Abstract
Background
One of the largest cross-sectional study in recent years was carried out to investigate the prevalence of intestinal parasitic infections among urban and rural school children from five states namely Selangor, Perak, Pahang, Kedah and Johor in Peninsula Malaysia. This information would be vital for school authorities to influence strategies for providing better health especially in terms of reducing intestinal parasitism.
Methods and Principal Findings
A total of 3776 stool cups was distributed to 26 schools throughout the country. 1760 (46.61%) responded. The overall prevalence of intestinal parasitic infection in both rural and urban areas was 13.3%, with Blastocystis sp (10.6%) being the most predominant, followed by Trichuris trichiura (3.4%), Ascaris lumbricoides (1.5%) and hook worm infection (0.9%). Only rural school children had helminthic infection. In general Perak had the highest infection (37.2%, total, n = 317), followed by Selangor (10.4%, total, n = 729), Pahang (8.6%, total, n = 221), Kedah (6.2%, total, n = 195) and Johor (3.4%, total, n = 298). School children from rural schools had higher infection (13.7%, total, n = 922) than urban school children (7.2%, total, n = 838). Subtype (ST) 3 (54.3%) is the most predominant ST with persons infected with only ST1 and ST3 showing symptoms. Blastocystis sp infection significantly associated with low household income, low parent’s education and presence of symptoms (p<0.05).
Conclusion
It is critical that we institute deworming and treatment to eradicate the parasite especially in rural school children.
Introduction
Intestinal parasitic infection (IPI) is common with an estimation of 3 billion people infected worldwide. It is a major public health problem in Southeast Asia particularly among poor children living in urban squats and rural communities. In Malaysia, intestinal parasitic infection is endemic among Orang Asli communities [1,2]. High infection rates are associated with high human population density, low socio-economic status, inadequate supplies of clean water, insanitary disposal of feces and larger families [2]. Infection distribution in a community follows a negative binomial pattern, although everybody is susceptible, most individuals are uninfected or have low infection intensity, whilst only a small proportion carry a heavy parasitic load [3].
Epidemiological studies carried out previously showed that the socioeconomic situation can be one of the major contributors to disease transmission caused by parasitic diseases. Blastocystis sp. have been shown to be the commonest intestinal parasite found in most stool surveys[4]. Most of the prevalence studies carried out in Malaysia focused on aborigines [1,5,6], HIV infected patients or immunocompromised patients[7], closed communities namely high-rise flat dwellers [8,9], patients diagnosed with gastrointestinal disorders such as Irritable Bowel Syndrome (IBS) [10] and colorectal cancer patients [11]. The cohort group never exceed sample of 300 except two studies which examined 500 stool samples form the aborigine community. There are increasing reports implicating that the parasite causes diarrhoea and stomach bloating [12–14]. The prevalence of Blastocystis sp. varies with different types of population however it can be seen that a large scale survey has never been carried out (Table 1). Furthermore, extensive studies on this parasite showed that Blatocystis sp. infection is not restricted to or just affecting developing countries such as Bangladesh [15], China [16], Nepal [17], Pakistan [15], Thailand [18], Turkey [19] but also developed countries such as Denmark [20], France [21], Germany [15], Japan [15], Singapore [22] and United States [4].
Table 1. Prevalence studies on Intestinal parasitic infection in Malaysia from 1999 to 2013.
| No | Type of parasite | Type of population | Location | Total sample size | Year | Reference |
|---|---|---|---|---|---|---|
| 1 | Intestinal parasite | aborigine children | Kelantan | 111 | 2013 | [23] |
| 2 | Blastocystis sp. | Orang Asli | Negeri Sembilan, Perak and Pahang | 500 | 2014 | [24] |
| 3 | Giardia sp. | Orang Asli | Selangor, Perak and Pahang | 500 | 2012 | [25] |
| 4 | Blastocystis sp. | rural primary schoolchildren | Pahang | 300 | 2012 | [26] |
| 5 | Intestinal parasite | rural community | West Malaysia | 550 | 2012 | [27] |
| 6 | Intestinal parasite | HIV-infected individuals | Malaysia | 346 | 2011 | [28] |
| 7 | Cryptosporidium sp | Orang Asli | Selangor | 276 | 2011 | [29] |
| 8 | Giardia sp. | Orang Asli | Pahang | 321 | 2008 | [30] |
| 9 | soil-transmitted helminths | Orang Asli | Selangor | 281 | 2007 | [31] |
| 10 | Intestinal parasite | Orang Asli | Cameron highland | 262 | 2007 | [32] |
| 11 | intestinal protozoa | Orang Asli | Pahang | 130 | 2007 | [6] |
| 12 | Cryptosporidium sp.sp | HIV-infected | Kajang Hospital, Selangor | 66 | 2005 | [33] |
| 13 | Intestinal parasite | public | Kuala Lumpur | 246 | 2005 | [9] |
| 14 | Intestinal parasite | interior communities | Rejang River, Sarawak | 355 | 2002 | [34] |
| 15 | Intestinal parasite | aborigine children | Kelantan | 162 | 1997 | [35] |
| 16 | Giardia duodenalis | rural community | Malaysia | 917 | 1998 | [36] |
| 17 | Blastocystis sp. | animal handlers | local research institutions and zoo | 105 | 1999 | [37] |
There has not been a pervasive prevalence study on IPI throughout the nation. As the nation is progressing towards a developed status, one of the indicators of achievement is health. Prevalence of parasitic infections in primary school children from rural and urban schools will provide health indicators. The questionnaire reflecting the association of these children to socio-demographic factors, environmental factors, behavioural habits and gastrointestinal complaints would further provide a gauge to assess health and the respective risks among children aged 5–12 years in the country.
