Abstract
Activation of adenosine receptors (ARs) has been implicated in the modulation of renal and cardiovascular systems, as well as erectile functions. Recent studies suggest that adenosine-mediated regulation of erectile function is mainly mediated through A2BAR activation. However, no studies have been conducted to determine the contribution of AR subtype in the regulation of the vascular tone of the pudendal artery (PA), the major artery supplying and controlling blood flow to the penis. Our aim was to characterize the contribution of AR subtypes and identify signaling mechanisms involved in adenosine-mediated vascular tone regulation in the PA. We used a DMT wire myograph for muscle tension measurements in isolated PAs from wild-type, A2AAR knockout, A2BAR knockout, and A2A/A2BAR double-knockout mice. Real-time reverse transcription–polymerase chain reaction was used to determine the expression of the AR subtypes. Data from our pharmacologic and genetic approaches suggest that AR activation–mediated vasodilation in the PA is mediated by both the A2AAR and A2BAR, whereas neither the A1AR nor A3AR play a role in vascular tone regulation of the PA. In addition, we showed that A2AAR- and A2BAR-mediated vasorelaxation requires activation of nitric oxide and potassium channels; however, only the A2AAR-mediated response requires protein kinase A activation. Our data are complemented by mRNA expression showing the expression of all AR subtypes with the exception of the A3AR. AR signaling in the PA may play an important role in mediating erection and represent a promising therapeutic option for the treatment of erectile dysfunction.
Introduction
Penile erection consists of multiple neurovascular processes that all simultaneously involve the nerves, blood vessels, and endothelium in the sinusoids and trabecular smooth muscle cells of the penis. Those factors, which regulate contraction and relaxation of vascular smooth muscle, determine the state of the penis (flaccidity versus erection) (Andersson, 2001; Tostes et al., 2007; Nunes et al., 2012). The flaccid state is mainly mediated by the release of norepinephrine from adrenergic nerve terminals and other vasoconstrictors, such as endothelin-1 and angiotensin II (Sáenz de Tejada et al., 1989, 2004). The erection is mainly mediated by nitric oxide (NO) released from the endothelium and nonadrenergic-noncholinergic nerves in addition to other neurotransmitters and endothelium-derived hyperpolarizing factors (EDHFs), such as adenosine (Chiang et al., 1994; Tostes et al., 2007; Wen et al., 2011b).
Adenosine, a signaling nucleoside, is produced during conditions of metabolic stress and high cellular activity, resulting in increased oxygen supply and decreased oxygen consumption. Adenosine is mainly generated by the 5′-nucleotidases CD73 that catalyze the dephosphorylation of AMP into adenosine. Intracellular adenosine levels are primarily regulated by adenosine kinase, which converts adenosine to AMP, whereas extracellular adenosine levels are critically regulated by adenosine deaminase, which degrades adenosine to inosine (Blackburn, 2003; Ham and Evans, 2012; Wen and Xia (2012)). Recently, adenosine was described as an EDHF because of its ability to relax and hyperpolarize vascular smooth muscle cells (VSMC) (Ohta et al., 2013). The activation of adenosine receptors (ARs) is implicated in the modulation of renal and cardiovascular functions, as well as erectile function, with both in vivo and in vitro studies demonstrating that, like NO, adenosine is a potent vasodilator that may regulate penile erection in humans and animals (Takahashi et al., 1991, 1992; Chiang et al., 1994; Mantelli et al., 1995; Filippi et al., 2000; Noto et al., 2001; Tostes et al., 2007; Carneiro et al., 2008; Mi et al., 2008; Vallon and Osswald, 2009; Phatarpekar et al., 2010; Wen et al., 2010, 2011b, 2012; Ning et al., 2012; Headrick et al., 2013; Layland et al., 2014). Adenosine binds to a family of four P1 G protein-coupled AR subtypes: A1, A2A, A2B, and A3. Vascular studies from our laboratory and others have demonstrated that, whereas the activation of the A1AR and A3AR results in vasoconstriction, the activation of the A2AAR and A2BAR results in vasodilation (Tawfik et al., 2005; Ansari et al., 2007; Nayeem et al., 2008; El-Awady et al., 2011; Sanjani et al., 2011; El-Gowelli et al., 2013; Hein et al., 2013; Kunduri et al., 2013; Teng et al., 2013). The different contribution of each AR subtype to the physiology or pathophysiology of erection has been studied in the corpus cavernosum (CC). Studies in humans and animals reported that both the A2AAR and A2BAR mediate the CC’s vasorelaxation (Filippi et al., 2000; Noto et al., 2001; Faria et al., 2006; Tostes et al., 2007; Mi et al., 2008; Moura et al., 2015). Using quantitative real-time polymerase chain reaction (qPCR), Mi et al. (2008) showed that the A2BAR was the predominant receptor subtype expressed in murine cavernosal smooth muscle cells, whereas relatively low level expression of the A2AAR was observed. The subtypes promoting vasoconstriction, namely the A1AR and A3AR, were not detectable. In contrast, an earlier study by Tostes et al. (2007) suggested that activation of both the A2AAR and A2BAR mediate CC relaxation in mice. While the contribution of the A1AR to erectile function plays an important role in the release of neurotransmitters, the role of the A3AR in erectile function is still not known (Chiang et al., 1994; Tostes et al., 2007; Ning et al., 2012). Recent studies demonstrated that adenosine functions to promote penile erection; however, this research has focused solely on the CC.
In addition to the CC, an important player in regulating erectile function and blood flow to the penis is the pudendal artery (PA). The PA is an artery that branches from the internal iliac artery, providing oxygenated blood to the external genitals. The PA branches into cavernous arteries that further branch into tortuous helicine arteries, all feeding the cavernous sinuses. In the PA, the absence of capillaries allows for a rapid filling of cavernosal sinuses during erection (Hale et al., 2009). Recent studies have shown that the PA contributes approximately 70% of total pudendal-penile vascular resistance, whereas the intrapenile vasculature contributes less than 25% of total resistance in this bed (Manabe et al., 2000). In addition, it has been demonstrated that optimal erection requires vasodilation of both prepenile arteries, such as the PA, as well as intrapenile arteries (Manabe et al., 2000; Hale et al., 2009; Hannan et al., 2011). To date, no studies have characterized the contribution of adenosine and its receptors in the regulation of the PA vascular tone. In the present study, we characterized the contribution of AR subtypes to vascular tone in the PA and identified signaling mechanisms involved in adenosine-mediated vascular tone regulation.
Materials and Methods
Animals.
