Abstract
Objective
To investigate the potential for gene × environment interaction in hypertension by examining the extent to which educational attainment modifies the heritability of hypertension in male twins. Prior twin and family studies have established that hypertension runs in families and is heritable. In addition, epidemiological research indicates that the prevalence of hypertension differs by socioeconomic factors, such as educational attainment.
Methods
Twin structural equation modeling was used to examine educational attainment as a moderator of heritability of hypertension. Participants were 4301 monozygotic and 3414 dizygotic male Vietnam-era twins who provided data on both education (in years) and self-report of physician diagnosis of hypertension or medication usage.
Results
Heritability was 17 points lower among co-twins concordant for educational attainment of ≤14 years (0.46, 95% CI = 0.32–0.57) relative to co-twins concordant for >14 years of education (0.63, 95% CI = 0.54–0.71). The significant moderation of the heritability (p = .04) was confirmed in twin models examining educational attainment as a continuous moderator of hypertension.
Conclusions
These results demonstrate that the expression of genetic vulnerability to hypertension can vary as a function of environmental factors, including education level, and that nongenetic pathways may differentially contribute to risk among those with fewer years of education.
Keywords: hypertension, twins, education
INTRODUCTION
Twin and family studies provide a unique opportunity to partition the relative contributions of genes, environment, and gene × environment interaction to hypertension. From these studies, it is well established that familial transmission of blood pressure is primarily attributable to genetic factors (1) with an additional contribution of nonshared environment, or environmental factors not shared by the twins or family members. The role of shared environment, or environmental factors that contribute to similarity among twins and family members, remains more controversial. Although the majority of studies suggest little or no contribution of shared environment, it has recently been suggested that larger twin studies, with greater power to detect moderate effects, do identify effects of shared environment (1–3).
Another approach to examining environmental effects on hypertension risk is incorporating measures of environment directly into the twin model. This type of approach can build on the larger twin studies, suggestive of shared and nonshared environment, by identifying specific environmental measures that may account for the environmental variance seen in twin studies and by examining the extent to which the environmental measure may modify the heritability of hypertension (gene × environment interaction).
One environmental factor that seems to influence risk for hypertension is socioeconomic status (SES). In Western countries, hypertension is often overrepresented among individuals in lower socioeconomic strata (4–10). Educational attainment, one indicator of SES, was shown to predict incidence of hypertension among Caucasian men and women aged 25 to 44 years at 13-year follow-up in a nationally representative United States sample (11). Within a twin model, individual educational attainment can be decomposed into between-pair and within-pair components, the first component corresponding to the average educational attainment of the twin pair, the second component corresponding to individual deviations from this average. As shown in the study by Purcell (12), the between-pair component contributes to shared environment and/or gene × shared environment interaction, whereas the within-pair component contributes to nonshared environment and/or gene × nonshared environment interaction. Finally, it is also possible that educational attainment contributes directly to the heritability of hypertension, as educational attainment has previously been shown to be heritable (13).
To our knowledge, the role of educational attainment in hypertension has not been examined in the context of a twin design in which genetic and environmental factors can be partitioned. In this study, we examine years of education as a moderator of the heritability of self-report of physician diagnosed hypertension or use of antihypertensive medications in >3000 male-male twin pairs from the Vietnam-era Twin (VET) Registry.
METHODS
Sample
Participants were drawn from the VET Registry. The VET Registry is a nationally distributed cohort consisting of male-male twin pairs born between 1939 and 1957 in which both siblings served on active military duty during the Vietnam War era (14). Zygosity was determined using a questionnaire and blood group typing methodology that achieved 95% accuracy (14). Registry members are representative of all twins who served in the military during the Vietnam War on a variety of sociodemographic and other variables (15,16). The data used in the present study were derived from the Survey of Health (1987) and the National Heart, Lung and Blood Institute (NHLBI) survey (1990). The current study was approved by the Miriam Hospital Institutional Review Board and procedures that were followed were in accordance with The Miriam Hospital guidelines. All participants gave their informed consent at the time of the interviews.