Materials and Methods
Study area and population
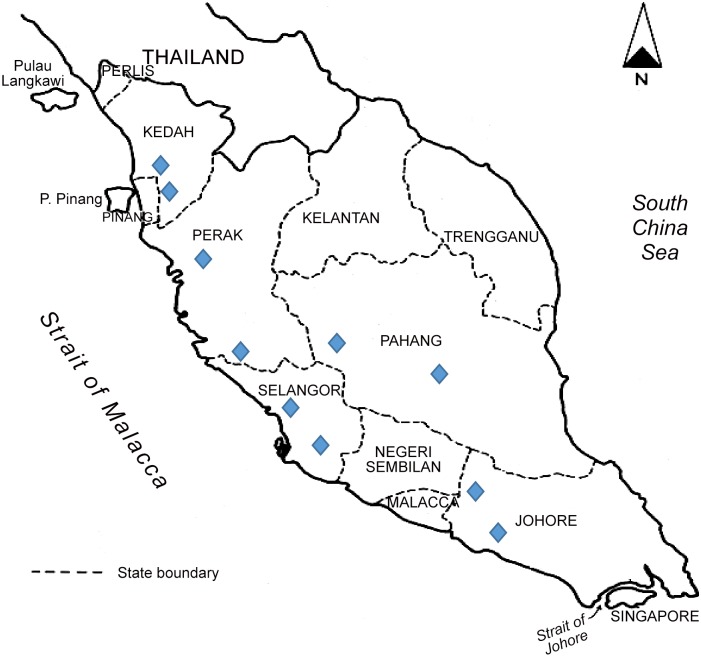
A cross-sectional survey was carried out between May 2012 and October 2013 among primary school children (from age 7 to 12) from five different states of Peninsular Malaysia i.e.: Perak, Selangor, Johor, Pahang and Kedah (Fig 1). Kedah and Perak represent northern region, Pahang and Selangor represent central region and Johor represents southern region of Peninsular Malaysia This study was approved by National Ministry of Education and the respective State Departments of Education. Rural and urban schools were randomly selected based on the official list of schools published in the respective State Education’s web portal. According to Ministry of Rural and Regional Development, Malaysia, rural areas are referred to areas with population less than 10,000 people having agriculture and natural resources in which its population either clustered, linear or scattered. In contrast, urban areas are known as gazetted areas with population of 10 000 and more. The school administrators (i.e. headmaster, school teachers and staff) were informed about the objectives and procedures involved in the study. Literate parents or legal guardians of school children were informed and consent (both written and verbal) were obtained prior to the surveys being carried out. Participation was voluntary, and hence, children could withdraw at any time from the study without any obligations. The list of school children names, age, class and gender were obtained prior to sample collection in order to categorize them accordingly.
Fig 1. Geographical map of Peninsular Malaysia and study areas.
—Indicates sample collection sites including both rural and urban areas. The rural and urban schools were identified based on the classification provided in the website of Ministry of Education, Malaysia.
Questionnaire
A structured questionnaire was prepared in English and Bahasa Malaysia (the national language of Malaysia). An oral briefing on IPI and hygiene practices to school children was given before the commencement of sample collection. The questionnaire was then distributed to children to be filled by their respective parents or guardian. school children were requested for demographic data (i.e., age, gender and education level), socioeconomic background of parents (household income and educational status) and also asked if they had any gastrointestinal symptoms or abnormalities (i.e. diarrhoea, abdominal pain or bloating, bloody or watery stools, constipation, vomiting).
Faecal examination
Sterile stool containers were distributed to all the school childrenon the day before sample collection. All school children were briefed on the procedure to collect and handle faecal samples. Faecal examination was performed by adding approximately pea size of faecal sample into Jones’ medium supplemented with 10% horse serum, incubated at 37°C and examined using light microscope for the subsequent 48 to 72 hours. Direct faecal examination and formal ether concentration techniques were used to examine the presence of other intestinal parasites using light microscope.
Subtyping of Blastocystis sp.
Faecal samples positive for Blastocystis sp. by in vitro cultivation in Jones’s medium and identified by light microscopy were subjected to DNA extraction using a commercial kit, QIAamp Deoxyribonucleic acid (DNA) Stool Mini Kit (Qiagen GmbH, Hilden, Germany) following the manufacturer’s instructions. Extracted DNA was stored at -20°C until used for genotyping of Blastocystis sp. isolates. The samples were subjected to polymerase chain reaction (PCR) amplification using sequenced tagged site (STS) primer with seven sets of primers (SB83, SB155, SB227, SB332, SB340, SB336 and SB337) for the genotyping of Blastocystis sp. from subtype 1 to subtype 7 (ST1 –ST7) (Yoshikawa et al. 2004). Blastocystis sp. ST1- ST7 are the most common subtypes that infect humans [21]. Therefore, the primer sequences specific for ST8 and ST9 were not used in the present study. Samples that were positive in in vitro cultivation but negative for subtyping classification using primer ST 1- ST 7 were labelled as unknown subtypes. The PCR conditions used for subtyping consisted of one initial denaturing cycle at 94°C for 3 min followed by 30 cycles that started with denaturing at 94°C for 30 seconds, annealing at 56.3°C for 30 seconds, extending at 72°C for 1 minute and an additional cycle of 10 minutes chain elongation at 72°C (Thermocycler, Bio-Rad). PCR products were separated in 1.5% agarose gel.
Statistical analysis
Data entry was carried out in Microsoft Excel 2007 spreadsheet and statistical analyses were carried out in SPSS Statistics version 17.0 software. Descriptive chi square test as appropriate was used to assess the relationship of the prevalence of Blastocystis sp. infection between demographic factor (i.e age, gender), socioeconomically status and symptoms caused by infection. Univariate analysis were used to identify the potential risk factor between each variable while multivariate analysis employing forward logistic regression model was used to identify the significant predictors. The frequency of the various subtypes of Blastocystis sp. was also assessed.
Ethical consideration
The study protocol was approved by the Ethics Committee of the University Malaya Medical Centre (UMMC), Malaysia (Reference Number: 848.28) prior to the commencement of the study. The participants were informed that the procedure used did not pose any potential risk and their identities and personal particulars would be kept strictly confidential.
Results
The response from the respective five states (Perak, Selangor, Johor, Pahang and Kedah) is shown in Table 2. Although 3776 questionnaires and faecal containers were distributed during the study, only 1760 (46.61%) school children turned up with their respective faecal samples. These school children consisted of 937 (53.20%) male and 823(46.8%) female with ages between 7 to 9 years (604 (34.3%) and 10 to 12 years (1156 (65.7%) respectively. The demographic data including background information of school children such as age, gender, household income of family, and parents’ education level are shown (Table 3).
