Abstract
3,4-Methylenedioxypyrovalerone (MDPV) is a common constituent of illicit “bath salts” products. MDPV is a chiral molecule, but the contribution of each enantiomer to in vivo effects in mice has not been determined. To address this, mice were trained to discriminate 10 mg/kg cocaine from saline, and substitutions with racemic MDPV, S(+)-MDPV, and R(−)-MDPV were performed. Other mice were implanted with telemetry probes to monitor core temperature and locomotor responses elicited by racemic MDPV, S(+)-MDPV, and R(−)-MDPV under a warm (28°C) or cool (20°C) ambient temperature. Mice reliably discriminated the cocaine training dose from saline, and each form of MDPV fully substituted for cocaine, although marked potency differences were observed such that S(+)-MDPV was most potent, racemic MDPV was less potent than the S(+) enantiomer, and R(−)-MDPV was least potent. At both ambient temperatures, locomotor stimulant effects were observed after doses of S(+)-MDPV and racemic MDPV, but R(−)-MDPV did not elicit locomotor stimulant effects at any tested dose. Interestingly, significant increases in maximum core body temperature were only observed after administration of racemic MDPV in the warm ambient environment; neither MDPV enantiomer altered core temperature at any dose tested, at either ambient temperature. These studies suggest that all three forms of MDPV induce biologic effects, but R(−)-MDPV is less potent than S(+)-MDPV and racemic MDPV. Taken together, these data suggest that the S(+)-MDPV enantiomer is likely responsible for the majority of the biologic effects of the racemate and should be targeted in therapeutic efforts against MDPV overdose and abuse.
Introduction
Synthetic analogs of cathinone are psychostimulant-like drugs of abuse found in commercial “bath salt” preparations, and racemic 3,4-methylenedioxypyrovalerone (MDPV) is a common constituent of these illicit products (Vircks and Mulligan, 2012; O’Byrne et al., 2013; Seely et al., 2013). Racemic MDPV (hereafter simply referred to as MDPV) potently inhibits the reuptake of dopamine (DA) at the dopamine transporter (DAT) but does not stimulate DA release (Baumann et al., 2013; Simmler et al., 2013). Consistent with this cocaine-like mechanism of action, in vivo studies of rats trained to discriminate cocaine or methamphetamine have revealed MDPV substitutes for both of these psychostimulants (Gatch et al., 2013). Previously, our laboratory trained mice to discriminate MDPV from saline and we reported that methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) fully substituted for MDPV; however, neither the synthetic CB1 cannabinoid agonist JWH-018 [(1-pentyl-1H-indol-3-yl)-1-naphthalenyl-methanone] nor the µ-opioid agonist morphine elicited MDPV-appropriate responding (Fantegrossi et al., 2013). In addition, in both rats and mice, induction of hyperactivity and locomotor stimulation has been observed after administration of MDPV (Fuwa et al., 2007; Huang et al., 2012; Aarde et al., 2013; Fantegrossi et al., 2013).
MDPV has two stereoisomers [designated as R or S based on absolute configuration, or (+) or (−) depending on their rotational effect on plane polarized light] that differ from one another only in three-dimensional orientation at the chiral center; these stereoisomers are referred to as enantiomers. Many pharmacological differences can exist between enantiomers, including potency differences, apparent inactivity of one enantiomer versus the other, or differences in mechanism of action. It should be noted that the (+) or (−) designation of a given isomer can change depending on whether the drug is in a free-base form or a salt, but the R or S designation will remain constant. Therefore, in older studies conducted before the adoption of absolute configuration designations for isomers, it is difficult to determine which enantiomer is which if the drug formulation is not well described.
Only the S-enantiomers of the DA releasers amphetamine and methamphetamine are intravenously self-administered, suggesting that only S-methamphetamine and S-amphetamine are centrally active (Yokel and Pickens, 1973). However, studies with MDMA have reported that although both enantiomers are centrally active across similar doses, each has a distinct mechanism of action (Fantegrossi et al., 2009; Murnane et al., 2009). Previous in vivo studies conducted with substituted phenethylamines such as cathinone (Glennon et al., 1984a,b; Schechter, 1986) and methcathinone (Glennon et al., 1995) have shown the S-enantiomers appear to be more potent as discriminative stimuli than either the R-enantiomers or the racemate in rats, whereas S-methcathinone has also been shown to be a more potent locomotor stimulant than R-methcathinone in mice (Glennon et al., 1995; Sparago et al., 1996). Evaluation of the enantiomers of monoamine reuptake inhibitor cocaine indicates that (−)-cocaine is centrally active, whereas (+)-cocaine is not (Katz et al., 1990). Although the R/S designation is not used in this study, the S-enantiomer is the only enantiomer naturally synthesized by the coca plant. As such, it is assumed that (−)-cocaine is actually S-cocaine and (+)-cocaine is R-cocaine; however, this rule is cocaine specific and may differ for other compounds. Bupropion (another reuptake inhibitor and cathinone analog) lacks stereospecificity (Musso et al., 1993). These contrasting results suggest that the contribution of each enantiomer to the observed effects of the racemate among a group of structurally related compounds may be compound and assay specific, emphasizing the need to conduct experiments with the MDPV enantiomers across multiple measures.