The Institutional Animal Care and Use Committee of West Virginia University approved all experimental protocols. We followed guidelines set forth by the American Physiologic Society and National Institutes of Health regarding the care and use of laboratory animals. A2AAR and A2BAR single knockout (KO) mice (A2AAR KO and A2BAR KO mice, respectively) were generously provided by Dr. C. Ledent (Université libre de Bruxelles, Brussels, Belgium) and Stephen Tilley (University of North Carolina, Chapel Hill, NC), respectively. A2AAR and A2BAR KO mice, both backcrossed 12 generations to wild-type (WT) C57BL/6 background mice (Jackson Laboratory, Bar Harbor, ME) were bred to generate A2A/A2BAR double-KO (DKO) heterozygotes. Double heterozygotes were intercrossed, and 1/16 of the offspring were A2A/A2BAR DKO mice. A2A/A2BAR DKO breeding pairs were then established (Zhou et al., 2014). Mice were caged on a 12-hour light/dark cycle with free access to standard chow diet, with water ad libitum.
Muscle Tension Studies in Pudendal Arteries.
Mice were euthanized using sodium pentobarbital (50 mg/kg i.p.). PAs were excised, transferred into an ice-cold physiologic salt solution (130 mM NaCl, 14.9 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4 ⋅ 7H2O, 1.6 mM CaCl2 ⋅ 2H2O), and dissected to remove loose connective tissue and fat. The arteries were then cut into 2-mm segments and mounted on a wire myograph in 5-ml chambers (DMT, Aarhus, Denmark) containing buffer at 37°C and continuously bubbled with a mixture of 95% O2 and 5% CO2. The tissues were stretched to a resting force of 200 mg and allowed to equilibrate for 60 minutes. Changes in isometric force were recorded using a PowerLab/8SP data acquisition system (Chart software, version 5.0; ADInstruments, Colorado Springs, CO). After equilibration, rings were precontracted with 50 mM KCl to check the contractility of the individual PA rings. Arterial and endothelial integrity were assessed by contracting with phenylephrine (Phe, 10−6 M), followed by relaxation with acetylcholine (ACh, 10−6 M). Concentration-response curves (CRC) for AR agonists [5′-N-ethylcarboxamidoadenosine (NECA), 2-chloro-N6-cyclopentyladenosine (CCPA), and 1-[2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide (Cl-IBMECA), 10−9 M to 10−5 M] were performed. Endothelium-dependent relaxation was assessed by measuring the dilatory response to ACh (10−9 M to 10−5 M) in Phe-contracted vessels.
Drugs and Solutions.
ACh, Phe, NECA, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; an A1AR antagonist), KT 5720 [a protein kinase A (PKA) inhibitor], MRS1523 (an A3AR antagonist), NG-nitro-l-arginine methyl ester [L-NAME; a nitric oxide synthase (NOS) inhibitor], and Cl-IBMECA (an A3AR agonist) were purchased from Sigma-Aldrich/MilliporeSigma (St. Louis, MO). CCPA (an A1AR agonist), SCH-58261 (a selective A2AAR antagonist), and CVT-6883 (a selective A2BAR antagonist) were purchased from Tocris Bioscience (Minneapolis, MN). Stock solutions were prepared in deionized water or dimethyl sulfoxide (DMSO) and stored in aliquots at –20°C. Dilutions were prepared immediately before use. Rings were preincubated with antagonist or inhibitors 30 minutes prior to Phe preconstruction for the CRC. Stock solutions originally diluted in DMSO were used with a final concentration of less than 0.003% v/v in the muscle bath; this concentration has been demonstrated to have no effect on vascular reactivity.
Real-Time Reverse Transcription–Polymerase Chain Reaction.
Total RNA was isolated from PAs of WT mice using an RNAEasy Total RNA isolation kit from Qiagen (Valencia, CA). This was followed by conversion of 0.5 μg of total RNA into complementary DNA (cDNA) using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions in a total volume of 100 μl. Each sample contained PAs pooled from seven to nine mice. Because of the relatively low expression of ARs, a polymerase chain reaction (PCR) PreAmplification Kit (Applied Biosystems) was used. Reverse transcription–polymerase chain reaction (qPCR) was performed using an ABI PRISM 7300 Detection System (Applied Biosystems) with TaqMan Universal Master Mix (Applied Biosystems, Branchburg, NJ) according to manufacturer’s instructions. The reaction volume (25 μl) included 12.5 μl of 2× TaqMan Universal Master Mix, 1 μl of cDNA, and 1.25 μl of 20× FAM-labeled TaqMan Gene Expression Assay Master Mix solution. For real-time PCR of AR genes, the TaqMan-inventoried gene expression product was purchased from Applied Biosystems. The 18S ribosomal RNA was used as an endogenous control. The fold-difference in expression of target cDNA was determined using the comparative CT method. The ΔCT value was determined in each experiment by subtracting the average 18S CT value from the corresponding average CT for the A1, A2A, A2B, and A3AR in coronary arteries, as previously described (Teng et al., 2013).
Statistical Analysis.
Student’s t test was used for the comparison between two groups, and one-way analysis of variance was used for groups of three or more. CRC data were analyzed between groups at the same concentrations. In addition, an F-test was used for the estimation of EC50 values obtained from best-fit analysis using a nonlinear, interactive fitting program (GraphPad Prism; Graph Pad Software Inc. San Diego CA). Data are expressed as means ± S.E.M. (N), where N is the number of mice. Values of P < 0.05 were considered a statistically significant difference.
Results
NECA-Mediated Relaxation in the PA Is Dependent on A2AAR and A2BAR Activation.
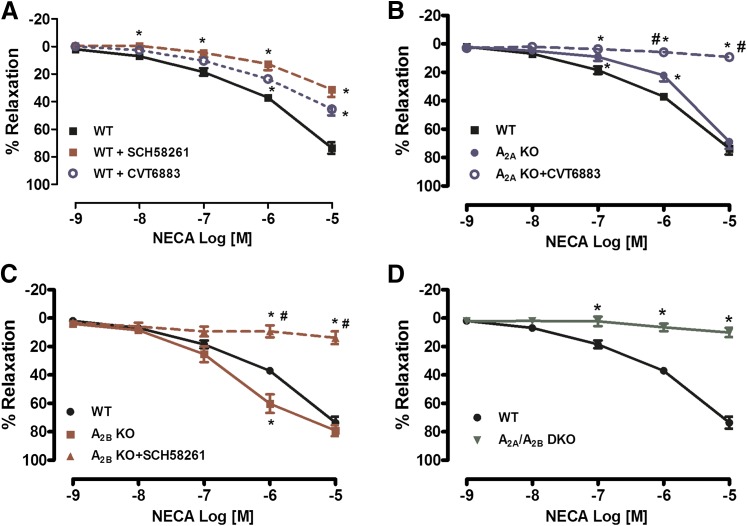
In preconstricted PA rings isolated from WT mice, NECA (a nonselective AR agonist) produced a concentration-dependent relaxation, suggesting a role for either the A2AAR or A2BAR, since both ARs are associated with vascular relaxation. Preincubation with either the A2AAR antagonist SCH-58261 or A2BAR antagonist CVT-6883 significantly decreased the NECA-mediated relaxation (Fig. 1A). Furthermore, PAs isolated from A2AAR KO mice exhibited a decreased relaxation response to NECA (EC50 –5.88 ± 0.10 in WT versus –5.40 ± 0.17 in A2AAR KO mice, P < 0.05) (Fig. 1B). Incubation of PAs isolated from A2AAR KO mice with the A2BAR antagonist abolished the NECA-mediated vasorelaxation (Fig. 1B). Contrary to A2AAR KO mice, PAs isolated from A2BAR KO mice exhibited an increase in sensitivity to NECA compared with PAs from WT mice (–5.88 ± 0.10 versus –6.50 ± 0.15, P < 0.05) (Fig. 1C). Incubation of PAs isolated from A2BAR KO mice with the A2AAR antagonist abolished the NECA-mediated vasorelaxation (Fig. 1C). Furthermore, genetic deletion of both the A2AAR and A2BAR resulted in the absence of vasodilation in response to increased NECA concentrations (Fig. 1D). Taken together, our data suggest that both the A2AAR and A2BAR contribute to relaxation in the PA.