Table 2. The rate of response of school children from different states.
| State | Rural | Urban | ||
|---|---|---|---|---|
| Distributed, N | Responded, N (%) | Distributed, N | Responded, N (%) | |
| Perak | 302 | 188 (62.25%) | 301 | 129 (42.86%) |
| Selangor | 766 | 349 (45.56%) | 789 | 380 (48.16%) |
| Johor | 340 | 148 (43.53%) | 150 | 47 (31.33%) |
| Pahang | 243 | 92 (37.86%) | 225 | 129 (57.33%) |
| Kedah | 329 | 145 (44.07%) | 331 | 153 (46.22%) |
| Total | 1760 / 3776 (46.61%) | |||
Table 3. The general characteristics of the school children such as household income of family, and parent’s education background.
| Factors | Rural, n = 922 | Urban, n = 838 | Overall, n = 1760 |
|---|---|---|---|
| Gender | |||
| Male | 463(50.2%) | 474(56.6%) | 937(53.20%) |
| Female | 459(49.8%) | 364(43.4%) | 823(46.8%) |
| Age group | |||
| Age (7–9) | 320(34.7%) | 284(33.9%) | 604(34.3%) |
| Age (10–12) | 602(65.3%) | 554(66.1%) | 1156(65.7%) |
| Household Income | |||
| Low | 438(47.5%) | 147(17.5%) | 585(33.2%) |
| Middle | 419(45.4%) | 487(58.1%) | 906(51.5%) |
| High | 64(6.9%) | 204(24.3%) | 268(15.2%) |
| Father’s education level | |||
| Primary | 214(23.2%) | 25(3.0%) | 239(13.7%) |
| secondary | 673(73.0%) | 662(79.0%) | 1335(75.9%) |
| Tertiary | 34(3.7%) | 151(18%) | 185(10.5%) |
| Mother’s education level | |||
| Primary | 257(27.9%) | 34(4.1%) | 291(16.5%) |
| secondary | 638(69.2%) | 710(84.7%) | 1348(76.6%) |
| Tertiary | 27(2.9%) | 94(11.2%) | 121(6.9%) |
Prevalence of Intestinal parasitic infection
The overall prevalence of intestinal parasite infection (IPI) among 1760 school children was 13.3% (n = 234), the parasites found were Blastocystis sp 10.6% (n = 186), Trichuris trichiura 3.4% (n = 59), Ascaris lumbricoides 1.5% (n = 26) and hookworm 0.9% (n = 16). Blastocystis sp. was found in both rural (13.70%; n = 922) and urban (3.40%; n = 838) school children. Whereas other parasites such as Trichuris trichiura (6.40%), Ascaris lumbricoides (2.80%), hookworm (1.70%) Giardia sp. (0.002%) and Taenia sp. (0.001%) can be found only in rural areas (n = 922) (Table 4). Blastocystis sp. is the most predominant parasite among all the parasites.
Table 4. Percentage of intestinal parasitic infection among school children from rural and urban in Peninsular Malaysia.
| Name of intestinal | Percentage of positive sample | ||
|---|---|---|---|
| parasite | Urban, n = 838 | Rural, n = 922 | Total, n = 1760 |
| Blastocystis sp. | 3.40% | 13.70% | 10.6% (n = 186) |
| Trichuris trichiura | 0% | 6.40% | 3.4% (n = 65) |
| Ascaris lumbricoides | 0% | 2.80% | 1.5% (n = 26) |
| Hookworm | 0% | 1.70% | 0.9% (n = 16) |
| Giardia sp | 0% | 0.002% | 0.001% (n = 2) |
| Taenia sp. | 0% | 0.001% | 0.0005% (n = 1) |
Prevalence of Blastocystis sp. infection
The overall prevalence of Blastocystis sp. infection was 10.6%. The highest prevalence rate was evident in the rural area (13.7%; n = 126) whereas the urban area had 7.2% (n = 60). In total, Perak showed highest prevalence rate with 24.0% followed by Selangor (9.5%), Pahang (8.6%), Kedah (6.2%) and Johor (3.4%) (Table 5). Subtype (ST) 3 (54.3%) had been reported to be the most predominant among all the subtypes, followed by ST 1 (22.6%), ST 2 (7.0%), ST 4 (7.0%) and ST 5(3.2%). There were no ST6 and ST7 found in these subjects (Table 6). In addition, 2.2% (4/186) were mixed infections consisting of two subtypes, which are ST1 and ST2 (n = 1) as well as ST1 and ST3 (n = 3). Unknown subtypes (negative for subtyping classification using primer ST1-ST7) also found in this study which is 1.6% (3/186). Generally, school children with ST3 and ST1 infection had symptoms such as diarrhoea, abdominal pain, abdominal bloating and constipation whereas other subtypes did not show any symptoms
Table 5. Prevalence of Blastocystis sp. infection among school children from different states, namely Selangor, Perak, Johor, Pahang and Kedah.
| State | Rural | Urban | Total |
|---|---|---|---|
| Selangor | 37/349 (10.6%) | 32/380 (8.2%) | 69/729(9.5%) |
| Perak | 58/188 (30.9%) | 18/129 (14%) | 76/317(24.0%) |
| Johor | 10/145 (6.9%) | 0/153 (0%) | 10/298(3.4%) |
| Pahang | 11/92 (12.1%) | 8/129 (6.2%) | 19/221(8.6%) |
| Kedah | 10/148 (6.8%) | 2/47 (4.3%) | 12/195(6.2%) |
| Total | 126 (13.7.7%) | 60 (7.2%) | 186/1760(10.6%) |
Table 6. Subtype classification from Blastocystis sp isolates from different states.