Measures
Twin educational attainment (highest grade or year of school completed) was extracted from the Survey of Health (1987), whereas demographic information and hypertension data were derived from the NHLBI 1990 survey. The hypertension variable reflected responses to two questions: 1) “Have you ever been told by a doctor that you have hypertension?” and 2) “Are you currently medicated for hypertension?” An ordinal variable was created categorizing those with no evidence of hypertension as “0,” those who report being told by a physician that they have hypertension but who are not currently medicated as “1,” and those who report being currently medicated for hypertension as “2.” Of note, these self-report questions are similar to those included in Third National Health and Nutrition Survey (NHANES III) and have previously been shown to be sensitive to measures of clinical blood pressure and medication use (17).
Statistical Analyses
Twin structural equation modeling, which aims to explain the observed total phenotypic variation and covariation between monozygotic (MZ) and dizygotic (DZ) twins in terms of latent causes due to additive (A) or nonadditive (D) genetic effects and shared (C) or nonshared (E) environmental effects, was the primary method of analysis. We focused on additive genetic effects and shared and nonshared environmental genetic effects as the prior twin literature had found little evidence for nonadditive genetic effects on hypertension (18). For illustrative purposes, we first stratified the sample using a median split at 14 years of education to explore differences in A, C, and E by educational attainment. In this initial round of analyses, twin pairs who were either concordant low or concordant high on education attainment were supplemented by singleton twins from pairs in which education attainment was known for one twin, but missing for the second.
Next, we fit full gene × measured environment interaction models, using education level as a continuous moderator of hypertension incorporating all available twin pairs. Gene × measure environment interaction may be detected within twin models by examining differences in estimates of variance and variance components (A, C, D, and E) attributable to genetic and environmental effects in interaction with a continuous moderator (12). Both effects on the mean and on the variance are modeled. As we were primarily interested in the effects of educational attainment on variance components, we opted to guard against model misspecification by deliberately overparameterizing the mean structure. Therefore, linear and quadratic effects were forced into the model (e.g., B × M1 + F × M12 ), where M1 represents the value of the moderator and B and F represent linear and quadratic effects on the outcome, respectively, irrespective of the statistical significance of the two regression coefficients. This mean structure encompasses any phenotypic correlation between educational attainment and hypertension, permitting analyses of gene × educational attainment interaction independent of any gene-education correlation.
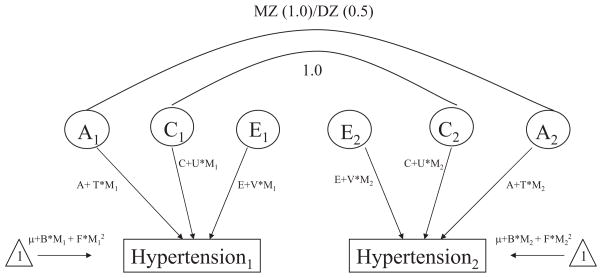
In addition, variability in susceptibility to hypertension is described in terms of three latent variables, A, C, and E with the path coefficients associated with each variable expressed as linear functions of the moderator (e.g., A + T × M1, C + U × M1, E + V × M1), where T represents the effects of the moderator on additive genetic variance and U and V represent the effects of the moderator on shared and nonshared environmental variance, respectively. The full model is presented in Figure 1. A significant compromise of model fit when parameters T, U, and V are fixed to zero reflects evidence of significant moderation of additive genetic, shared environmental, and nonshared environmental variance by the continuous moderator, respectively.
Figure 1.
Gene × environment interaction model adapted from Purcell, 2002 (12). A = additive genetic effects; C = shared environmental effects; E = nonshared environmental effects; M = moderator; T = moderated component of A; U = moderated component of C; V = moderated component of E; B, F = linear and quadratic effects of moderator on mean (forced entry).
Twin modeling for the ordinal hypertension variable was based on a liability model, which presumes an underlying, normally distributed susceptibility for the expression of the phenotype of interest with zero mean and unit variance (19). Categories were defined via thresholds on this underlying curve that reflect sample prevalence. Further, although both the overall mean and the variance components were allowed to vary by educational level, the thresholds in the liability scale were assumed to be fixed across educational levels. Educational attainment was treated as a continuous variable based on nationally representative data indicating a linear relationship between years of education and risk for hypertension (11). Nonetheless, given that the preponderance of participants in VET Registry reported between 12 and 16 years of education, we based the interpretation of our results on this range.