Fig. 1.
Adenosine receptor (AR)-mediated relaxation in the pudendal artery (PA) is mediated through activation of both the A2AAR and A2BAR. (A) NECA induced a concentration- dependent relaxation, and treatment with either an A2AAR antagonist (SCH-58261; 1 μM) or A2BAR antagonist (CVT-6883; 1 μM) resulted in a decrease in NECA-mediated vasorelaxation. (B) Genetic deletion of the A2AAR resulted in a decrease in NECA-mediated relaxation compared with wild type (WT), and treatment with an A2BAR antagonist (CVT-6883) completely abolished the NECA response. (C) Genetic deletion of the A2BAR resulted in significantly altered NECA-mediated relaxation compared with WT, and treatment with an A2AAR antagonist (SCH-58261) abolished the NECA response. (D) In A2A/A2BAR DKO mice, the vasorelaxation response to the adenosine receptor agonist NECA was completely abolished. Data are represented as mean ± S.E.M. (N = 5–7). *P < 0.05 versus WT and #P < 0.05 versus corresponding KO (A2AAR KO or A2BAR KO).
Neither A1AR Nor A3AR Activation Resulted in Vasoconstriction in the PA.
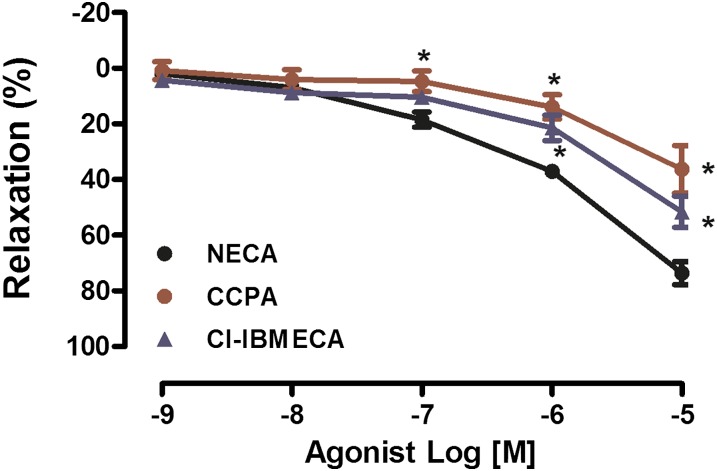
Activation of either A1AR or A3AR is known to result in vasoconstriction. Cumulative addition of CCPA (an A1AR agonist) or Cl-IBMECA (an A3AR agonist) did not cause vasoconstriction in PAs isolated from WT mice. Furthermore, both CCPA and Cl-IBEMCA caused vasorelaxation of PAs at high concentrations (10−6 M and 10−5 M), possibly owing to nonselectivity of the agonists; however, the vasodilatory response to CCPA and Cl-IBMECA was significantly lower in comparison with that elicited by NECA (Fig. 2).
Fig. 2.
A1AR and A3AR agonists caused vasorelaxation in PAs isolated from WT mice. A CRC to NECA, CCPA, and Cl-IBMECA was performed in PAs isolated from WT mice. Data are represented as mean ± S.E.M. (N = 5–7). *P < 0.05 versus NECA.
Neither A1AR nor A3AR is Required for AR-Mediated Vasorelaxation.
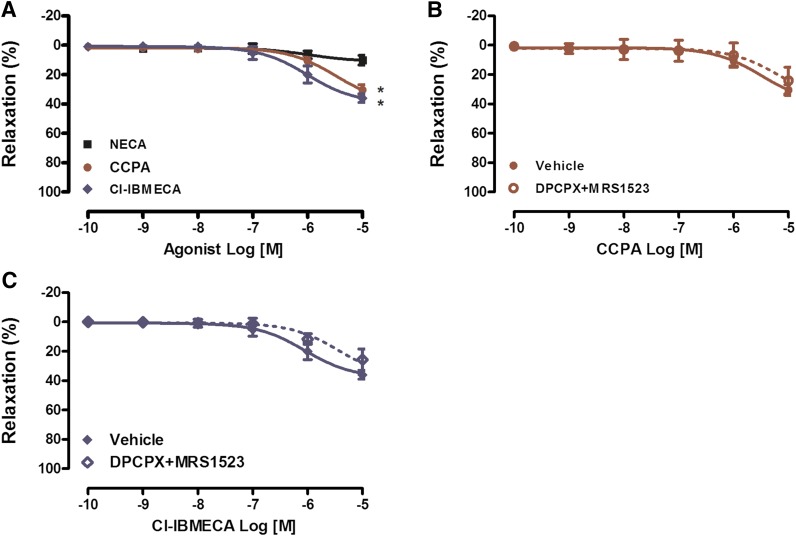
Since A1AR or A3AR agonists were shown to activate either the A2AAR or A2BAR at high concentrations, we used PAs isolated from A2A/A2BAR DKO mice (Fig. 3). In preconstricted PAs isolated from A2A/A2BAR DKO mice, NECA did not affect the vascular relaxation, whereas both CCPA and Cl-BMECA caused dilation at the highest dose (10−5 M) (Fig. 3A). Incubation of PAs isolated from A2A/A2BAR DKO mice with both the A1AR antagonist DPCPX and the A3AR antagonist MRS-1593 did not affect CCPA- and Cl-BMECA-mediated relaxation at the 10−5 M concentration, suggesting that the dilation mediated by CCPA and Cl-BMECA at the 10−5 M concentration is not dependent on ARs (Fig. 3, B and C).
Fig. 3.