| Area | No of Blastocystis sp.infection | ST1 | ST2 | ST3 | ST4 | ST5 | UNKNOWN*/ Co-INFECTION# |
|---|---|---|---|---|---|---|---|
| Rural | |||||||
| Selangor | 37 | 10 | 3 | 22 | 1 | 1 | 0 |
| Perak | 58 | 12 | 7 | 32 | 5 | 2 | 0 |
| Johor | 10 | 2 | 0 | 5 | 2 | 0 | 1 |
| Pahang | 11 | 2 | 1 | 7 | 0 | 0 | 1 |
| Kedah | 10 | 4 | 0 | 4 | 1 | 0 | 1 |
| Total | 126 | 28(22.2%) | 10(7.9%) | 69(54.8%) | 9(7.1%) | 3(2.4%) | 3(2.4%) |
| Urban | |||||||
| Selangor | 32 | 6 | 2 | 17 | 3 | 2 | 2 |
| Perak | 18 | 5 | 0 | 10 | 1 | 0 | 2 |
| Johor | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pahang | 8 | 3 | 1 | 3 | 0 | 1 | 0 |
| Kedah | 2 | 0 | 0 | 2 | 0 | 0 | 0 |
| Total | 60 | 14(23.3%) | 3(5%) | 32(53.3%) | 4(6.7%) | 3(5%) | 4(6.7%) |
| Overall | 186 | 42(22.6%) | 13(7.0%) | 101(54.3%) | 13(7.0%) | 6(3.2%) | 7(3.8%) |
* Unknown: samples are positive in in vitro cultivation but negative for subtyping classification using primer ST 1- ST 7.
#Co-infection: samples that are positive for more than one subtype.
Risk factors of Blastocystis sp. infection
The risk factors associated with Blastocystis sp. infection in relation to demographic, geographical location and socioeconomic factors among rural and urban communities were examined using univariate analysis. The results showed that demographic factors of the school children age (7–9 years old (10.3%) vs 10–12 years old (10.70%), p = 0.807) and gender (male (12.0%) vs female (9.0%), p = 0.052) were not significantly associated with Blastocystis sp. infection. Risk factors were identified which include family’s low household income (OR = 4.223, 95% CI = 3.072–5.806; p<0.001), fathers’ low education level (OR = 5.321, 95% CI = 3.803–7.445; p<0.001), mothers’ low education level (OR = 5.011, 95% CI = 3.623–6.929; p<0.001), presence of symptoms (OR = 5.963, 95% CI = 4.304–8.261; p<0.001) and rural area (OR = 2.053, 95% CI = 1.486–2.835; p<0.001). (Table 7) Multivariate analysis using forward logistic regression model further confirmed that school childrenwith low household income had 2 times (95% CI = 1.279–2.869, p<0.001) and presence of symptoms had 6 times (95% CI = 4.281–8.838, p<0.001) possibilities of suffering from Blastocystis sp. infections, respectively.
Table 7. Potential risk factor associated with Blastocystis sp. infection (Univariate analysis, n = 1760).
| Variables | N | No | % | OR (95%CI) | p value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 937 | 112 | 12.00% | 1.374(.008–1.873) | 0.052 |
| Female | 823 | 74 | 9.00% | 1 | |
| Age group | |||||
| Age (7–9) | 604 | 62 | 10.30% | 0.952(0.690-.1.314) | 0.807 |
| Age (10–12) | 1156 | 124 | 10.70% | 1 | |
| Household Income | |||||
| <2000 | 585 | 119 | 20.30% | 4.223(3.072–5.806) | <0.001** |
| >2000 | 1175 | 67 | 5.70% | 1 | |
| Presence of symptoms | |||||
| Symptomatic | 254 | 83 | 32.70% | 5.963(4.304–8.261) | <0.001** |
| Asymptomatic | 1506 | 103 | 6.80% | 1 | |
| Education Of father | |||||
| Primary | 239 | 72 | 30.10% | 5.321(3.803–7.445) | <0.001* |
| Secondary | 1521 | 114 | 7.50% | 1 | |
| Education of Mother | |||||
| Primary | 291 | 81 | 27.80% | 5.011(3.623–6.929) | <0.001* |
| Secondary | 1469 | 105 | 7.10% | 1 | |
| Area | |||||
| Rural | 922 | 126 | 16.40% | 5.391(3.564–8.154) | <0.001 |
| Urban | 838 | 60 | 7.20% | 1 |
N: Number examined; no: Number positive.
Reference group marked as OR = 1; CI: Confidence interval.
*Significant association (p<0.05).
**Variables were confirmed by multivariate analysis as significant predictors of Blastocystis infection.
Discussion
To the best of our knowledge, this is the first large scale study done in Peninsular Malaysia to provide a prevalence data on intestinal parasitic infection namely Blastocystis sp. among urban and school children. In present study, the prevalence of Blastocystis sp. were determined based on in vitro culture technique. Generally, in vitro culture technique is used for routine laboratory screening for the rapid detection of Blastocystis sp as it is reported in vitro culture using Jones' medium is more sensitive than the formalin-ether concentration technique for detecting B. hominis [38] We agree that other molecular techniques namely PCR [20] and qPCR [39] which have been reported to be more sensitive for Blastocystis sp. detection could have increased the prevalence rate in this study. However, in our study the in vitro technique would be the best choice as it is more economical in screening large sample size.
The results showed that out of 1760school children, 234 (13.3%) were infected with at least one type of intestinal parasite with infection rate of 18.9% and 7.2% in rural and urban area respectively. The data showed that intestinal parasitic infection (IPI) is one of the major public health problems among underprivileged and socioeconomically deprived communities in developing countries such as Malaysia. Our study also showed that IPI is highly prevalent in Perak (24%). This is due to the fact that majority of school children of this state were from the aborigine community and had low household income compared to other states. Many previous studies reported that aborigine communities had high IPI as a consequence of their poor socio-economic environment.
The present study revealed high prevalence of intestinal protozoa infection namely Blastocytis sp. (13.70%) followed by Soil Transmitted Helminths (STH) such as Trichuris trichiura (6.4%), Ascaris lumbricoides (2.8%) and hookworm (1.7%) among school children from rural area. According to a previous study, prevalence of STH infection was at 41.4% among Malaysian children (from 0 to 15 years old). The present study showed that the prevalence of STH had decreased compared to previous study [40]. The present study showed that Giardia sp. (0.002%) and Taenia sp. (0.001%) were present but had the least infection rate. Between year 1992 and 1994 a study carried out to determine the prevalence of giardiasis among Malaysian primary school children (n = 7557) from lower socio-economic group showed 0.21% [41]. The present study showed that there is almost a 100-fold reduction in the infection rate of Giardia sp. in the past 10 years and this can be considered as a reflection of development especially in terms of proper toilet facilities and sanitation, water supply and hygiene practice in Malaysia.