All models and maximum likelihood parameter estimates were calculated from raw data compiled within the Mx program (20). Comparative model fit is derived by comparing twice the log likelihood (−2lnL) of a reduced model with that of the full model. This resulting log-likelihood ratio is asymptotically distributed as a χ2 variable with degrees of freedom reflecting the difference in the number of parameters between the full and reduced models. However, for tests of the variance components, the corresponding p values are halved because their value under the null hypothesis is on the boundary of the parameter space (21).
Although the full VET Registry comprises 9548 male twins (4774 male-male twin pairs), 4301 MZ twins and 3414 DZ twins entered our analyses. This sample included 1924 complete MZ pairs (3848 individuals), 453 MZ singletons, 1472 complete DZ pairs (2944 individuals), and 470 DZ singletons. Complete pairs contributed to the estimation of cross-twin correlations, whereas singletons were incorporated into estimates of means and variances. The primary reason for the reduced sample size relative to the full VET Registry was refusal to participate in the NHLBI survey. Additional reasons included death and loss of contact with the twin at the time of the NHLBI survey. Those who participated in the NHLBI survey completed an average of an additional half year of schooling than those who did not participate, indicating some response bias in the NHLBI survey with regard to educational attainment.
To obtain confidence intervals for parameter estimates, thresholds, and genetic and environmental correlations, bootstrapping methods were employed. Specifically, at each bootstrap iteration, complete pairs were drawn with replacement from the original sample of the same size, so as to accurately represent missingness patterns in the original dataset. All runs in which Mx gave warning messages were dropped, and the process was repeated until 1000 bootstrap iterations had converged successfully. For each parameter of interest, the estimates were ordered and end points of 95% bootstrap confidence intervals were obtained from the 2.5 and 97.5 bootstrap sample percentiles. An inspection of parameter estimates from the original Mx model and the median for each parameter from the bootstrapping runs suggested that the results were exceedingly close, indicating little bias in the maximum likelihood estimate and that bootstrap confidence intervals are well centered.
RESULTS
Sample and Demographics
Demographic information and descriptive statistics for education level and self-reported hypertension are presented in Table 1. Participants were on average 41 years of age at the time of the NHLBI survey; 94% were Caucasian; 90% currently married. Participants had obtained an average of 13.85 (SD = 2.04) years of education, consistent with approximately 2 years of college or technical school; 81% reported never being told by a physician that they have hypertension; 13% reported being told by a physician that they have hypertension but no current medications; and 6% reported current medication for hypertension. Educational attainment was moderately correlated among twins, with intraclass correlation coefficients of 0.60 for MZ twins and 0.47 for DZ twins, resulting in additive genetic variance (a2) of 0.25, shared environmental variance (c2) of 0.35, and nonshared environmental variance (e2) of 0.40. The polyserial correlation between years of education and hypertension was not significant (r = −.02; p = .25), indicating little to no gene-environment correlation, and that educational attainment does not contribute directly to genetic, shared environmental, or nonshared environmental variance in hypertension.
TABLE 1.
Demographics and Descriptive Statistics for Self-Reported Hypertension
| MZ(n = 4301) | DZ (n = 3414) | |
|---|---|---|
| Age, mean ± SD | 41.12 ± 3.12 | 41.09 ± 2.77 |
| Education, mean ± SD, y | 13.95 ± 2.04 | 13.75 ± 2.05 |
| Race, n (%) | ||
| Caucasian | 4061 (94.49) | 3226 (94.55) |
| African | 217 (5.05) | 177 (5.19) |
| Hispanic | 2 (0.05) | 2 (0.06) |
| Other | 18 (0.42) | 7 (0.21) |
| Married, n (%) | 3829 (89.69) | 3078 (90.64) |
| Hypertension, n (%) | ||
| None | 3476 (80.82) | 2785 (81.58) |
| Yes | 575 (13.37) | 432 (12.65) |
| Medicated | 250 (5.81) | 197 (5.77) |
MZ = monozygotic; DZ = dizygotic; SD = standard deviation.