In this regard, the literature pertaining to the biologic effects of the enantiomers of MDPV remains sparse. A recent study determined that S(+)-MDPV is a more potent inhibitor of DAT and norepinephrine transporters than either MDPV or R(−)-MDPV, and the same study also demonstrated that S(+)-MDPV produced a more potent facilitation of intracranial self-stimulation (ICSS) in rats than the racemate, whereas R(−)-MDPV failed to alter ICSS at doses up to 100 times greater than the lowest effective dose of the S(+)-enantiomer (Kolanos et al., 2015). To our knowledge, no previous studies have assessed the relative contribution of each individual MDPV enantiomer to in vivo effects in mice. Therefore, the purpose of this study was to determine the relative contribution of each enantiomer to the discriminative stimulus, locomotor stimulant, and hyperthermic effects of MDPV. Because we have previously observed ambient temperature-dependent effects of MDPV (Fantegrossi et al., 2013), core temperatures and locomotor responses to MDPV, S(+)-MDPV, and R(−)-MDPV were studied under both warm (28°C) and cool (20°C) ambient temperatures.
Materials and Methods
Animals
All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences approved all of the experimental protocols. Adult male NIH Swiss mice (Harlan Laboratories, Indianapolis, IN) weighing 20–25 g on delivery were housed three animals per cage (15.24 × 25.40 × 12.70 cm3) in a temperature-controlled room in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facility. Room conditions were maintained at 22 ± 2°C and 45%–50% humidity, with lights set to a 12-hour light/dark cycle. Animals were fed Laboratory Diet rodent chow (Laboratory Rodent Diet no. 5001; PMI Feeds, St. Louis, MO), and, with the exception of mice used in drug discrimination experiments, animals were fed ad libitum until immediately before testing. Mice used in drug discrimination experiments were food restricted throughout all of the studies, and their weights were maintained at approximately 30 g with appropriate supplemental feedings after the completion of daily behavioral sessions. The drug discrimination studies were conducted using one group of mice, whereas the telemetric studies were conducted using a separate group. Mice were randomly assigned to experimental groups before the study and the order of both drug and dose were randomized for all testing conditions. All mice were drug-naive (with the exception of surgical anesthetics) before testing.
Procedures
Drug Discrimination.
Mice (n = 5) were trained to discriminate 10 mg/kg cocaine from saline in standard operant chambers for mice that were individually enclosed in larger lightproof Malaguard sound-attenuating cubicles (Med Associates, St. Albans, VT). The side wall of each chamber used in these studies was equipped with an aperture through which liquid reinforcement was delivered, driven by a dipper mounted outside the chamber but within the cubicle. The reinforcement aperture was centered between two retractable levers and contained an amber stimulus light, which was illuminated during reinforcer delivery.
Lever training.
Mice were trained 7 days per week to respond in two-lever operant conditioning boxes, reinforced by 2 seconds of access to a palatable liquid reinforcer (approximately 0.02 ml evaporated milk diluted 1:1 with water). Upon completion of the response requirement on either lever, that lever was retracted and reinforcement was delivered. After a brief (10-second) timeout, mice were required to complete the response requirement on the remaining lever. Both levers were reintroduced into the chamber after the 10-second timeout. In this manner, mice received equivalent reinforcement from each lever, and no subsequent biases for one lever or the other were noted. Animals were initially maintained on a fixed ratio (FR) 1 schedule of reinforcement in a session lasting 60 minutes or until 60 reinforcers had been earned (whichever came first.) The FR value increased by one increment every 20th reinforcer earned within a given session and the FR value achieved was carried over between sessions until mice were responding under an FR 10. This segment of the training was complete when mice reached an FR 10 and earned all 60 available reinforcers for at least 5 consecutive days.
Discrimination training.
Mice were trained during daily 60-minute sessions to discriminate their training dose (10 mg/kg cocaine) from saline vehicle. When animals were injected with the training dose, responses on the drug lever (right lever) produced the reinforcer. When animals were administered a saline injection, responses on the saline lever (left lever) were reinforced. Injections were administered intraperitoneally 10 minutes before extension of the response levers, signaling the start of the behavioral session. During discrimination training, any responses on the incorrect lever reset the FR on the injection-appropriate lever but had no other programmed consequences. Completion of the FR 10 on the injection-appropriate lever was reinforced. Percent drug-appropriate responding was calculated as the number of responses emitted on the drug-appropriate lever divided by the total number of responses on both levers, multiplied by 100. Training was composed of an alternating schedule of drug or saline injection. Subjects were switched from saline to drug or vice versa for the next day of training if they achieved a criterion of greater than 80% injection-appropriate responding. Drug-induced stimulus control was assumed to be present when, in five consecutive sessions, animals achieved 80% or better injection-appropriate responding.
Substitution testing.
After stimulus control was established with the training drug, tests were conducted twice per week in each animal so long as performance did not fall below the criterion level of 80% injection-appropriate responding in any one of the previous two training sessions. Approximately one-half of the test sessions were conducted the day after saline training sessions, with the remainder conducted after drug training sessions. During test sessions, a single dosing procedure was used, and no responses were reinforced. The session was terminated after the emission of 10 responses on either lever, or after 5 minutes, whichever occurred first. Mice were then removed from the chamber and returned to their home cages. The distribution of responses between the two levers was expressed as a percentage of total responses emitted on the drug-appropriate lever. The response rate was calculated for each test session by dividing the total number of responses emitted on both levers by the elapsed time prior to 10 responses on either lever. A saline test session was conducted to ensure discriminative performance was maintained and to obtain baseline response rates against which to compare the effects of cocaine, MDPV, S(+)-MDPV, R(−)-MDPV, and JWH-018. Both the order and corresponding dose of test drug were randomized for each mouse.
Radiotelemetry of Thermoregulation and Locomotor Activity.