To date, most of the studies reported on IPI among Malaysian school children had focused only on aborigine communities in selected areas [1,6,26] (Table 1) and the high prevalence in these communities was associated with low socioeconomic status. A recent report showed that the IPI was present in 52.3% among the aborigine community in Pahang [6] and 20.7% among three different types of Orang Asli tribes [27]. The present study is the first to compare IPI among both urban and rural school children in Malaysia. In this study we found transmission of IPI only prevalent in rural schools. This cannot be obviously due to poor sanitary and contaminated water supplies as development has reached most parts of the rural areas. It is highly possible that playing in fields, bare-footed, contaminated with STH as well as low public health awareness may contribute to the transmission. However in the case of Blastocystis sp. the organism reflected highest prevalence (10.6%), which is evident in both rural (13.7%) and urban (7.2%) school children. In the past, prevalence of Blastocystis sp. in Malaysia only focused on cohort groups of children in aborigines or rural communities (25.7%) [26], in cancer patients (4.0%) [42] and patients with diarrhoea (4.4%) [1]. The only other study showing a prevalence in urban population was the report of 10.2% in high rise flat dwellers in Kuala Lumpur [8]. In addition to that, epidemiological studies [43,44] also provided molecular evidence supporting possible human-to-human and waterborne zoonoses of Blastocystis sp. within communities living in close proximity with animals and in the vicinity of water sources.
In a previous study, 615 water samples were collected from May 2008 till April 2010 including bottled drinking water, filtered water, lakes, ponds, rivers, tap and well water in Peninsular Malaysia and 11.7% of samples were contaminated with Blastocystis sp. (unpublished work). This study also showed that Blastocystis sp. was detected in 37.0% of river water samples from Selangor state and Federal Territory of Kuala Lumpur where most of the rivers were surrounded with housing and commercial properties which may potentially contribute to the contamination. Accidental consumption of recreational river water contaminated with Blastocystis sp. by visitors may be a cause of transmission. The present study shows almost a similar prevalence of Blastocystis sp. in rural (10.6%) and urban schools (8.2%) in the state of Selangor. The statistics reveal that despite changing landscape in these two settings similar transmission patterns appear to be going on. Moreover the high population influx of people from other states to Selangor state seeking study and employment opportunities could be a contributory factor.
Blastocystis sp. is classified into 17 subtypes (ST1- ST17) based on nomenclature established by Stensvold et al. [45]. In the current study, Blastocystis sp. ST3 had the highest prevalence (54.3%) followed by ST1 (22.6%), ST2 (7.0%), ST4 (7.0%) and ST5 (3.2%). ST 1 –ST 4 are common among humans of which ST3 being the most predominant subtype. ST5—ST9 have been sporadically isolated from humans while ST10 –ST17 have not been found in humans [17]. In Asian countries such as Bangladesh [15], China [15], Iran [46], Japan [15], Malaysia [47], Pakistan [48], Singapore [22] and Thailand [18,49], ST1 as well as ST3 are the most predominant subtypes.
The epidemiological characteristics such as reservoir and transmission methods imply the variation of subtypes in different locations. In relation to this, Blastocystis sp. was also detected in drinking water[26], sewage[50] and rivers from recreational areas [51,52] in Malaysia. The World Health Organisation publications on drinking water quality have included Blastocystis sp. as one of the pathogens to be considered for waterborne zoonosis and thus providing evidence for waterborne transmission by this parasite [26]. Children in the rural schools may come from families with agricultural and farming background. This can also be a route of acquiring the infection as it was shown previously that Blastocystis was highly prevalent among animal handlers (41%) [37]. A study pertaining to zoonotic transmission with molecular evidence has shown the transmission of this parasite from animals to animal handlers in Philippines and Australia [53,54]. Besides that, Blastocystis sp. ST2 was detected in children and monkeys living within the same area in Kathmandu, Nepal and presence of Blastocystis sp. subtype 4 was seen in those who were raring animals next to their dwellings [17]
Subsequently, risk factors such as age, gender were analysed using univariate and multivariate analysis to identify the predictors of Blastocystis sp. infection among children in Malaysia. Based on our result, age did not show any significant value. This shows that there was no difference in socio behaviour between age groups 7–9 (10.3%) and 10–12 (10.7%) though the infection rates of children aged 10–12 was slightly higher than aged 7–9. With regards to gender, Blastocystis sp infection rate among male (12.0%) is slightly higher than female (9.0%) and shows marginally significant (p = 0.052). In previous studies, Blastocystis sp. infection were associated with numerous factors such as consumption of contaminated food and water, close contact with animals, poor personal hygiene, inadequate sanitation, geographical distribution and seasonal influences [4,37,55].
Our findings confirmed that, family’s low household income especially in rural communities and presence of symptoms were significant risk predictors of IPIs namely Blastocystis sp. infection. Previous studies substantiate that poor and socioeconomically deprived rural communities and the poor people of under developed nations are highly possible to acquire the infection due to active transmission within the community [2,27]. Health education and sanitation are two important aspects of primary health care system introduced by the World Health Organization (WHO) as a basis for the prevention and control of communicable diseases. Lower level of education in the present study possess a risk as parents due to lack of awareness on hygiene practices may play a major role in facilitating a transmission.
Besides that, Blastocystis sp. infection was found to be significantly associated with gastrointestinal symptoms among these school children. Interestingly in present study, we found that isolates from symptomatic participants belonged to ST1 and ST3, whereas ST2, ST4 and ST5 were isolated from asymptomatic participants. There was a significant difference in the distribution of subtypes between the symptomatic and asymptomatic groups (p<0.001)., A previous study did show the association of ST1 and ST3 with pathogenicity [56]. Furthermore, a study in Denmark showed that most of the patients with suspected parasitic gastrointestinal infection were positive for Blastocystis sp. ST1 and ST3 [20]. In addition, previous studies revealed that ST3 and ST1 respectively correlates with gastrointestinal symptoms [57] [58]. Our study concur with the previous findings that the pathogenic potential of this parasite is attributed to subtype variation. [59].