Gene × Environment Interaction 1: Heritability Above and Below the Median Split of Educational Attainment
For illustration, the additive genetic, shared environmental, and nonshared environmental effects on hypertension above and below the median split for this sample are presented in Table 2. As each of these components are standardized to sum to 1, the additive genetic variance is equivalent to the heritability. For >14 years of education, 1978 MZ and 1337 DZ individuals entered into the analyses, including concordant twin pairs as well as some singletons for whom education level was >14 years for one twin and the education level for the second was missing. For ≤14 years of education, 1160 MZ and 1005 DZ individuals entered into the analyses. As can be seen from Table 2, hypertension was influenced predominantly by heritability and nonshared environmental factors both above and below the median split. Nonetheless, the heritability of hypertension seems stronger among those with >14 years of education, indicative of gene × educational attainment interaction. Shared environmental variance could be dropped from the model without significantly compromising model fit both above (Δχ2 = 0.00; p = .50) and below (Δχ2 = 0.11; p = .37) the sample median for educational attainment. After fixing the shared environmental paths to zero (AE model), the heritability of hypertension among those with ≤14 years was 0.46, (95% CI = 0.32–0.57), whereas the heritability among those with >14 years of education was 0.63 (95% CI = 0.54–0.71).
TABLE 2.
Twin Pair Polychoric Correlations and Univariate Twin Structural Equation Model Parameter Estimates for Self-Reported Hypertension Above and Below the Median Split of Years of Education (≤14 years or >14 years)
| Variables | Twin Pair Correlations
|
a2 | e2 | Model Fit
|
||
|---|---|---|---|---|---|---|
| Rmz | Rdz | −2lnL | df | |||
| ≤14 years of education | 0.46 | 0.23 | 0.46 | 0.54 | 2573.34 | 2159 |
| Hypertension | 0.32 to 0.58a | 0.16 to 0.29 | 0.32 to 0.58 | 0.42 to 0.68 | ||
| >14 years of education | 0.63 | 0.32 | 0.63 | 0.37 | 3863.41 | 3308 |
| Hypertension | 0.54–0.71 | 0.27–0.35 | 0.54–0.71 | 0.29–0.46 | ||
−2lnL = twice the log likelihood; df = degrees of freedom.
95% Confidence Interval.
Gene × Environment Interaction 2: Educational Attainment as a Continuous Moderator of Self-Reported Hypertension
Comparative model fits testing the extent to which education level serves as a moderator of self-reported hypertension are presented in Table 3. Each model includes parameters B and F, representing linear and quadratic effects of years of education on the mean of the respective variables. The full model (ACETUVBF), including additive genetic (A), shared environmental (C), nonshared environmental (E) effects as well as terms for the moderation of each by years of education (T, U, and V, respectively), is presented first. Next, a backwards stepwise elimination procedure is followed for the individual parameters, testing the extent to which the parameter associated with the smallest change in log-likelihood ratio contributes significantly to the model. Moderation terms are tested first, followed by variance components with no evidence for moderation by educational attainment. In the final model, each parameter contributes significantly to model fit, with the possible exception of B and F, which are forced into each model.
TABLE 3.
Comparative Model Fits for Education Level as a Continuous Moderator of Hypertension
| Model Fit
|
Comparative Model Fit
|
||||||
|---|---|---|---|---|---|---|---|
| Model | −2LL | df | Δ−2LL | df | p | Test | |
| Hypertension | |||||||
| 1. Full | ACETUVBF | 9258.98 | 7913 | ||||
| 2. U = 0 | ACETVBF | 9258.99 | 7914 | 0.01 | 1 | 0.92 | 2 versus 1 |
| 3. V = 0 U = 0 | ACETBF | 9259.36 | 7915 | 0.37 | 1 | 0.54 | 3 versus 2 |
| 4. T = 0 U = V = 0 | ACEBF | 9263.65 | 7916 | 4.29 | 1 | 0.04 | 4 versus 3 |
| 5. C = 0 U = V = 0 | AETBF | 9259.80 | 7916 | 0.44 | 1 | 0.25 | 5 versus 3 |
Δ−2LL = log likelihood; df = degrees of freedom; A = additive genetic variance; C = shared environmental variance; E = nonshared environmental variance; T = moderation of additive genetic variance by education level; U = moderation of shared environmental variance by education level; V = moderation of nonshared environmental variance by education level; B = linear effects of education level on mean; F = quadratic effects of education level on mean.