After appropriate anesthetization with inhaled isoflurane, the abdominal area of each mouse (n = 6) was shaved and sanitized with iodine swabs. A rostral-caudal cut approximately 1.5 cm in length was made with sterile skin scissors, providing access to the intraperitoneal cavity. A cylindrical glass-encapsulated radiotelemetry probe (model ER-4000 E-Mitter; Mini Mitter Co., Inc., Bend, OR) was then inserted, and the incision was closed using 5-0 absorbable suture material. Surgeries were carried out at least 7 days before initiation of experimental conditions, allowing time for incisions to heal and for mice to recover normal body weights. After surgery, all implanted mice were individually housed in 15.24 cm × 25.40 cm × 12.70 cm cages for the duration of all telemetry experiments.
Implanted transmitters produced activity- and temperature-modulated signals that were sent to a receiver (model ER-4000 Receiver; Mini Mitter Co., Inc.) underneath each cage. These receivers were situated inside standard light- and sound-attenuating chambers (model ENV-022M; Med Associates) to minimize environmental variability during tests. Every 5 minutes, the computer collected two data updates from the probes: core temperature (in degrees Celsius) on one channel, and locomotor counts on the other. Each chamber was equipped with a house light (to establish a photoperiod), an exhaust fan, and a warm air heater (to increase the ambient temperature.) The heaters attached to each chamber were used to maintain the “warm” condition at 28°C, and the heating, ventilation, and air conditioning system of the room was sufficient to maintain the desired “cool” ambient temperature of 20°C (Fantegrossi et al., 2013). Ambient temperatures were monitored every 5 minutes by data loggers located within the chambers (Lascar EL-USB-1; MicroDAQ, Contoocook, NH) and could be read at a glance on digital thermometers attached to each chamber. After at least 60 minutes of baseline data collection, mice were removed from the chambers, injected with test compound (or saline), returned to the home cage, and then returned to the chambers for 24 hours of data collection. Each mouse received all of the doses of each test compound. The order of each compound and dose was randomized across animals.
Data Analysis
Graphical presentation of all drug discrimination and radiotelemetry data depict means ± S.E.M. Drug discrimination data are expressed as percent drug-appropriate responding, which is the number of responses emitted on the drug-appropriate lever as a percentage of the total number of responses emitted. Generalization was operationally defined as: 1) 80% or more of the group responses having been made on the drug-appropriate lever and 2) the group mean was significantly different [via Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks, followed by pairwise comparisons using the Holm–Sidak method] from saline. In telemetry experiments, although 24 hours of core temperature and locomotor data were collected after all injections, figures are truncated at 6 hours as measures had returned to control values. Core temperature data are presented as 30-minute means, whereas locomotor activity data have been binned in 30-minute summation averages. To minimize the number of multiple comparisons and maintain statistical power, locomotor data were subjected to Kruskal–Wallis one-way ANOVA, whereas temperature data were subjected to two-way ANOVA; both were followed by pairwise comparisons using the Holm–Sidak method to correct for multiple analyses.
Drugs
MDPV, S(+)-MDPV, and R(−)-MDPV were synthesized by one of the authors (K.C.R.) in the Laboratory of Medicinal Chemistry at the National Institute on Drug Abuse (Bethesda, MD). Cocaine was obtained from the National Institute on Drug Abuse Drug Supply Program. JWH-018 was synthesized in the Department of Medicinal Chemistry at the University of Kansas (Lawrence, KS) and was provided as a generous gift by Dr. Thomas Prisinzano. Cocaine, MDPV, S(+)-MDPV, and R(−)-MDPV were weighed as salts and dissolved in 0.9% physiologic saline, whereas JWH-018 was dissolved in a solution of 7.8% Tween 80 and 92.2% sterile water. All solutions of S(+)-MDPV and R(−)-MDPV were prepared daily to prevent racemization (see Suzuki et al., 2015). Injections were administered intraperitoneally at a volume of 0.1 ml/10 g. Saline vehicle and all other experimental supplies were obtained from standard commercial sources.
Results
Drug Discrimination
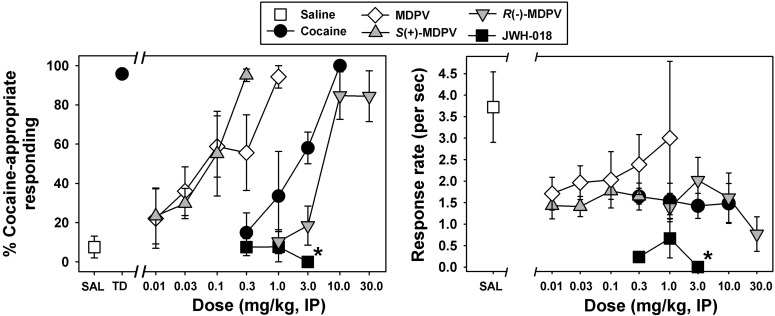
Mice reliably discriminated 10 mg/kg cocaine from saline (Fig. 1, left, black circle and white square, respectively). When saline was administered in training sessions, mice primarily responded on the saline lever; similarly, when the training dose was administered, mice responded almost exclusively on the cocaine lever. Response rates during substitution tests were generally consistent with those observed during training sessions, with saline sessions resulting in slightly higher rates than drug sessions (data not shown). During substitution test sessions with cocaine (Fig. 1, left, black circles), dose-dependent and full substitution for the training dose was observed, with > 80% of the total responses emitted on the drug lever at a dose of 10 mg/kg. Responding engendered by this dose of cocaine was significantly different from the discriminative responding elicited by saline (t = 9.422, P < 0.001), and the interpolated ED50 for cocaine was 2.20 ± 0.25 mg/kg. No systematic effects of cocaine on response rate were observed across the doses tested (Fig. 1, right, black circles).