Neither A1AR nor A3AR is required for the AR-mediated vasorelaxation in PAs. (A) CRC to NECA, CCPA and Cl-IBMECA in A2A/A2BAR DKO mice. (B) CRC to CCPA in A2A/A2BAR DKO mice in the presence of vehicle or the A1AR antagonist DPCPX (0.1 μM) and the A3AR antagonist MRS1523 (1 μM). (C) CRC to Cl-IBMECA in A2A/A2BAR DKO mice in the presence of vehicle or DPCPX and MRS1523. Data are represented as mean ± S.E.M. (N = 5–8). *P < 0.05 versus NECA.
Genetic Deletion of A2AAR or A2BAR Resulted in Decreased Endothelium-Mediated Vasorelaxation.
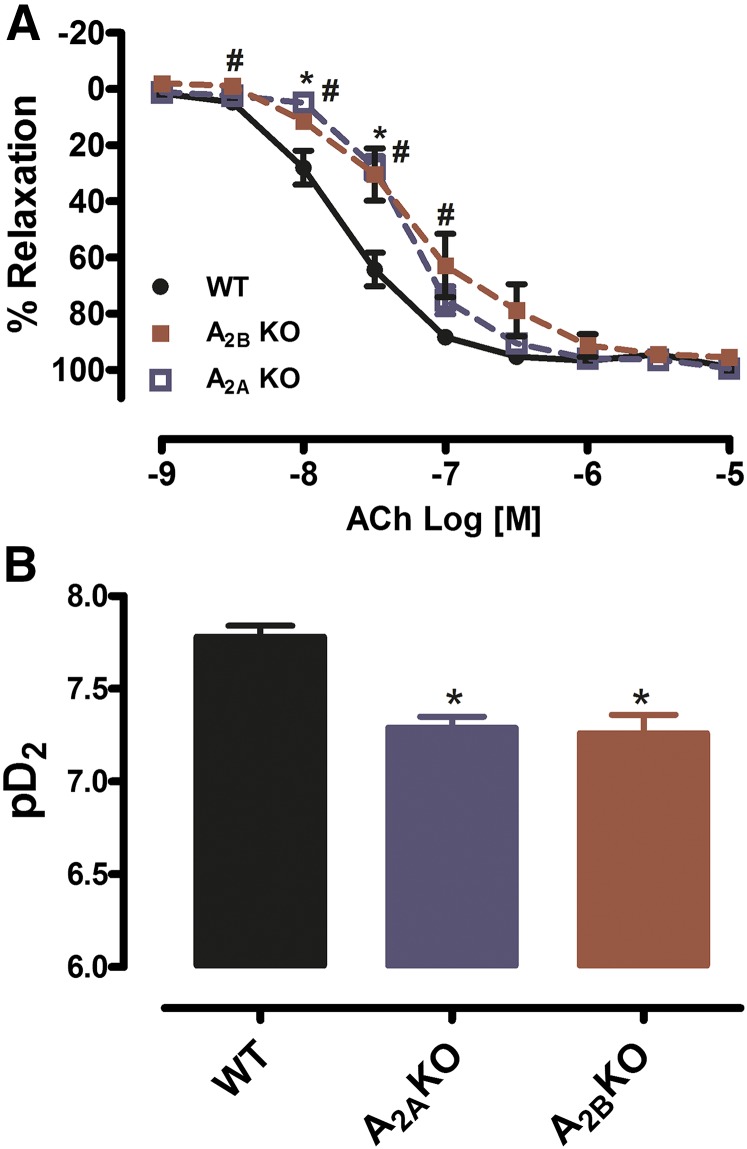
Recently, adenosine was described as an EDHF owing to its ability to relax and hyperpolarize VSMCs. To test the effect of either A2AAR or A2BAR deletion on the endothelium-mediated dilation, increased concentration of ACh resulted in a concentration- dependent relaxation in PAs isolated from WT, A2AAR KO, and A2BAR KO mice (Fig. 4A). However, PAs isolated from A2A KO or A2B KO mice exhibited a decrease in EC50 in response to ACh compared with WT mice: EC50 –7.78 ± 0.06 in WT versus –7.29 ± 0.06 in A2A KO, P < 0.05; and EC50 –7.78 ± 0.06 in WT versus –7.26 ± 0.10 in A2B KO, P < 0.05 (Fig. 4B). Taken together, our data suggest that both the A2AAR and A2BAR may play a role in the endothelium-mediated relaxation.
Fig. 4.
Genetic deletion of either the A2AAR or A2BAR resulted in decreased sensitivity of the PA to endothelium-mediated relaxation. (A) A2AAR KO and A2BAR KO mice exhibit decreases in ACh-mediated relaxation (*P < 0.05 A2A KO versus WT and #P < 0.05 A2B KO versus WT). (B) Representative graph of EC50 (expressed as potency or pD2) from both A2AAR KO and A2BAR KO mice exhibited a decrease in EC50 (*P < 0.05 KOs versus WT). Data are represented as mean ± S.E.M. (N = 5–7).
Mechanism of A2AAR- and A2BAR-Mediated Relaxation in the PA.
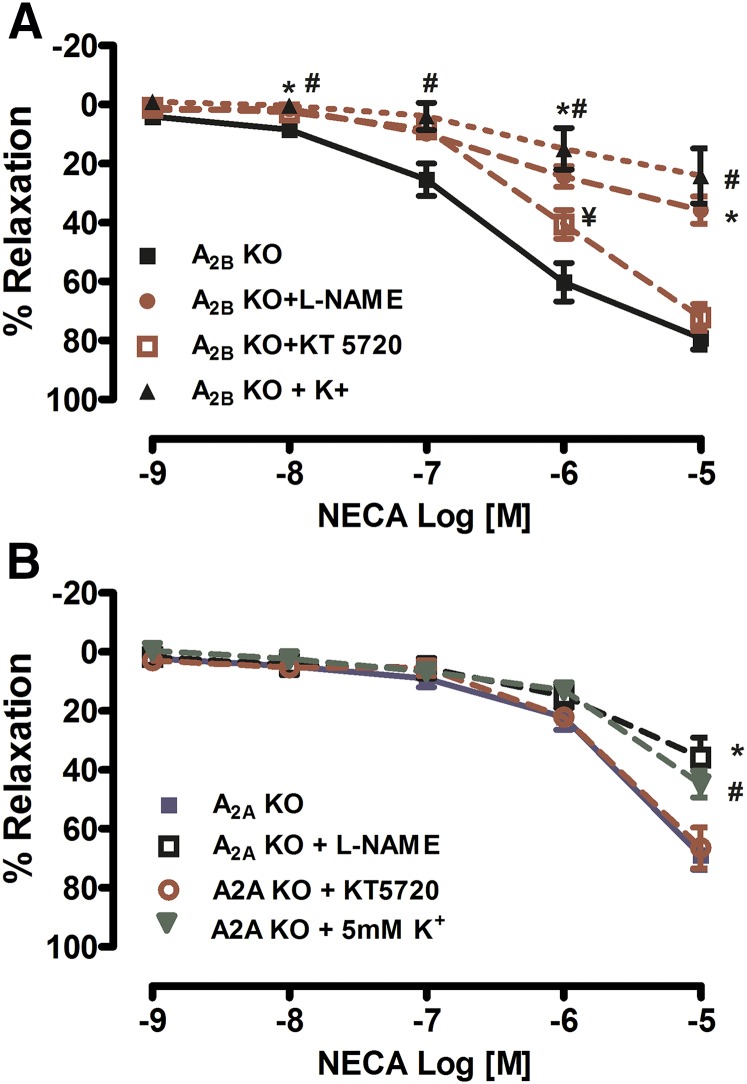
To look at the mechanisms of A2AAR-mediated relaxation, we used PAs isolated from A2B KO mice (Fig. 5A). Preincubation with either an NOS inhibitor (L-NAME), protein kinase A inhibitor (KT 5720), or potassium channel inhibition (5 mM KCl) significantly decreased A2A-mediated vasorelaxation in PAs isolated from A2A KO mice. To elucidate the mechanisms of A2BAR-mediated relaxation, we used PAs isolated from A2A KO mice (Fig. 5B). Pretreatment with the either L-NAME or increased extracellular K+ significantly decreased the A2B-mediated vasorelaxation, whereas treatment with KT 5720 did not affect relaxation in PAs isolated from A2A KO mice. Taken together, our data suggest that both A2AAR- and A2BAR-mediated vasorelaxation requires activation of NO and K+ channels; however, only the A2AAR-mediated response requires PKA activation.