Although in early years some clinical studies concluded that Blastocystis sp is a commensal and may not be responsible for clinical symptoms [60], recently, several epidemiological studies have reported on the positive correlation between gastrointestinal symptoms with Blastocystis sp. Infection [13, 56]. Sheehan et al. [61] had reported that a total of five or more B. hominis cells per 40x magnification field seen in a direct microscopic examination of stool smear wet mount is suggestive of a pathogen that causes clinical illnesses. A recent study conducted on patients with confirmed positive Blastocystis sp. and aimed to assess the frequency, clinical symptoms and skin manifestations, found that 73.75% of the patients had gastrointestinal symptoms[62]. In another study conducted among Malaysian primary rural school children revealed that infection of Blastocystis sp. regardless of single or multiple infection was found to be significantly associated with symptoms such as abdominal pain (58.8%) and diarrhoea (50.0%) [26]. On the other hand, predominance of amoeboid forms of B. hominis in isolates from symptomatic patients[63] had been demonstrated and this amoebic forms of Blastocystis sp. could play a major role in exacerbating the gastrointestinal symptoms[64]
Conclusion
Intestinal parasitic infections namely Blastocystis sp. are highly prevalent among school children from poor and socioeconomically deprived rural communities in Peninsular Malaysia. Our findings may serve as baseline data to the government authorities in order to eradicate intestinal parasitic infection such as STHs and protozoan infection. There is a need to conduct screening of IPIs namely for Blastocystis sp. among school children as it may suppress the immune system and subsequently leading to secondary infectious diseases. Therefore, in order to control the infection, it is necessary to promote health awareness and hygiene practices especially in school children.
Acknowledgments
The authors would like to thank all the children and their parents for their participation in this study. We thank Ministry of Education, headmasters and teachers of the respective schools at which specimens were collected, for their kind help and cooperation.
Data Availability
All relevant data are available in the paper.
Funding Statement
This research was supported by High Impact Research (HIR) Grant – Ministry of High Education (MOHE) - (Project UM.C/625/1/HIR/044) and Postgraduate Research Grant (PPP), Institute Graduate Studies, University Malaya - (PV133-2012A).
References
- 1.Sinniah B, Sabaridah I, Soe MM, Sabitha P, Awang IP, Ong GP, et al. (2012) Determining the prevalence of intestinal parasites in three Orang Asli (Aborigines) communities in Perak, Malaysia. Trop Biomed 29: 200–206. [PubMed] [Google Scholar]
- 2.Rajeswari B, Sinniah B, Hussein H (1994) Socio-economic factors associated with intestinal parasites among children living in Gombak, Malaysia. Asia-Pacific Journal of Public Health 7: 21–25. [DOI] [PubMed] [Google Scholar]
- 3.Bundy D, Guyatt H (1990) Global distribution of parasitic worm infections. Report to the.
- 4.Amin OM (2002) Seasonal prevalence of intestinal parasites in the United States during 2000. The American journal of tropical medicine and hygiene 66: 799–803. [DOI] [PubMed] [Google Scholar]
- 5.Anuar TS, Ghani MK, Azreen SN, Salleh FM, Moktar N (2013) Blastocystis infection in Malaysia: evidence of waterborne and human-to-human transmissions among the Proto-Malay, Negrito and Senoi tribes of Orang Asli. Parasit Vectors 6: 40 10.1186/1756-3305-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noor Azian MY, San YM, Gan CC, Yusri MY, Nurulsyamzawaty Y, Zuhaizam AH, et al. (2007) Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop Biomed 24: 55–62. [PubMed] [Google Scholar]
- 7.Lono A, Kumar S, Chye TT (2011) Detection of microsporidia in local HIV-positive population in Malaysia. Transactions of the Royal Society of Tropical Medicine and Hygiene 105: 409–413. 10.1016/j.trstmh.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim J (2001) Blastocystis hominis in high-rise flat dwellers in Kuala Lumpur, Malaysia. Transactions of the Royal Society of Tropical Medicine and Hygiene 95: 377–378. [DOI] [PubMed] [Google Scholar]
- 9.Jamaiah I, Rohela M (2005) Prevalence of intestinal parasites among members of the public in Kuala Lumpur, Malaysia. Southeast Asian J Trop Med Public Health 36: 68–71. [PubMed] [Google Scholar]
- 10.Giacometti A, Cirioni O, Fiorentini A, Fortuna M, Scalise G (1999) Irritable bowel syndrome in patients with Blastocystis hominis infection. European Journal of Clinical Microbiology and Infectious Diseases 18: 436–439. [DOI] [PubMed] [Google Scholar]
- 11.Kumarasamy V, Roslani AC, Rani KU, Govind SK (2014) Advantage of using colonic washouts for Blastocystis detection in colorectal cancer patients. Parasit Vectors 7: 162–166. 10.1186/1756-3305-7-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Shazly A, Abdel-Magied A, El-Beshbishi S, El-Nahas H, Fouad M, Monib M (2005) Blastocystis hominis among symptomatic and asymptomatic individuals in Talkha Center, Dakahlia Governorate, Egypt. Journal of the Egyptian Society of Parasitology 35: 653–666. [PubMed] [Google Scholar]
- 13.Kaya S, Cetin ES, Aridogan B, Arikan S, Demirci M (2007) Pathogenicity of Blastocystis hominis, a clinical reevaluation. Turkiye Parazitol Derg 31: 184–187. [PubMed] [Google Scholar]
- 14.Vennila GD, Suresh Kumar G, Khairul Anuar A, Rajah S, Saminathan R, Sivanandan S, et al. (1999) Irregular shedding of Blastocystis hominis. Parasitol Res 85: 162–164. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IK, Hossain M, et al. (2004) Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res 92: 22–29. [DOI] [PubMed] [Google Scholar]