Fig. 1.
(Left) Discriminative stimulus effects of cocaine, MDPV, S(+)-MDPV, R(−)-MDPV, and JWH-018 in mice trained to discriminate 10 mg/kg cocaine from saline. On the abscissa, SAL represents test injection of saline and TD represents administration of the cocaine training dose. Numbers refer to doses of drugs during substitution sessions, expressed as milligram per kilogram on a log scale. The ordinate provides the percentage of total responses emitted on the cocaine-appropriate lever. (Right) Response rates engendered by administration of saline, cocaine, MDPV, S(+)-MDPV, R(−)-MDPV, or JWH-018 during substitution sessions. The abscissa is as described above. The ordinate shows response rates, expressed as lever presses per second. Asterisks adjacent to points indicate that all animals failed to respond at this dose. Errors bars depict means ± S.E.M. IP, intraperitoneally.
Treatment with MDPV elicited a dose-dependent and full substitution for the cocaine training dose (Fig. 1, left, white diamonds), with near exclusive responding on the drug lever at a dose of 1.0 mg/kg. This highest tested dose of MDPV elicited significantly different discriminative responding than saline (t = 8.840, P < 0.001), and the interpolated ED50 for MDPV was 0.20 ± 0.05 mg/kg. Substitution doses of MDPV did not dose-dependently alter response rates (Fig. 1, right, white diamonds). Similar to the racemate, injections of S(+)-MDPV also engendered dose-dependent and full substitution for the cocaine training dose (Fig. 1, left, gray upward triangles), with > 80% of the total responses emitted on the drug lever at a dose of 0.3 mg/kg. Discriminative responding elicited by this highest tested dose of S(+)-MDPV was significantly different from that elicited by saline (t = 8.923, P < 0.001), and the interpolated ED50 for S(+)-MDPV was 0.10 ± 0.03 mg/kg. S(+)-MDPV did not dose-dependently alter response rates (Fig. 1, right, gray upward triangles). Injections of R(−)-MDPV also engendered dose-dependent and full substitution for the cocaine training dose (Fig. 1, left, gray downward triangles), with > 80% of the total responses emitted on the drug lever at a dose of 10 mg/kg. Discriminative responding elicited by this dose of R(−)-MDPV was significantly different from saline (t = 7.830, P < 0.001). The interpolated ED50 for R(−)-MDPV was 7.3 ± 1.81 mg/kg, and R(−)-MDPV suppressed response rates at the highest tested dose (Fig. 1, right, gray downward triangles). Substitutions with the negative control compound JWH-018 (Fig. 1, left, black squares) resulted primarily in responding on the saline lever, up to doses that suppressed response rates (Fig. 1, right, black squares). Importantly, the ED50 value for R(−)-MDPV was statistically different from that of cocaine (t = 3.954, P = 0.001), MDPV (t = 5.499, P < 0.001), and S(+)-MDPV (t = 5.581, P < 0.001); however, there were no statistical differences between the pairwise comparisons of cocaine, S(+)-MDPV, and MDPV.
Radiotelemetry
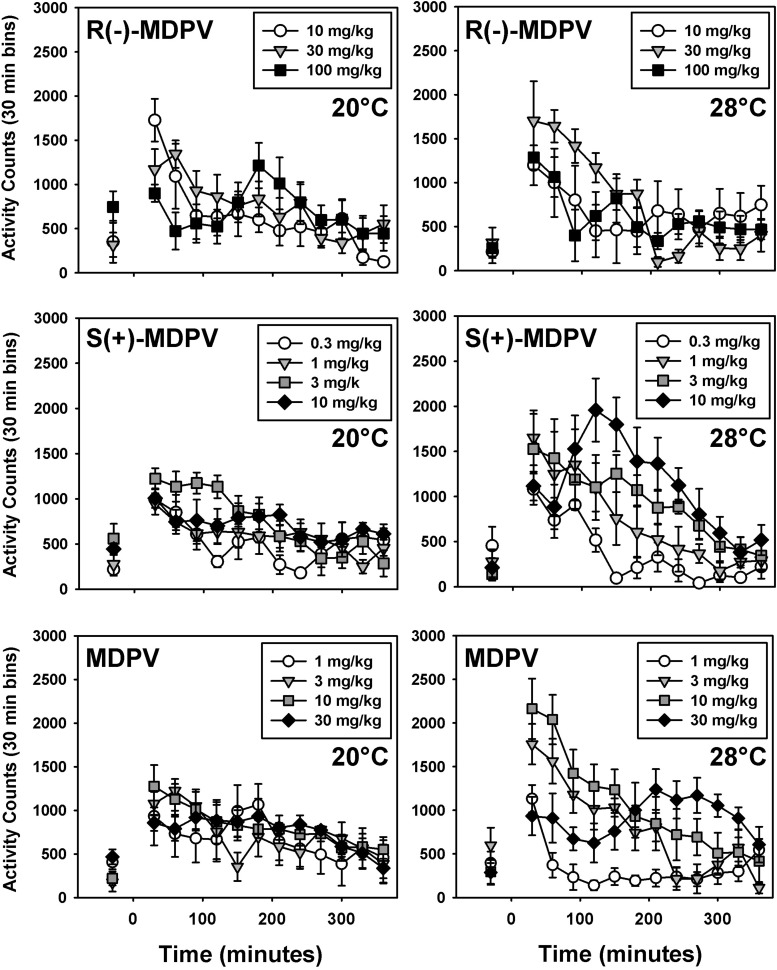
Administration of MDPV, S(+)-MDPV, or R(−)-MDPV increased locomotor activity from baseline levels, at both ambient temperatures (Fig. 2). At an ambient temperature of 20°C, these locomotor effects for all three forms of MDPV were not dose dependent across the dose range studied; however, at 28°C, MDPV and S(+)-MDPV generated dose-dependent increases in locomotor activity (Fig. 2, right, middle and bottom panels, respectively), whereas similar dose-dependent increases in activity levels were not observed after administration of R(−)-MDPV (Fig. 2, right, top panel).