Fig. 5.
Both A2AAR- and A2BAR-mediated vasorelaxation is dependent on nitric oxide (NO) in PAs. (A) Treatment with a nitric oxide synthase (NOS) inhibitor (L-NAME; 100 μM), increased K+ (5 mM KCl), or with a protein kinase A (PKA) inhibitor (KT 5720; 0.1 μM) significantly decreased A2AAR-mediated vasorelaxation from A2BAR KO mice. (B) Treatment with the either the NOS inhibitor or increased K+ significantly decreased the A2BAR-mediated vasorelaxation, whereas treatment with KT 5720 (0.1 μM) did not affect relaxation in A2AAR KO mice. Data are represented as mean ± S.E.M. (N = 4–7). *P < 0.05 L-NAME versus vehicle, #P < 0.05 K+ versus vehicle and ¥P < 0.05 KT5720 versus vehicle.
Message RNA Expression of ARs in the PA.
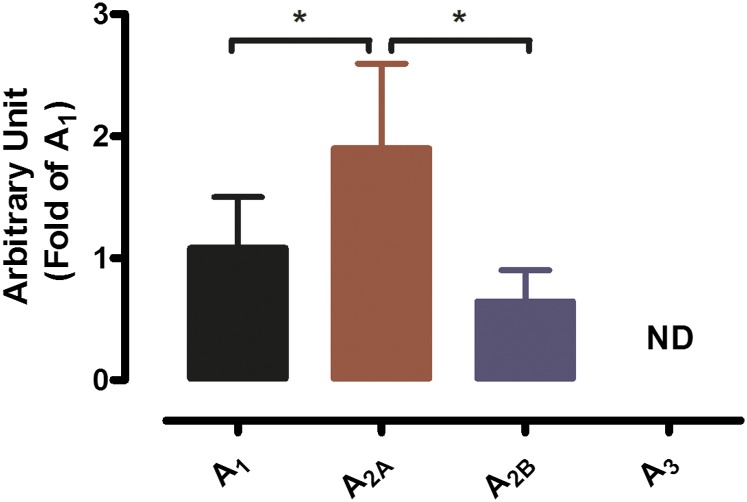
In support of our observations made in the functional studies, semiquantitative real-time PCR was used to determine mRNA expression of the AR subtypes in PAs isolated from WT mice. Of the four AR subtypes, only the A1R, A2AAR, and A2BAR were expressed, with the A2AAR having the highest mRNA expression level compared with the A1R and A2BAR (Fig. 6).
Fig. 6.
Messenger RNA expression of A1, A2A, A2B, and A3 AR subtypes as determined by real-time reverse-transcriptase PCR using total RNA extracted from WT mouse PAs. Values were normalized by the corresponding 18S ribosomal RNA of each sample. Results are represented as mean ± S.E.M. (N = 7), with each N representing pooled PAs from seven to nine mice. *P < 0.05.
Discussion
Using pharmacological and genetic approaches, our study is the first to investigate transcriptional expression and to characterize the contribution of the four AR subtypes to vascular tone regulation of the PA. Herein, we showed that: 1) of the four AR subtypes, only the A1R, A2AAR, and A2BAR were expressed in the PA; 2) although the A1AR was expressed in the PA, only the A2AAR and A2BAR played a role in vascular tone regulation of the PA; and 3) both A2AAR- and A2BAR-mediated vasorelaxation requires activation of NOS and K+ channels; however, only A2AAR-mediated responses requires PKA activation. In this paper, we sought to fill a gap in the understanding of AR signaling in erectile function and define the roles of AR subtypes in prepenile vascular tone regulation.
Numerous studies have investigated the contribution of adenosine to erectile function, looking at the effect of AR activation in the CC. However, this study is the first to characterize the contribution of ARs in the regulation of PA vascular tone, the major artery supplying and controlling blood flow to the penis (Manabe et al., 2000; Hale et al., 2009). In the first set of experiments, we observed that the adenosine analog NECA caused a concentration-dependent vasorelaxation in murine PAs. As we already know, A2AAR and A2BAR activation results in vasorelaxation, whereas A1AR and A3AR activation results in vasoconstriction. In this study, our results suggested that NECA-induced relaxation is mediated through A2AAR and A2BAR activation. Indeed, preincubation of PA rings with specific A2AAR (SCH-58261) or A2BAR (CVT-6883) antagonists significantly decreased the NECA-mediated relaxation, suggesting that both A2AAR and A2BAR activation are required for the response to NECA in PAs (Fig. 1A). Furthermore, PAs isolated from A2A KO mice exhibited a decreased relaxation to NECA, which was abolished using an A2BAR antagonist. Contrary to A2A KO mice, PAs isolated from A2B KO mice exhibited an increase in sensitivity to NECA compared with PAs from WT mice; however, when incubated with an A2AAR antagonist, the relaxation response to NECA was completely abolished. The difference in PA responses using pharmacological inhibition, compared with other genetic KO models, can be explained by the fact that global gene deletion usually results in compensatory upregulation of other genes or other compensatory signaling pathways. Regarding A2A and A2B ARs, although it has been shown that a single gene KO results in upregulation of the other AR in mesenteric artery (Teng et al., 2013), this was not the case in aorta, where KO of A2AAR gene did not affect the expression of A2BAR (Ponnoth et al., 2009). This also confirms the importance of pharmacological studies in addition to studies using gene deletion. In addition, the differences in the affinity of the receptors to the agonist could also explain the different response to NECA in the PA isolated from the KO mice, since A2BAR possesses the lowest affinity for adenosine and NECA. Our results were confirmed using PAs isolated from A2A/A2BAR DKO mice, where NECA failed to cause vasorelaxation in the PA rings. Our data confirm the important role played by both the A2AAR and A2BAR in CC relaxation and, thus, erection. In fact, several studies reported the role of both the A2AAR and A2BAR in mediating CC vasorelaxation (Filippi et al., 2000; Noto et al., 2001; Faria et al., 2006; Tostes et al., 2007). In mice, a recent study using a genetic approach by Mi and colleagues (2008) showed high A2BAR expression in the CC compared with expression of the A2AAR. They also showed that vasorelaxation of the CC is mediated solely through the activation of the A2BAR, whereas the A2AAR did not seem to play a role. However, Tostes et al. (2007) showed that both A2AAR antagonist SCH-58261 and A2BAR antagonist MRS-1706 resulted in decreased CC relaxation to the nonspecific agonist 2-chloroadenosine, with the combination of both antagonists resulting in almost total inhibition of the CC’s relaxation, supporting our data in the present study, whereas both the A2AAR and A2BAR contribute to relaxation of the CC. In addition, other groups also have shown the contribution of the A2AAR to vasorelaxation in the CC (Mantelli et al., 1995; Filippi et al., 2000; Noto et al., 2001; Faria et al., 2006; Moura et al., 2015).