- 16.Li LH, Zhang XP, Lv S, Zhang L, Yoshikawa H, Wu Z, et al. (2007) Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitology research 102: 83–90. [DOI] [PubMed] [Google Scholar]
- 17.Lee IL, Tan TC, Tan PC, Nanthiney DR, Biraj MK, et al. (2012) Predominance of Blastocystis sp. subtype 4 in rural communities, Nepal. Parasitol Res 110: 1553–1562. 10.1007/s00436-011-2665-0 [DOI] [PubMed] [Google Scholar]
- 18.Leelayoova S, Siripattanapipong S, Thathaisong U, Naaglor T, Taamasri P, Piyaraj P, et al. (2008) Drinking water: a possible source of Blastocystis spp. subtype 1 infection in schoolchildren of a rural community in central Thailand. Am J Trop Med Hyg 79: 401–406. [PubMed] [Google Scholar]
- 19.Dogruman-Al F, Dagci H, Yoshikawa H, Kurt Ö, Demirel M (2008) A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitology research 103: 685–689. 10.1007/s00436-008-1031-3 [DOI] [PubMed] [Google Scholar]
- 20.Stensvold CR, Arendrup MC, Jespersgaard C, Mølbak K, Nielsen HV (2007) Detecting< i> Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagnostic microbiology and infectious disease 59: 303–307. [DOI] [PubMed] [Google Scholar]
- 21.Souppart L, Sanciu G, Cian A, Wawrzyniak I, Delbac F, Capron M, et al. (2009) Molecular epidemiology of human Blastocystis isolates in France. Parasitology research 105: 413–421. 10.1007/s00436-009-1398-9 [DOI] [PubMed] [Google Scholar]
- 22.Wong KH, Ng GC, Lin RT, Yoshikawa H, Taylor MB, Tan KS. (2008) Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol Res 102: 663–670. [DOI] [PubMed] [Google Scholar]
- 23.Hartini Y, Geishamimi G, Mariam AZ, Mohamed-Kamel AG, Hidayatul FO, et al. (2013) Distribution of intestinal parasitic infections amongst aborigine children at Post Sungai Rual, Kelantan, Malaysia. Trop Biomed 30: 596–601. [PubMed] [Google Scholar]
- 24.Anuar TS, Ghani M, Azreen SN, Salleh FM, Moktar N (2013) Blastocystis infection in Malaysia: Evidence of waterborne and human-to-human transmissions among the Proto-Malay, Negrito and Senoi tribes of Orang Asli. Parasit Vectors 6: 40 10.1186/1756-3305-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anuar TS, Al-Mekhlafi HM, Ghani MK, Osman E, Yasin AM, Nordin A, et al. (2012) Giardiasis among different tribes of Orang Asli in Malaysia: highlighting the presence of other family members infected with Giardia intestinalis as a main risk factor. Int J Parasitol 42: 871–880. 10.1016/j.ijpara.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 26.Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Ahmed A, Surin J, Mak JW (2012) Drinking water is a significant predictor of Blastocystis infection among rural Malaysian primary schoolchildren. Parasitology 139: 1014–1020. 10.1017/S0031182012000340 [DOI] [PubMed] [Google Scholar]
- 27.Ngui R, Lim YA, Chong Kin L, Sek Chuen C, Jaffar S (2012) Association between anaemia, iron deficiency anaemia, neglected parasitic infections and socioeconomic factors in rural children of West Malaysia. PLOS Negl Trop Dis 6: e1550 10.1371/journal.pntd.0001550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asma I, Johari S, Sim BL, Lim YA (2011) How common is intestinal parasitism in HIV-infected patients in Malaysia? Trop Biomed 28: 400–410. [PubMed] [Google Scholar]
- 29.Al-Mekhlafi HM, Mahdy MA, Azlin MY, Fatmah MS, Norhayati M (2011) Childhood Cryptosporidium infection among aboriginal communities in Peninsular Malaysia. Ann Trop Med Parasitol 105: 135–143. 10.1179/136485911X12899838683368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed Mahdy AK, Lim YA, Surin J, Wan KL, Al-Mekhlafi MS (2008) Risk factors for endemic giardiasis: highlighting the possible association of contaminated water and food. Trans R Soc Trop Med Hyg 102: 465–470. 10.1016/j.trstmh.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Aini UN, Al-Mekhlafi MS, Azlin M, Shaik A, Sa'iah A, Fatimah MS, et al. (2007) Serum iron status in Orang Asli children living in endemic areas of soil-transmitted helminths. Asia Pac J Clin Nutr 16: 724–730. [PubMed] [Google Scholar]
- 32.Hakim SL, Gan CC, Malkit K, Azian MN, Chong CK, Shaari N, et al. (2007) Parasitic infections among Orang Asli (aborigine) in the Cameron Highlands, Malaysia. Southeast Asian J Trop Med Public Health 38: 415–419. [PubMed] [Google Scholar]
- 33.Lim YA, Rohela M, Sim BL, Jamaiah I, Nurbayah M (2005) Prevalence of cryptosporidiosis in HIV-infected patients in Kajang Hospital, Selangor. Southeast Asian J Trop Med Public Health 36 Suppl 4: 30–33. [PubMed] [Google Scholar]
- 34.Sagin DD, Mohamed M, Ismail G, Jok JJ, Lim LH, Pui JN (2002) Intestinal parasitic infection among five interior communities at upper Rejang River, Sarawak, Malaysia. Southeast Asian J Trop Med Public Health 33: 18–22. [PubMed] [Google Scholar]
- 35.Rahmah N, Ariff RH, Abdullah B, Shariman MS, Nazli MZ, Rizal MZ (1997) Parasitic infections among aborigine children at Post Brooke, Kelantan, Malaysia. Med J Malaysia 52: 412–415. [PubMed] [Google Scholar]
- 36.Norhayati M, Penggabean M, Oothuman P, Fatmah MS (1998) Prevalence and some risk factors of Giardia duodenalis infection in a rural community in Malaysia. Southeast Asian J Trop Med Public Health 29: 735–738. [PubMed] [Google Scholar]
- 37.Rajah Salim H, Suresh Kumar G, Vellayan S, Mak JW, Khairul Anuar A, Init I, et al. (1999) Blastocystis in animal handlers. Parasitol Res 85: 1032–1033. [DOI] [PubMed] [Google Scholar]
- 38.Suresh K, Smith H (2004) Comparison of methods for detecting Blastocystis hominis. European Journal of Clinical Microbiology and Infectious Diseases 23: 509–511. [DOI] [PubMed] [Google Scholar]