Fig. 2.
Locomotor effects of R(−)-MDPV (top), S(+)-MDPV (middle), and MDPV (bottom) at an ambient temperature of 20°C (left) or 28°C (right) in mice. On the abscissae, points to the left of the 0 denote baseline locomotor data before injection, presented as the mean activity observed over 30 minutes. Numbers refer to time, in minutes, after drug administration. Ordinates show mean activity counts calculated in 30-minute bins. Errors bars depict means ± S.E.M.
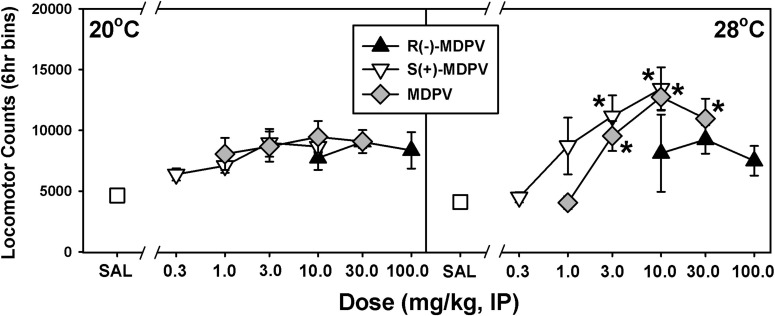
To statistically compare these data, locomotor activity counts for each animal were summed over a 6-hour period after injection and plotted as a function of dose (Fig. 3). At the low ambient temperature, locomotor counts for each form of MDPV were not statistically different from those observed after administration of saline (Fig. 3, left). For R(−)-MDPV, this same lack of dose-dependent locomotor effects was also observed at 28°C (Fig. 3, right, black triangles). In contrast, S(+)-MDPV and MDPV generated more typical dose-dependent effects on locomotor activity at 28°C (Fig. 3, right, white triangles and gray diamonds). At this higher ambient temperature, S(+)-MDPV induced significant increases in locomotor activity at doses of 3 mg/kg and 10 mg/kg (t = 4.463, P < 0.001; and t = 5.104, P < 0.001, respectively) compared with saline, whereas MDPV elicited significant locomotor stimulant effects at doses of 3 mg/kg, 10 mg/kg, and 30 mg/kg (t = 3.619, P < 0.001; t = 5.296, P < 0.001; and t = 4.232, P < 0.001, respectively).
Fig. 3.
Effects of R(−)-MDPV, S(+)-MDPV, and MDPV on locomotor activity at an ambient temperature of 20°C (left) or 28°C (right) in mice. On the abscissae, SAL represents saline administration and numbers refer to doses of drug, expressed as milligram/kilogram on a log scale. The ordinate shows the mean total activity recorded over 6 hours postinjection, via radiotelemetry. Asterisks indicate significant differences between locomotor effects of drug doses and saline, within a given ambient temperature. Errors bars depict means ± S.E.M. IP, intraperitoneally.
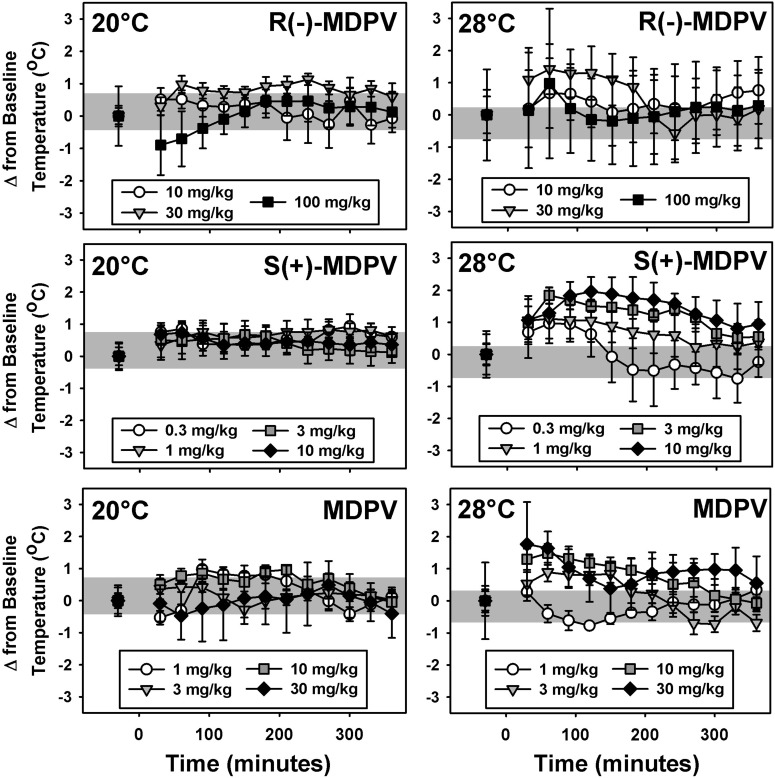
At an ambient temperature of 20°C, mean core temperatures of mice treated with saline changed only slightly compared with baseline preinjection values (ranging from −0.35 to +0.78°C and represented as the gray-shaded regions in Fig. 4, left). Similar results were obtained at an ambient temperature of 28°C, where changes from baseline values ranged from −0.78 to 0.20°C, over 6 hours after saline administration (Fig. 4, right, gray-shaded regions). Because these baseline temperature ranges overlap, the ambient temperature did not affect normal thermoregulation in these subjects. Doses of all three forms of MDPV altered core temperature in mice at the 20°C ambient temperature (Fig. 4, left), but these core temperatures did not systematically deviate from the operationally defined normal range after handling and saline administration. At 28°C, large variability accounted for no observed changes from saline treatment, despite apparently higher mean temperatures at some time points, in the R(−)-MDPV mice (Fig. 4, right, top panel). However, all doses of S(+)-MDPV (Fig. 4, right, middle panel) and doses of 3 mg/kg, 10 mg/kg, and 30 mg/kg MDPV (Fig. 4, right, bottom panel) transiently increased temperatures outside the normal thermoregulatory range.