In the backwards elimination procedure of moderation terms, the moderation of the additive genetic component by education level (T) contributed significantly to the model (p = .04), whereas the moderation terms association with the shared environment (U) and nonshared environment (V) did not (p > .54). In further modeling, it was also determined that the shared environment component (C) did not contribute significantly to variance in hypertension (p = .25), resulting in a final model of AETBF. At the mean level of education, heritability (additive genetic variance/total variance) of hypertension was estimated at 0.57 (95% CI = 0.52–0.63).
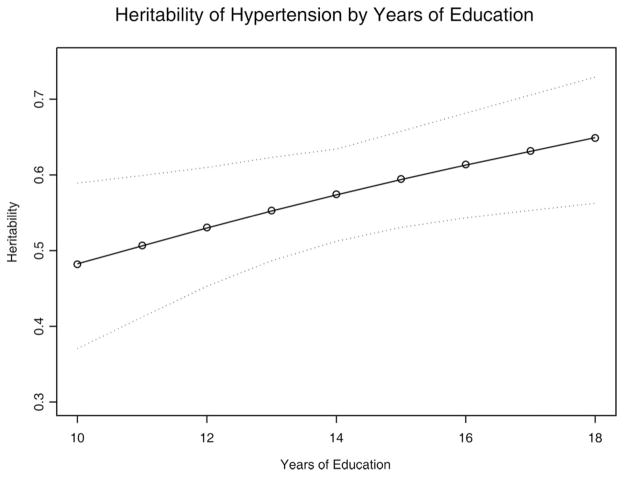
The moderation of additive genetic effects on hypertension by education level is depicted in Figure 2. Although survey participants reported between 6 and 20 years of education, 5/6 of the sample reported between 12 and 16 years of education. This 4-year difference in educational attainment—roughly corresponding with completion of a college degree—was associated with an 8-point increase in heritability (95% CI = 0.01–0.16), from 0.53 to 0.61. These results differ somewhat from median split analyses due to the inclusion of twin pairs discordant for educational attainment, and the narrowing of the range over which differences in educational attainment are evaluated. Nonetheless, these results illustrate greater heritability of self-reported hypertension at higher levels of educational attainment, or gene × educational attainment interaction.
Figure 2.
Heritability and 95% Confidence Interval for hypertension by years of education.
DISCUSSION
Prior twin studies have indicated that familial transmission of hypertension is largely attributable to genetic and non-shared environmental factors with a possible lesser influence of shared environmental factors. In addition, epidemiological investigations document an association between SES and hypertension. In this study, we use twin methods to show that the relationship between educational attainment and hypertension may, in part, reflect previously undetected gene × environment interaction. Specifically, among Vietnam-era twins, the heritability of self-reported hypertension differed by educational attainment. Among twin pairs selected for relatively high and low educational attainment, the heritability was greater among twins with higher educational attainment. Further, using educational attainment as a continuous predictor, the heritability of hypertension increased from 0.53 to 0.61 from 12 to 16 years of education, with a compensatory decrease in environmental factors not shared by twins, or non-shared environment. These results suggest that nongenetic factors may be more important at lower education levels and underscore the importance of including measured environmental factors, such as SES, in molecular genetic studies of hypertension.
At the mean of education level (14 years), the additive genetic and nonshared environmental components of 0.57 and 0.43, with no significant contribution of shared environment, are largely consistent with prior twin studies of blood pressure and hypertension. For example, in the Snieder review (1), encompassing 15 studies of adult blood pressure and >2500 twin pairs, heritability estimates for systolic and diastolic blood pressure were largely in the range of 0.50 to 0.60 with the remaining variance attributable to nonshared environment.
However, in this study, the estimate of the heritability of hypertension varied by years of education attained, indicating that the expression of genetic vulnerability to hypertension varies by sociodemographic factors, or gene × environment interaction. Our results are consistent with reduced genetic variation and heritability in the higher risk environment (as indexed by lower education levels). These results seem to suggest that lower educational attainment may reduce the penetrance of genetic vulnerability to hypertension, perhaps due to additional environmental pathways also leading to risk in the context of lower educational attainment. Although the specific mechanism by which education level affects the heritability of hypertension cannot be determined from this study, it is well documented that several environmental and behavioral factors that predict hypertension are more prevalent among groups with little formal education. Within the twin design, this type of effect may manifest itself as an attenuation of genetic effects, relative to environmental effects, and reduced heritability in lower socioeconomic environments.
