following intradermal administration of SLIGRL (Fig. 7). Inhibition of SLIGRL-induced c-Fos activation by RC-3095, but not by A71915, strongly supports the behavioral findings. These results indicate that an anatomic approach evaluating c-Fos expression strengthens validity of behavioral studies assessing antipruritic effects of these candidates. Although pruriceptive circuitry in the dorsal horn is still not clear, on the basis of our present findings we hypothesized that intradermal SLIGRL might induce the release of GRP from some GRP-expressing neurons, but only a small amount of BNP might be released in the dorsal horn.
In summary, this pharmacological study provides functional and anatomic evidence that BNP-NPRA system may function upstream of the GRP-GRPR system to regulate neurotransmission of itch in the mouse spinal cord. In addition, spinal blockade of GRPR is partially effective in regulating peripherally elicited itch. It is interesting to note that activation of supraspinal GRPR can also elicit excessive scratching in rodents (Su and Ko, 2011). Given that nonhuman primate behavioral models have been used to distinguish spinal versus supraspinal actions of different ligand-receptor systems for regulating itch and pain (Ding et al., 2015; Lee and Ko, 2015), it is essential to further investigate the functional relationship of central GRP-GRPR and BNP-NPRA systems in primates. Spinal delivery of GRP elicited robust scratching responses in primates (Lee and Ko, 2015), which can be used to compare the onset, magnitude, and duration of scratching activity elicited by any ligands, including BNP, and to determine if BNP-induced scratching depends on the GRP-GRPR system. More importantly, these pharmacological studies in nonhuman primates will provide a translational bridge to discover the effectiveness of NPRA and GRPR antagonists and other therapeutic candidates against centrally or/and peripherally elicited itch.
Acknowledgments
The authors thank Colette Cremeans, Erin Gruley, Jade Lackey, Emily Whitaker, and Jillian Odom for technical assistance.
Abbreviations
- A71915
(Arg6,β-cyclohexyl-Ala8,D-Tic16,Arg17,Cys18)-atrial natriuretic factor(6-18) amide
- BAM8-22
bovine adrenal medulla 8-22
- BNP
B-type natriuretic peptide
- DAPI
4′,6-diamidino-2-phenylindole
- DRG
dorsal root ganglion
- GRP
gastrin-releasing peptide
- GRPR
gastrin-releasing peptide receptor
- NPRA
natriuretic peptide receptor A
- RC-3095
(D-Tpi6,Leu13ψ(CH2-NH)-Leu14)bombesin(6-14)
Authorship Contributions
Participated in research design: Kiguchi, Sukhtankar, Ding, Tanaka, Ko.
Conducted experiments: Kiguchi, Sukhtankar, Ding.
Performed data analysis: Kiguchi, Sukhtankar, Ding, Kishioka, Peters, Ko.
Wrote or contributed to the writing of the manuscript: Kiguchi, Sukhtankar, Ding, Ko.
Footnotes
Research reported in this publication was supported by the National Institutes of Health, National Institute of Arthritis and Muskuloskeletal and Skin Diseases [R01-AR059193, R21-AR064456]. The content is solely the responsibility of the authors and does not necessarily represent the official views of U.S. federal agencies.
 This article has supplemental material available
at jpet.aspetjournals.org.
This article has supplemental material available
at jpet.aspetjournals.org.
References
- Akiyama T, Carstens E. (2013) Neural processing of itch. Neuroscience 250:697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. (2013) Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J Neurophysiol 109:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. (2014) Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 155:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Nishikawa Y, Yamaguchi-Miyamoto T, Nojima H, Narumiya S, Kuraishi Y. (2007) Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J Invest Dermatol 127:2042–2047. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, Hoon MA. (2014) Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 17:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biró T, Ko MC, Bromm B, Wei ET, Bigliardi P, Siebenhaar F, Hashizume H, Misery L, Bergasa NV, Kamei C, et al. (2005) How best to fight that nasty itch - from new insights into the neuroimmunological, neuroendocrine, and neurophysiological bases of pruritus to novel therapeutic approaches. Exp Dermatol 14:225–240. [DOI] [PubMed] [Google Scholar]
- Bishop JF, Moody TW, O’Donohue TL. (1986) Peptide transmitters of primary sensory neurons: similar actions of tachykinins and bombesin-like peptides. Peptides 7:835–842. [DOI] [PubMed] [Google Scholar]
- Ding H, Hayashida K, Suto T, Sukhtankar DD, Kimura M, Mendenhall V, Ko MC. (2015) Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol 172:3302–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. (2012) The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain 8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, Marks R, Naldi L, Weinstock MA, Wulf SK, et al. (2014) The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 134:1527–1534. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. (1987) Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328:632–634. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. (2009) TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA 106:11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A. (2011) Investigation of gastrin-releasing peptide as a mediator for 5′-guanidinonaltrindole-induced compulsive scratching in mice. Peptides 32:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. (2008) International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Sugaya M, Suga H, Morimura S, Kai H, Ohmatsu H, Fujita H, Tsunemi Y, Sato S. (2013) Serum gastrin-releasing peptide levels correlate with pruritus in patients with atopic dermatitis. J Invest Dermatol 133:1673–1675. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Dong X, Ringkamp M. (2014) Sensory neurons and circuits mediating itch. Nat Rev Neurosci 15:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere WR, Chesler EJ, Mogil JS. (2001) Transgenic studies of pain and analgesia: mutation or background genotype? J Pharmacol Exp Ther 297:467–473. [PubMed] [Google Scholar]