Fig. 4.
Thermoregulatory effects of R(−)-MDPV (top), S(+)-MDPV (middle), and MDPV (bottom) at an ambient temperature of 20°C (left) or 28°C (right) in mice. On the abscissae, points to the left of the 0 denote baseline data before injection, presented as the mean change in temperature observed over 30 minutes. Numbers refer to time, in minutes, after MDPV administration. Ordinates indicate the mean change in core temperature from baseline values in degrees Celsius. Gray regions represent the range of temperature fluctuation observed over 6 hours after saline injection. Errors bars depict means ± S.E.M.
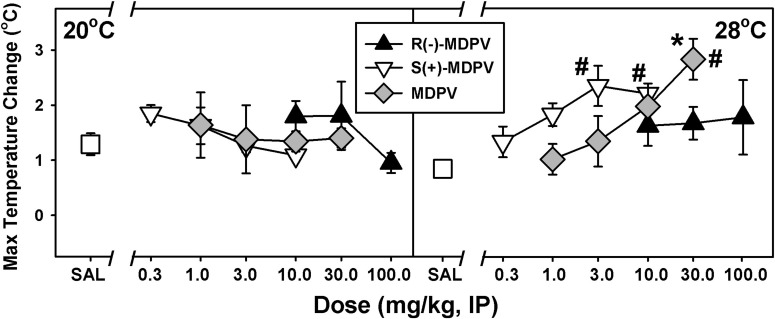
To statistically compare these temperature data, the maximum core temperature observed for each animal over 6 hours after drug or saline administration was determined and the mean difference between this highest temperature obtained and baseline preinjection temperature was plotted as a function of dose (Fig. 5). Compared with saline injection, no significant changes in core temperature were observed at the cool ambient temperature at any dose with any form of MDPV (Fig. 5, left), but mean maximum temperature changes increased with dose in the warm ambient environment for both S(+)-MDPV (Fig. 5, right, white triangles) and MDPV (Fig. 5, right, gray diamonds). Despite these apparent dose-dependent effects on core temperature at 28°C, only a dose of 30 mg/kg MDPV was significantly different from saline-treated animals (t = 4.239, P < 0.001). Because there was a significant interaction (P = 0.001) between dose/drug administered and ambient temperature, main effects of dose and ambient temperature could not be determined. However, doses of 30 mg/kg MDPV (t = 3.046, P = 0.003) and 3 mg/kg and 10 mg/kg S(+)-MDPV (t = 2.539, P = 0.013; t = 2.499, P = 0.014, respectively) resulted in significantly larger differences in mean maximum temperature changes at the warm ambient temperature compared with the same doses under cool ambient conditions.
Fig. 5.
Effects of R(−)-MDPV, S(+)-MDPV, and MDPV on core temperature at an ambient temperature of 20°C (left) or 28°C (right) in mice. On the abscissae, SAL represents saline administration and numbers refer to doses of drug, expressed as milligram/kilogram on a log scale. The ordinate shows the mean maximum change in core temperature from baseline values over 6 hours postinjection, via radiotelemetry. Asterisks indicate significant differences between dose of drug administered and saline. Hash marks indicate significant differences compared with the effect observed at the same dose administered at 20°C. Errors bars depict means ± S.E.M. IP, intraperitoneally.
Discussion
The studies reported here include the first evaluations of the discriminative stimulus effects of the S(+)- and R(−)-enantiomers of MDPV compared with those of cocaine and racemic MDPV in the mouse. We previously showed that MDPV was readily trained as a discriminative stimulus and that the discrimination was pharmacologically specific, resulting in dose-dependent, full generalization of the structurally related psychostimulants MDMA and methamphetamine but not the pharmacologically dissimilar μ-opioid receptor agonist morphine nor the CB1 cannabinoid receptor agonist JWH-018 to the MDPV training dose (Fantegrossi et al., 2013). Concurrently, it was demonstrated that MDPV differs from other “bath salt” constituents because MDPV functions as a cocaine-like reuptake inhibitor (Baumann et al., 2013; Simmler et al., 2013), unlike 4-methyl methcathinone and methylone, which were previously shown to function as amphetamine-like releasers (Baumann et al., 2012). We also previously reported that cocaine fully substitutes in mice trained to discriminate S(+)-MDMA or R(−)-MDMA from saline, although a significant potency difference for cocaine substitution was apparent between the two MDMA enantiomer groups (Murnane et al., 2009). Although similarly training each individual MDPV enantiomer would have been interesting for this study, quantities of each enantiomer were limited and in vivo data on their potencies were not available in the mouse, so it was unknown which doses would function as a discriminative stimulus for each enantiomer. As such, we decided that a more prudent approach would be to train with cocaine as a discriminative stimulus and then test the trained animals with not only MDPV, but with each of the individual enantiomers of MDPV as well. Again, to verify pharmacological specificity of the assay, we also tested a negative control (JWH-018), which, as expected, did not substitute for cocaine.