Of note, this heritability by educational attainment interaction is independent of any gene-educational attainment correlation, or direct genetic effects of educational attainment on hypertension. Further, educational attainment did not account directly for shared or nonshared environmental variance in the absence of interaction with genetic effects. In this sample, the association between educational attainment and hypertension was r = −.02, which would have reflected the composite of genetic, shared environment and nonshared environmental correlation across educational attainment and hypertension. Nonetheless, we were careful to account for the potential for both linear and nonlinear effects of educational attainment on the mean of hypertension in moderation analyses.
An alternative explanation of reduced genetic variation in the high-risk environment is differential MZ twin participation in the high-risk environment as a result of a disease state, e.g., hypertension. As MZ twins are more likely to share hypertension relative to DZ twins, and lower educational level is a risk factor for hypertension, it is possible that more MZ twins in the high-risk environment—hereby indexed by lower educational attainment—may have failed to participate because they were either deceased or disabled due to hypertension. This would result in an attenuation of the overall association between educational attainment and hypertension and a lower MZ:DZ ratio in the high-risk environment. In the present study, the overall association of education level with hypertension was not significant, potentially suggesting an attenuation of a true effect. However, when examined across the entire sample, education level did not predict the ratio of MZ:DZ twin pairs, indicating that there was no differential loss of MZ twins in the high-risk environment. Further, to influence the gene × educational attainment interaction, a lower MZ:DZ ratio would need to result in an attenuated correlation among MZ twins relative to DZ twins specific to the high-risk environment. In Table 2, a reduction in correlation is seen in both MZ and DZ twins concordant for ≤14 years of education, relative to the correlations among their counterparts with >14 years of education. This suggests that the drop in heritability with educational attainment does not solely reflect a loss of association among the MZ twins with ≤14 years of education, reducing the likelihood that our results are accounted for by this type of ascertainment bias.
It is important to recognize important limitations to the present study. First, our hypertension data were based on self-report, leaving the possibility that our results pertain to hypertension awareness and not hypertension per se. Nonetheless, the prevalence of hypertension is similar to that seen in NHANES III, a survey of health risk factors in the United States conducted during a similar time period as the VET Registry surveys (1988–1991). The prevalence of self-reported hypertension in the VET Registry sample with a mean age of 41 years and age range of 33 to 55 years was 19%. In the NHANES III sample, the prevalence of hypertension based on measured blood pressure >140/90 mm Hg or self-report of antihypertensive medications was approximately 13% among male Caucasians, ages 30 to 39 years, and approximately 23% among male Caucasians, ages 40 to 49 years (22). Thus, the prevalence of self-reported hypertension in VET Registry does not seem largely discrepant from the prevalence of hypertension among Caucasian males in the US population based on clinical measurement of blood pressure or self-report of antihypertensive medications.
Furthermore, in additional analyses of NHANES III, it was concluded that self-reported hypertension can be an appropriate indicator of hypertension prevalence among Caucasian males (17). The overall sensitivity of self-reported hypertension to clinical measures of hypertension was 75%. In other words, in 75% of cases, the people who reported that they had hypertension were accurate relative to the criteria for documented hypertension as defined by measured blood pressure >140/90 mm Hg and/or controlling hypertension through pharmacological or nonpharmacological means. Importantly, for the present paper, this accuracy relative to clinical measures of hypertension, or “hypertension awareness,” did not differ significantly by education level, either across the entire NHANES III sample or within Caucasian males in that sample. Thus, based on the results from NHANES III, it does not seem the effects of education level on hypertension awareness accounted for the present results.