A1AR and A3AR activation has been shown to mediate vascular contraction (Ansari et al., 2007; El-Awady et al., 2011; Kunduri et al., 2013). To test the contribution of the A1AR and A3AR in the regulation of PA vascular tone, we performed CRCs to both an A1AR agonist (CCPA) and A3AR agonist (Cl-IBMECA). Both agonists failed to mediate vasoconstriction in PAs, suggesting that neither A1AR nor A3AR contribute to the vascular tone in PAs. However, both drugs at high concentrations caused PA relaxation. These results were not surprising, as both drugs at high concentrations activate other receptors, including A2AAR and A2BAR (Teng and Mustafa, 2011). To further rule out the role of A1AR and A3AR, we performed the CRC to an A1AR agonist (CCPA) and A3AR agonist (Cl-IBMECA) in PAs isolated from A2A/A2BAR DKO mice. To our surprise, both agonists (CCPA and Cl-IBMECA) were able to mediate vasorelaxation in PAs from A2A/A2BAR DKO mice at high concentrations (10−5 M; Fig. 3A). Preincubation of PAs isolated from A2A/A2BAR DKO mice with both A1R and A3AR antagonists (DPCPX and MRS1523, respectively) did not affect vasorelaxation mediated by either CCPA or Cl-IBMECA at 10−5 M, suggesting that relaxations to both CCPA and Cl-IBMECA are not mediated by AR activation and may be attributable to nonspecific agonist effects (Fig. 3, B and C).
Recently, adenosine was described as an EDHF, with CD73 as an EDHF synthase, owing to its ability to relax and hyperpolarize VSMCs (Ohta et al., 2013). We looked at the effect of the genetic deletion of either A2AAR or A2BAR on endothelium-mediated vasorelaxation in PAs. CRCs to ACh in PAs isolated from A2AAR KO and A2BAR KO mice resulted in a rightward shift of CRC in A2AAR KO and A2BAR KO mice and decreased sensitivity to ACh compared with WT mice, suggesting that both receptors may play a role in endothelium-mediated relaxation (Fig. 4, A and B). Our data suggest that the A2AAR KO and A2BAR KO partially contributed to the endothelium-mediated vasorelaxation in the PA, corroborating the role of adenosine as an EDHF.
Next, we sought to elucidate the mechanism of AR-mediated relaxation in the PA. To dissect the signaling pathways for A2AR-mediated relaxation, we used PAs isolated from A2BAR KO mice to study A2AAR-mediated relaxation and A2AAR KO mice to study A2BAR-mediated relaxation in the PA (Fig. 5). Previous studies suggested a role for PKA, the NO signaling pathway, and potassium (K+) channels in mediating adenosine-mediated vasorelaxation (Mi et al., 2008; Sanjani et al., 2011; El-Gowelli et al., 2013; Hein et al., 2013). Our data showed that PKA inhibition caused a rightward shift of the CRC, resulting in a decrease in relaxation to NECA. In addition, inhibition of NOS and increasing extracellular K+ resulted in a decrease in NECA-mediated vasorelaxation in PAs (Fig. 5A). Our data suggest that A2AAR-mediated relaxation in the PA is dependent on PKA, NO, and K+ channel activation. On the other hand, we found that A2BAR-mediated relaxation in the PA is dependent on NO and K+ channel activation, but not PKA, as PKA inhibition did not result in decreased NECA-mediated vasorelaxation in PAs isolated from A2AAR KO mice. In fact, a recent study showed that A2BAR contributed to penile erection via an alternative signaling pathway, which is dependent on PI3K/AKT signaling (Wen et al., 2011b).
Several studies have looked at the contribution of adenosine to erectile function both in vivo and in vitro. However, limited studies have tried to characterize AR expression in the erectile system. Using qPCR, Mi et al. (2008) demonstrated that the A2BAR is the predominant receptor, with relatively low expression of the A2AAR, whereas mRNA levels of both the A1AR and A3AR were nondetectable in CC smooth muscle cells. A similar pattern of AR expression was found in another study using primary CC fibroblast cells (Wen et al., 2011b). However, the expression level of ARs at the whole tissue level or other cell types in the CC (i.e., endothelial cells) was not evaluated. In the present study, we used isolated PAs to evaluate the expression pattern of AR subtypes using qPCR. We found that A1AR, A2AAR, and A2BAR were expressed in PAs (with higher expression of A2AAR compared with A1AR and A2BAR), whereas A3AR mRNA levels were not detectable (Fig. 6). These results are in accordance with our functional data in the case of A2AAR and A2BAR; however, it did not translate to vascular response in the case of A1AR, where there was a lack of response to the A1AR agonist CCPA (Fig. 3B). Although unexpected, this was not surprising, as previous studies also showed a lack of A2AAR-mediated vascular response despite the presence of this receptor in rat and mouse mesenteric arteries (Rubino et al., 1995; Teng et al., 2013). In addition, one limitation of the present study is that, whereas the mRNA levels are important to determine, it was difficult to assess the protein expression and level owing to the size of the tissue, which would be more functionally relevant. Although A1AR activation did not affect vascular reactivity, A1AR may still contribute to the overall PA vascular tone, as it was shown to play an important role in the release of neurotransmitters (Chiang et al., 1994; Ning et al., 2012). As in recent studies using CC smooth muscle cells and CC fibroblast cells, we were unable to detect A3AR expression in the PA, suggesting that A3AR may not play an important role in erectile function (Mi et al., 2008; Wen et al., 2010).