In these studies, we chose to test discrete bolus doses of MDPV and its enantiomers rather than utilizing a cumulative dosing paradigm. The ED50 values for S(+)-MDPV and MDPV were similar in this discrimination assay, with S(+)-MDPV having a slightly lower ED50 (0.1 mg/kg) than MDPV (0.2 mg/kg), suggesting a higher potency to induce cocaine-like discriminative stimulus effects for the S(+)-enantiomer, although this potency difference was not statistically significant. This potency trend in the discriminative stimulus effects of S(+)-MDPV and MDPV is strikingly similar to recently published data demonstrating that S(+)-MDPV is roughly twice as potent at inhibiting DA reuptake as MDPV (Kolanos et al., 2015). Importantly, although S(+)-MDPV, R(−)-MDPV, and MDPV all fully substituted for the cocaine training dose in our studies, R(−)-MDPV barely met our 80% threshold criteria for “generalization,” whereas both S(+)-MDPV and MDPV produced nearly 100% responding on the drug lever. In addition, R(−)-MDPV had a much higher ED50 (7.3 mg/kg) than the S(+)-enantiomer or MDPV, together suggesting a lower potency and perhaps a lower effectiveness to induce cocaine-like interoceptive effects for this enantiomer. To determine whether a higher dose of R(−)-MDPV would also elicit 100% responding on the drug lever, 30 mg/kg R(−)-MDPV was also tested. However, this resulted in the same degree of cocaine-like responding as 10 mg/kg R(−)-MDPV (80%) but also resulted in decreased response rates. As such, even higher doses of R(−)-MDPV were not tested. Perhaps similar to these findings, it was recently shown that R(−)-MDPV does not block DA reuptake as potently as S(+)-MDPV or MDPV (Kolanos et al., 2015). Thus, the cocaine-like discriminative stimulus effects of MDPV and its enantiomers are consistent with their interactions with DAT, which is thought to be the primary site mediating cocaine-like discriminative stimulus effects for other psychostimulant-like drugs (Cook et al., 2001; Loland et al., 2008).
Because our previous work examined the role of ambient temperature on the locomotor stimulant and thermoregulatory effects of MDPV (Fantegrossi et al., 2013), we again used radiotelemetry to simultaneously monitor both of these end points in response to various doses of MDPV and its enantiomers at either 20°C or 28°C. Interestingly, we found that R(−)-MDPV had no significant effects on locomotor activity or core temperature regardless of ambient temperature. Admittedly, intersubject variability was high in many of the R(−)-MDPV experiments conducted. Nevertheless, this apparent lack of central nervous system activity in the biotelemetry experiments contrasts sharply with the results observed in the drug discrimination studies, where R(−)-MDPV met our criteria for full substitution for the cocaine training dose [albeit possibly not to the same extent as S(+)-MDPV or MDPV]. Since R(−)-MDPV was relatively inactive in assays investigating the previously demonstrated locomotor stimulant and hyperthermic effects of MDPV, it appears that S(+)-MDPV is likely responsible for these effects after administration of the racemate. Indeed, the pattern observed here after administration of S(+)-MDPV closely resembled that of MDPV regardless of ambient temperature. Since S(+)-MDPV was roughly twice as potent as MDPV in our drug discrimination experiments, and because pronounced self-injurious behavior had previously been observed after administration of 30 mg/kg MDPV under warm ambient conditions (Fantegrossi et al., 2013), we were hesitant to push the dose of S(+)-MDPV in our study. Perhaps for this reason, a biphasic dose-response curve was not observed for the S(+)-MDPV 6-hour locomotor activity data at an ambient temperature of 28°C. Similarly, the only significant thermoregulatory effects observed in this study were seen after administration of 30 mg/kg MDPV, again at an ambient temperature of 28°C. Although increasing doses of S(+)-MDPV trended toward statistically significant increases in maximum temperature changes, significance was not obtained at any dose; yet it is possible that increasing the dose beyond the range studied here may have been sufficient to produce a significant effect. Aside from these minor differences between MDPV and S(+)-MDPV, dose-response curves were similar between the two, suggesting that S(+)-MDPV is primarily (if not solely) responsible for the effects of the racemate on locomotor activity and core temperature. The data presented here coupled with the DA reuptake data parallel nicely with literature suggesting that the locomotor effects of stimulants are related to their actions at DAT. Thus, as a consequence of the DA reuptake profile for R(−)-MDPV, one may have anticipated no systematic increases in locomotor activity after administration of R(−)-MDPV.
Although our study presents substantial data to suggest that S(+)-MDPV may be an important target for pharmacotherapy development to combat MDPV overdose and abuse, hyperthermia [elicited by MDPV but not by S(+)-MDPV] is likely an effect of particular concern to clinicians. As such, it is possible that treatments aimed solely at blocking the action of S(+)-MDPV may fail in this clinically relevant domain. Because administration of neither enantiomer was sufficient to produce the observed hyperthermic effects of MDPV, it may be the case that the individual MDPV enantiomers somehow synergize in vivo to elicit this action, either through yet-undetermined pharmacodynamic or pharmacokinetic interactions. Similarly, mechanistic studies to investigate the role of various monoamine transporters and receptors in the mediation of MDPV-induced hyperthermia and locomotor stimulation are likely necessary to elucidate important underlying mechanisms of this emerging drug of abuse and may lead to new insights for clinically relevant pharmacotherapeutics useful in cases of MDPV overdose and abuse.