- Lee H, Ko MC. (2015) Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci Rep 5:11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER, Gardner DG, Samson WK. (1998) Natriuretic peptides. N Engl J Med 339:321–328. [DOI] [PubMed] [Google Scholar]
- Liu XY, Wan L, Huo FQ, Barry DM, Li H, Zhao ZQ, Chen ZF. (2014) B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol Pain 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui A, Kato N, Itoshima T, Tsunashima K, Nakajima T, Yanaihara N. (1993) Scratching behavior induced by bombesin-related peptides. Comparison of bombesin, gastrin-releasing peptide and phyllolitorins. Eur J Pharmacol 238:297–301. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Jörnvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. (1979) Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun 90:227–233. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. (2013) The cells and circuitry for itch responses in mice. Science 340:968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misono KS, Philo JS, Arakawa T, Ogata CM, Qiu Y, Ogawa H, Young HS. (2011) Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase. FEBS J 278:1818–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. (1989) Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci 12:459–462. [DOI] [PubMed] [Google Scholar]
- Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. (1991) Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 87:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Andoh T, Lee JB, Kuraishi Y. (2008) Different dorsal horn neurons responding to histamine and allergic itch stimuli. Neuroreport 19:723–726. [DOI] [PubMed] [Google Scholar]
- Nattkemper LA, Zhao ZQ, Nichols AJ, Papoiu AD, Shively CA, Chen ZF, Yosipovitch G. (2013) Overexpression of the gastrin-releasing peptide in cutaneous nerve fibers and its receptor in the spinal cord in primates with chronic itch. J Invest Dermatol 133:2489–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Simons CT, Cuellar JM, Carstens MI, Moore JA, Carstens E. (2003) Opioid modulation of scratching and spinal c-fos expression evoked by intradermal serotonin. J Neurosci 23:10784–10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PJ, Lazarotto LF, Leal PC, Lopes TG, Morrone FB, Campos MM. (2011) Inhibition of phosphatidylinositol-3 kinase γ reduces pruriceptive, inflammatory, and nociceptive responses induced by trypsin in mice. Pain 152:2861–2869. [DOI] [PubMed] [Google Scholar]
- Potter LR, Yoder AR, Flora DR, Antos LK, and Dickey DM (2009) Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications, in Handb Exp Pharmacol pp 191:341–366, Springer Berlin-Heidelberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley RW, Menétrey D, Levine JD, Basbaum AI. (1990) Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci 10:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Adhikari S, Andoh T, Kuraishi Y. (2013) BB2 bombesin receptor-expressing spinal neurons transmit herpes-associated itch by BB2 receptor-independent signaling. Neuroreport 24:652–656. [DOI] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. (2006) Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 530:281–283. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Sonoda A, Nogi C, Ogushi Y, Kanda R, Yamaguchi S, Nohara N, Aoki T, Yamada K, Nakata J, et al. (2014) B-type (brain) natriuretic peptide and pruritus in hemodialysis patients. Int J Nephrol Renovasc Dis 7:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Dong X, LaMotte RH. (2011) BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci 31:7563–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano C, Villafuerte D, Meda K, Cevikbas F, Bráz J, Sharif-Naeini R, Juarez-Salinas D, Llewellyn-Smith IJ, Guan Z, Basbaum AI. (2015) Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci 35:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su PY, Ko MC. (2011) The role of central gastrin-releasing peptide and neuromedin B receptors in the modulation of scratching behavior in rats. J Pharmacol Exp Ther 337:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Ko MC. (2013) Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS One 8:e67422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. (2007) A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448:700–703. [DOI] [PubMed] [Google Scholar]
- Takanami K, Sakamoto H, Matsuda KI, Satoh K, Tanida T, Yamada S, Inoue K, Oti T, Sakamoto T, Kawata M. (2014) Distribution of gastrin-releasing peptide in the rat trigeminal and spinal somatosensory systems. J Comp Neurol 522:1858–1873. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Kazerouni K, Chaplan SR, and Greenspan AJ (2015) Antihistamines and itch, in Handb Exp Pharmacol pp226:257–290, Springer Berlin-Heidelberg. [DOI] [PubMed] [Google Scholar]
- Trentin PG, Fernandes MB, D’Orléans-Juste P, Rae GA. (2006) Endothelin-1 causes pruritus in mice. Exp Biol Med (Maywood) 231:1146–1151. [PubMed] [Google Scholar]
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, Jupiter A, Matterne U, Weisshaar E. (2014) No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar E, Dalgard F. (2009) Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol 89:339–350. [DOI] [PubMed] [Google Scholar]
- Yao GL, Tohyama M, Senba E. (1992) Histamine-caused itch induces Fos-like immunoreactivity in dorsal horn neurons: effect of morphine pretreatment. Brain Res 599:333–337. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Bernhard JD. (2013) Clinical practice. Chronic pruritus. N Engl J Med 368:1625–1634. [DOI] [PubMed] [Google Scholar]
- Zhang FX, Liu XJ, Gong LQ, Yao JR, Li KC, Li ZY, Lin LB, Lu YJ, Xiao HS, Bao L, et al. (2010) Inhibition of inflammatory pain by activating B-type natriuretic peptide signal pathway in nociceptive sensory neurons. J Neurosci 30:10927–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]