Early studies looked at the potential role of adenosine signaling in erectile function at the level of the penile tissue; however, our study is the first one to look at its role in the regulation of PA vascular tone, which is an important regulator of blood flow to the penis. Decreased adenosine levels or signaling results in erectile dysfunction (ED), whereas increased adenosine levels and signaling through the A2BAR are associated with priapism (Mi et al., 2008; Wen et al., 2011a). The use of adenosine as treatment of ED has been previously evaluated; however, the rapid degradation of adenosine may interfere with its ability to maintain erection (Chiang et al., 1994; Kilic et al., 1994).
Wen and Xia (2012) suggested A2BAR activation as a potential therapeutic pathway for ED; however, further studies looking at the change of AR expression in diseases associated with vasculogenic ED, such as hypertension and diabetes, are crucial. In fact, using an angiotensin II model of hypertension, the same group demonstrated that in this model, which was also shown to be associated with ED, A2BAR expression and signaling increased in the kidney, resulting in progression of hypertension associated with increased renal fibrosis (Jin et al., 2008; Labazi et al., 2013; Zhang et al., 2013). Increased A2BAR signaling in hypertension together with its association with penile fibrosis will make the use of A2BAR agonist as a potential therapeutic target for ED, at least in this model, counterproductive. An alternative therapeutic target could be the use of an A2AAR agonist, since our study showed that both A2AAR and A2BAR signaling contributed to the regulation of PA vascular tone, in addition to studies showing the involvement of A2AAR signaling in mediating human CC relaxation. Furthermore, a recent study demonstrated a synergic effect of a novel A2AAR agonist and sildenafil (a PDE5 inhibitor), suggesting that combined treatment may reduce side effects and increase efficacy in ED patients who do not respond to sildenafil alone (Moura et al., 2015). Together, A2AAR activation may represent an alternative clinical target for the treatment of ED, although future studies looking at the change of AR expression and signaling in diseases associated with vasculogenic ED would be critical to evaluate the safety and efficacy of A2AAR agonists use in ED treatment.
Acknowledgments
The authors thank Dr. Brandi Talkington for help with manuscript editing.
Abbreviations
- Ach
acetylcholine
- AR
adenosine receptors
- CC
corpus cavernosum
- CCPA
2-chloro-N6-cyclopentyladenosine
- Cl-IBMECA
1-[2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide
- CRC
concentration-response curves
- CVT 6883 (GS 6201)
3-ethyl-3,9-dihydro-1-propyl-8-[1-[[3-(trifluoromethyl)phenyl]methyl]-1H-pyrazol-4-yl]-1H-purine-2,6-dione
- DKO
double-KO
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- ED
erectile dysfunction
- EDHF
endothelium-derived hyperpolarizing factors
- K+
potassium
- KO
knockout
- KT 5720
(9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg,3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester
- L-NAME
NG-nitro-l-arginine methyl ester
- MRS1523
6-ethyl-5-[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylic acid propyl ester
- NECA
5′-N-ethylcarboxamidoadenosine
- NO
nitric oxide
- NOS
nitric oxide synthase
- PA
pudendal artery
- Phe
phenylephrine
- PKA
protein kinase A
- qPCR
quantitative real-time polymerase chain reaction
- SCH-58261
5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo(4,3-e)-1,2,4-triazolo(1,5-c)pyrimidine
- VSMC
vascular smooth muscle cells
- WT
wild type
Authorship Contributions
Participated in research design: Labazi.
Conducted experiments: Labazi.
Contributed new reagents or analytic tools: Tilley, Ledent.
Performed data analysis: Labazi.
Wrote or contributed to the writing of the manuscript: Labazi, Mustafa.
Footnotes
This study was supported by the National Institutes of Health [Grants HL027339, HL094447, and U54GM104942 to S.J.M.) and by a Sexual Medicine Society of North America Mini-Grant (Grant 1006727R to H.L.).
References
- Andersson KE. (2001) Pharmacology of penile erection. Pharmacol Rev 53:417–450. [PubMed] [Google Scholar]
- Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. (2007) Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol 293:H3448–H3455. [DOI] [PubMed] [Google Scholar]
- Blackburn MR. (2003) Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci 24:66–70. [DOI] [PubMed] [Google Scholar]
- Carneiro FS, Giachini FR, Lima VV, Carneiro ZN, Leite R, Inscho EW, Tostes RC, Webb RC. (2008) Adenosine actions are preserved in corpus cavernosum from obese and type II diabetic db/db mouse. J Sex Med 5:1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PH, Wu SN, Tsai EM, Wu CC, Shen MR, Huang CH, Chiang CP. (1994) Adenosine modulation of neurotransmission in penile erection. Br J Clin Pharmacol 38:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Awady MS, Ansari HR, Fil D, Tilley SL, Mustafa SJ. (2011) NADPH oxidase pathway is involved in aortic contraction induced by A3 adenosine receptor in mice. J Pharmacol Exp Ther 338:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gowelli HM, El-Gowilly SM, Elsalakawy LK, El-Mas MM. (2013) Nitric oxide synthase/K+ channel cascade triggers the adenosine A(2B) receptor-sensitive renal vasodilation in female rats. Eur J Pharmacol 702:116–125. [DOI] [PubMed] [Google Scholar]
- Faria M, Magalhães-Cardoso T, Lafuente-de-Carvalho JM, Correia-de-Sá P. (2006) Corpus cavernosum from men with vasculogenic impotence is partially resistant to adenosine relaxation due to endothelial A(2B) receptor dysfunction. J Pharmacol Exp Ther 319:405–413. [DOI] [PubMed] [Google Scholar]
- Filippi S, Mancini M, Amerini S, Bartolini M, Natali A, Mancina R, Forti G, Ledda F, Maggi M. (2000) Functional adenosine receptors in human corpora cavernosa. Int J Androl 23:210–217. [DOI] [PubMed] [Google Scholar]
- Hale TM, Hannan JL, Carrier S, deBlois D, Adams MA. (2009) Targeting vascular structure for the treatment of sexual dysfunction. J Sex Med 6 (Suppl 3):210–220. [DOI] [PubMed] [Google Scholar]
- Ham J, Evans BA. (2012) An emerging role for adenosine and its receptors in bone homeostasis. Front Endocrinol (Lausanne) 3:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan JL, Blaser MC, Pang JJ, Adams SM, Pang SC, Adams MA. (2011) Impact of hypertension, aging, and antihypertensive treatment on the morphology of the pudendal artery. J Sex Med 8:1027–1038. [DOI] [PubMed] [Google Scholar]
- Headrick JP, Ashton KJ, Rose’meyer RB, Peart JN. (2013) Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol Ther 140:92–111. [DOI] [PubMed] [Google Scholar]