Acknowledgments
The authors thank the University of Arkansas for Medical Sciences Department of Laboratory Animal Medicine for expert husbandry and veterinary services.
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- DAT
dopamine transporter
- FR
fixed ratio
- ICSS
intracranial self-stimulation
- JWH-018
(1-pentyl-1H-indol-3-yl)-1-naphthalenyl-methanone
- MDPV
3,4-methylenedioxypyrovalerone
- MDMA
3,4-methylenedioxymethamphetamine
Authorship Contributions
Participated in research design: Gannon, Fantegrossi.
Conducted experiments: Gannon, Williamson.
Contributed new reagents or analytic tools: Suzuki, Rice.
Performed data analysis: Gannon, Williamson, Fantegrossi.
Wrote or contributed to the writing of the manuscript: Gannon, Williamson, Fantegrossi.
Footnotes
This research was supported by the University of Arkansas for Medical Sciences Center for Translational Neuroscience [Grant RR020146], the University of Arkansas for Medical Sciences Translational Research Institute [Grant RR029884], and the National Institutes of Health National Institute on Drug Abuse [Grant T32DA022981]. A portion of this work was supported by the National Institutes of Health Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Institute of Alcohol Abuse and Alcoholism, or the National Institutes of Health.
This work was presented, in part, in B.M.G.’s doctoral dissertation defense as follows: Gannon BM (2015) In Vivo Characterization of Major ‘Bath Salt’ Constituent 3,4-Methylenedioxypyrovalerone (MDPV) in Mice. Doctoral dissertation, University of Arkansas for Medical Sciences, Little Rock, AR.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, et al. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Carroll IF, Beardsley PM. (2001) Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat. Psychopharmacology (Berl) 159:58–63. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. (2013) In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Murai N, Mathúna BO, Pizarro N, de la Torre R. (2009) Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine and its enantiomers in mice: pharmacokinetic considerations. J Pharmacol Exp Ther 329:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuwa T, Fukumori N, Tanaka T, Kubo Y, Ogata A, Uehara S, Honda Y, Kodama T. (2007) Microdialysis study of drug effects on central nervous system - changes of dopamine levels in mice striatum after oral administration of methylenedioxypyrovalerone. Ann Rep Tokyo Metr Inst PH 58:287–292. [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Schechter MD, Rosecrans JA. (1984a) Discriminative stimulus properties of S(-)- and R(+)-cathinone, (+)-cathine and several structural modifications. Pharmacol Biochem Behav 21:1–3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Hauck AE, McKenney JD. (1984b) Structure-activity studies on amphetamine analogs using drug discrimination methodology. Pharmacol Biochem Behav 21:895–901. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Martin BR, Dal Cason TA. (1995) Methcathione (“cat”): an enantiomeric potency comparison. Pharmacol Biochem Behav 50:601–606. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. (2012) Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend 126:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Tirelli E, Witkin JM. (1990) Stereoselective effects of cocaine. Behav Pharmacol 1:347–353. [DOI] [PubMed] [Google Scholar]
- Kolanos R, Partilla JS, Baumann MH, Hutsell BA, Banks ML, Negus SS, Glennon RA. (2015) Stereoselective actions of methylenedioxypyrovalerone (MDPV) to inhibit dopamine and norepinephrine transporters and facilitate intracranial self-stimulation in rats. ACS Chem Neurosci 6:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73:813–823. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Murai N, Howell LL, Fantegrossi WE. (2009) Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(-)-MDMA trained mice. J Pharmacol Exp Ther 331:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso DL, Mehta NB, Soroko FE, Ferris RM, Hollingsworth EB, Kenney BT. (1993) Synthesis and evaluation of the antidepressant activity of the enantiomers of bupropion. Chirality 5:495–500. [DOI] [PubMed] [Google Scholar]
- O’Byrne PM, Kavanagh PV, McNamara SM, Stokes SM. (2013) Screening of stimulants including designer drugs in urine using a liquid chromatography tandem mass spectrometry system. J Anal Toxicol 37:64–73. [DOI] [PubMed] [Google Scholar]
- Schechter MD. (1986) Discriminative properties of l-cathinone compared to dl- and d-cathinone. Pharmacol Biochem Behav 24:1161–1165. [DOI] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, et al. (2013) Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int 233:416–422. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparago M, Wlos J, Yuan J, Hatzidimitriou G, Tolliver J, Dal Cason TA, Katz J, Ricaurte G. (1996) Neurotoxic and pharmacologic studies on enantiomers of the N-methylated analog of cathinone (methcathinone): a new drug of abuse. J Pharmacol Exp Ther 279:1043–1052. [PubMed] [Google Scholar]
- Suzuki M, Deschamps JR, Jacobson AE, Rice KC. (2015) Chiral resolution and absolute configuration of the enantiomers of the psychoactive “designer drug” 3,4-methylenedioxypyrovalerone. Chirality 27:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vircks KE, Mulligan CC. (2012) Rapid screening of synthetic cathinones as trace residues and in authentic seizures using a portable mass spectrometer equipped with desorption electrospray ionization. Rapid Commun Mass Spectrom 26:2665–2672. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens R. (1973) Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther 187:27–33. [PubMed] [Google Scholar]