- 39.Poirier P, Wawrzyniak I, Albert A, El Alaoui H, Delbac F, Livrelli V (2011) Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. Journal of clinical microbiology 49: 975–983. 10.1128/JCM.01392-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kan S, Poon G (1987) Prevalence, distribution and intensity of soil-transmitted helminthiases among Malaysian children. Public health 101: 243–251. [DOI] [PubMed] [Google Scholar]
- 41.Shekhar K, Prathapa S, Gurpreet K (1996) Prevalence of giardiasis among Malaysian primary school children. The Medical journal of Malaysia 51: 475–479. [PubMed] [Google Scholar]
- 42.Menon BS, Abdullah MS, Mahamud F, Singh B (1999) Intestinal parasites in Malaysian children with cancer. J Trop Pediatr 45: 241–242. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Owen H, Traub RJ, Cuttell L, Inpankaew T, Bielefeldt OH (2014) Molecular epidemiology of Blastocystis in pigs and their in-contact humans in Southeast Queensland, Australia, and Cambodia. Veterinary parasitology 203: 264–269. 10.1016/j.vetpar.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 44.Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Al-Mekhlafi AM, Ahmed A, Surin J (2013) Subtype distribution of Blastocystis isolates in Sebha, Libya. PLOS ONE 8: e84372 10.1371/journal.pone.0084372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stensvold CR, Suresh GK, Tan KS, Thompson RC, Traub RJ, Viscogliosi E, et al. (2007) Terminology for Blastocystis subtypes—a consensus. Trends Parasitol 23: 93–96. [DOI] [PubMed] [Google Scholar]
- 46.Motazedian H, Ghasemi H, Sadjjadi S (2008) Genomic diversity of Blastocystis hominis from patients in southern Iran. Annals of tropical medicine and parasitology 102: 85–88. 10.1179/136485908X252197 [DOI] [PubMed] [Google Scholar]
- 47.Tan TC, Ong SC, Suresh KG (2009) Genetic variability of Blastocystis sp. isolates obtained from cancer and HIV/AIDS patients. Parasitol Res 105: 1283–1286. 10.1007/s00436-009-1551-5 [DOI] [PubMed] [Google Scholar]
- 48.Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, et al. (2010) Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis. Parasitol Res 106: 1033–1038. 10.1007/s00436-010-1761-x [DOI] [PubMed] [Google Scholar]
- 49.Thathaisong U, Worapong J, Mungthin M, Tan-Ariya P, Viputtigul K, Sudatis A, et al. (2003) Blastocystis isolates from a pig and a horse are closely related to Blastocystis hominis. J Clin Microbiol 41: 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suresh K, Smith H, Tan T (2005) Viable Blastocystis cysts in Scottish and Malaysian sewage samples. Applied and environmental microbiology 71: 5619–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee LI, Chye TT, Karmacharya BM, Govind SK (2012) Blastocystis sp.: waterborne zoonotic organism, a possibility. Parasit Vectors 5: 130 10.1186/1756-3305-5-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ithoi I, Jali A, Mak J, Wan Sulaiman WY, Mahmud R (2011) Occurrence of Blastocystis in water of two rivers from recreational areas in Malaysia. Journal of parasitology research 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivera WL (2008) Phylogenetic analysis of Blastocystis isolates from animal and human hosts in the Philippines. Veterinary parasitology 156: 178–182. 10.1016/j.vetpar.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 54.Parkar U, Traub R, Kumar S, Mungthin M, Vitali S, Leelayoova S, et al. (2007) Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134: 359–367. [DOI] [PubMed] [Google Scholar]
- 55.Slifko TR, Smith HV, Rose JB (2000) Emerging parasite zoonoses associated with water and food. International journal for parasitology 30: 1379–1393. [DOI] [PubMed] [Google Scholar]
- 56.Jones MS, Whipps CM, Ganac RD, Hudson NR, Boroom K (2009) Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitology research 104: 341–345. 10.1007/s00436-008-1198-7 [DOI] [PubMed] [Google Scholar]
- 57.Tan T, Suresh K, Smith H (2008) Phenotypic and genotypic characterisation of Blastocystis hominis isolates implicates subtype 3 as a subtype with pathogenic potential. Parasitology research 104: 85–93. 10.1007/s00436-008-1163-5 [DOI] [PubMed] [Google Scholar]
- 58.El Safadi D, Meloni D, Poirier P, Osman M, Cian A, Gaayeb L, et al. (2013) Molecular epidemiology of Blastocystis in Lebanon and correlation between subtype 1 and gastrointestinal symptoms. The American journal of tropical medicine and hygiene 88: 1203–1206. 10.4269/ajtmh.12-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan KS, Mirza H, Teo JD, Wu B, MacAry PA (2010) Current views on the clinical relevance of Blastocystis spp. Current infectious disease reports 12: 28–35. 10.1007/s11908-009-0073-8 [DOI] [PubMed] [Google Scholar]
- 60.Sun T, Katz S, Tanenbaum B, Schenone C (1989) Questionable clinical significance of Blastocystis hominis infection. The American journal of gastroenterology 84: 1543–1547. [PubMed] [Google Scholar]
- 61.Sheehan DJ, Raucher B, McKitrick JC (1986) Association of Blastocystis hominis with signs and symptoms of human disease. Journal of clinical microbiology 24: 548–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balint A, Doczi I, Bereczki L, Gyulai R, Szucs M, Farkas K, et al. (2014) Do not forget the stool examination!-cutaneous and gastrointestinal manifestations of Blastocystis sp. infection. Parasitol Res 113: 1585–1590. 10.1007/s00436-014-3805-0 [DOI] [PubMed] [Google Scholar]
- 63.Tian Chye T (2006) Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitology research 98: 189–193. [DOI] [PubMed] [Google Scholar]
- 64.Rajamanikam A, Govind SK (2013) Amoebic forms of Blastocystis spp.—evidence for a pathogenic role. Parasit Vectors 6: 295 10.1186/1756-3305-6-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available in the paper.