- Hein TW, Xu W, Ren Y, Kuo L. (2013) Cellular signalling pathways mediating dilation of porcine pial arterioles to adenosine A₂A receptor activation. Cardiovasc Res 99:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Lagoda G, Leite R, Webb RC, Burnett AL. (2008) NADPH oxidase activation: a mechanism of hypertension-associated erectile dysfunction. J Sex Med 5:544–551. [DOI] [PubMed] [Google Scholar]
- Kiliç S, Salih M, Anafarta K, Baltaci S, Koşar A. (1994) Adenosine: a new agent in the diagnosis of impotence. Int J Impot Res 6:191–198. [PubMed] [Google Scholar]
- Kunduri SS, Mustafa SJ, Ponnoth DS, Dick GM, Nayeem MA. (2013) Adenosine A1 receptors link to smooth muscle contraction via CYP4a, protein kinase C-α, and ERK1/2. J Cardiovasc Pharmacol 62:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labazi H, Wynne BM, Tostes R, Webb RC. (2013) Metformin treatment improves erectile function in an angiotensin II model of erectile dysfunction. J Sex Med 10:2154–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Carrick D, Lee M, Oldroyd K, Berry C. (2014) Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv 7:581–591. [DOI] [PubMed] [Google Scholar]
- Manabe K, Heaton JPW, Morales A, Kumon H, Adams MA. (2000) Pre-penile arteries are dominant in the regulation of penile vascular resistance in the rat. Int J Impot Res 12:183–189. [DOI] [PubMed] [Google Scholar]
- Mantelli L, Amerini S, Ledda F, Forti G, Maggi M. (1995) The potent relaxant effect of adenosine in rabbit corpora cavernosa is nitric oxide independent and mediated by A2 receptors. J Androl 16:312–317. [PubMed] [Google Scholar]
- Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, et al. (2008) Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest 118:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura VJ, Alencar AK, Calasans-Maia Jde A, da Silva JS, Fraga CA, Zapata-Sudo G, (Barreiro EJ, (Sudo RT (2015) Novel agonist of adenosine receptor induces relaxation of corpus cavernosum in guinea pigs: an in vitro and in vivo study. Urology 85:1214 e1217–1221. [DOI] [PubMed]
- Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. (2008) Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol 295:H2068–H2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning C, Qi L, Wen J, Zhang Y, Zhang W, Wang W, Blackburn M, Kellems R, Xia Y. (2012) Excessive penile norepinephrine level underlies impaired erectile function in adenosine A1 receptor deficient mice. J Sex Med 9:2552–2561. [DOI] [PubMed] [Google Scholar]
- Noto T, Inoue H, Mochida H, Kikkawa K. (2001) Role of adenosine and P2 receptors in the penile tumescence in anesthetized dogs. Eur J Pharmacol 425:51–55. [DOI] [PubMed] [Google Scholar]
- Nunes KP, Labazi H, Webb RC. (2012) New insights into hypertension-associated erectile dysfunction. Curr Opin Nephrol Hypertens 21:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Toyama K, Gutterman DD, Campbell WB, Lemaître V, Teraoka R, Miura H. (2013) Ecto-5′-nucleotidase, CD73, is an endothelium-derived hyperpolarizing factor synthase. Arterioscler Thromb Vasc Biol 33:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatarpekar PV, Wen J, Xia Y. (2010) Role of adenosine signaling in penile erection and erectile disorders. J Sex Med 7:3553–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnoth DS, Sanjani MS, Ledent C, Roush K, Krahn T, Mustafa SJ. (2009) Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol 297:H1655–H1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino A, Ralevic V, Burnstock G. (1995) Contribution of P1-(A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br J Pharmacol 115:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz de Tejada I, Kim N, Lagan I, Krane RJ, Goldstein I. (1989) Regulation of adrenergic activity in penile corpus cavernosum. J Urol 142:1117–1121. [DOI] [PubMed] [Google Scholar]
- Sáenz de Tejada I, Angulo J, Cellek S, González-Cadavid N, Heaton J, Pickard R, Simonsen U. (2004) Physiology of erectile function. J Sex Med 1:254–265. [DOI] [PubMed] [Google Scholar]
- Sanjani MS, Teng B, Krahn T, Tilley S, Ledent C, Mustafa SJ. (2011) Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double-knockout mice. Am J Physiol Heart Circ Physiol 301:H2322–H2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Ishii N, Lue TF, Tanagho EA. (1991) Pharmacological effects of adenosine on canine penile erection. Tohoku J Exp Med 165:49–58. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Ishii N, Lue TF, Tanagho EA. (1992) Effects of adenosine on canine penile erection. J Urol 148:1323–1325. [DOI] [PubMed] [Google Scholar]
- Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. (2005) Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol 288:H1411–H1416. [DOI] [PubMed] [Google Scholar]
- Teng B, Mustafa SJ. (2011) A(2A) adenosine receptor-mediated increase in coronary flow in hyperlipidemic APOE-knockout mice. J Exp Pharmacol 2011:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng B, Fil D, Tilley SL, Ledent C, Krahn T, Mustafa SJ. (2013) Functional and RNA expression profile of adenosine receptor subtypes in mouse mesenteric arteries. J Cardiovasc Pharmacol 61:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostes RC, Giachini FR, Carneiro FS, Leite R, Inscho EW, Webb RC. (2007) Determination of adenosine effects and adenosine receptors in murine corpus cavernosum. J Pharmacol Exp Ther 322:678–685. [DOI] [PubMed] [Google Scholar]
- Vallon V and Osswald H (2009). Adenosine receptors and the kidney. Handb Exp Pharmacol (193): 443–470. [DOI] [PMC free article] [PubMed]
- Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Phatarpekar PV, Kellems RE, Blackburn MR, et al. (2010) Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J 24:740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Xia Y. (2012) Adenosine signaling: good or bad in erectile function? Arterioscler Thromb Vasc Biol 32:845–850. [DOI] [PubMed] [Google Scholar]
- Wen J, Dai Y, Zhang Y, Zhang W, Kellems RE, Xia Y. (2011a) Impaired erectile function in CD73-deficient mice with reduced endogenous penile adenosine production. J Sex Med 8:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Grenz A, Zhang Y, Dai Y, Kellems RE, Blackburn MR, Eltzschig HK, Xia Y. (2011b) A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J 25:2823–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, et al. (2013) Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112:1466–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Teng B, Tilley S, Ledent C, Mustafa SJ. (2014) Metabolic hyperemia requires ATP-sensitive K+ channels and H2O2 but not adenosine in isolated mouse hearts. Am J Physiol Heart Circ Physiol 307:H1046–H1055. [DOI] [PMC free article] [PubMed] [Google Scholar]