A second limitation is the ethnic and gender composition of the VET Registry. As the VET Registry is comprised entirely of men who are predominantly Caucasian, the generalizability of these results to civilians, women, and ethnic minorities remains to be determined. Finally, it should be noted that VET Registry members had higher levels of educational attainment than the national population samples. This was likely attributable to the “selective” criteria for entering the military, even though a draft was in effect during the Vietnam War, as well as a greater initial response rate among veterans with higher education levels for the initial survey (16) and the NHLBI survey. Nonetheless, the veterans initially targeted for inclusion in the sample were representative of all twins who served in the military in the Vietnam era in terms of education level and other demographic characteristics (15). The higher educational attainment in this sample suggests that the impact of years of education on the heritability of hypertension may operate across the range of years of education and that the present results may be stronger in a twin sample with greater representation of persons who did not complete high school. Finally, there are additional environmental factors that may contribute to hypertension that are not included in this first study and may account for the gene × educational attainment interaction reported in this paper.
The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution, this research would not have been possible. Numerous organizations have provided invaluable assistance in the conduct of this study, including the Department of Defense, National Personnel Records Center, National Archives and Records Administration, the Internal Revenue Service, National Opinion Research Center, National Research Council, National Academy of Sciences, and the Institute for Survey Research, Temple University.
Acknowledgments
This paper was supported by NIH Grant HL-72819 (JMM). The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the VET Registry.
Glossary
- SES
socioeconomic status
- VET
Vietnam-era twin
- NHANES III
Third National Health and Nutrition Survey
- MZ
monozygotic
- DZ
dizygotic
- A
additive genetic factor
- D
dominant genetic factor
- C
shared environmental factor
- E
nonshared environmental factor
- A2
additive genetic variance
- C2
shared environmental variance
- E2
nonshared environmental variance
- M1
moderator
- B
linear effects of the moderator on the mean
- F
quadratic effects of the moderator on the mean
- T
effects of the moderator on additive genetic variance
- U
effects of the moderator on shared environmental variance
- V
effects of the moderator on nonshared environmental variance
- NHLBI
National Heart, Lung and Blood Institute
- −2lnL
twice the log likelihood
References
- 1.Snieder H. Familial Aggregation of Blood Pressure. In: Portman RJ, Sorof JM, Ingelfinger JR, editors. Clinical Hypertension and Vascular Disease: Pediatric Hypertension. Totowa, NJ: Humana Press Inc; 2004. [Google Scholar]
- 2.Hong Y, de Faire U, Heller DA, McClearn GE, Pedersen N. Genetic and environmental influences on blood pressure in elderly twins. Hypertension. 1994;24:663–70. doi: 10.1161/01.hyp.24.6.663. [DOI] [PubMed] [Google Scholar]
- 3.Snieder H, Hayward CS, Perks U, Kelly RP, Kelly PJ, Spector TD. Heritability of central systolic pressure augmentation: a twin study. Hypertension. 2000;35:574–9. doi: 10.1161/01.hyp.35.2.574. [DOI] [PubMed] [Google Scholar]
- 4.Adler N, Ostrove J. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 5.Colhoun HM, Hemingway H, Poulter NR. Socio-economic status and blood pressure: an overview analysis. J Hum Hypertens. 1998;12:91–110. doi: 10.1038/sj.jhh.1000558. [DOI] [PubMed] [Google Scholar]
- 6.Grewen K, Girdler S, West S, Bradgon E, Costello N, Light K. Stable pessimistic attributions interact with socioeconomic status to influence blood pressure and vulnerability to hypertension. Journal of Women’s Health & Gender-Based Medicine. 2000;9:905–15. doi: 10.1089/152460900750020946. [DOI] [PubMed] [Google Scholar]
- 7.Kivimaki M, Kinnunen M, Pitkanen T, Vahtera J, Elovainio M, Pulkkinen L. Contribution of early and adult factors to socioeconomic variation in blood pressure: thirty-four-year follow-up study of school children. Psychosom Med. 2004;66:184–9. doi: 10.1097/01.psy.0000126821.33005.6b. [DOI] [PubMed] [Google Scholar]
- 8.Matthews K, Kiefe C, Lewis C, Liu K, Sidney S, Yunis C. Socioeconomic trajectories and incident hypertension in a biracial cohort of young adults. Hypertension. 2001;39:772–6. doi: 10.1161/hy0302.105682. [DOI] [PubMed] [Google Scholar]
- 9.Paeratakul S, Lovejoy JC, Ryan DH, Bray GA. The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. International Journal of Obesity. 2002;26:1205–10. doi: 10.1038/sj.ijo.0802026. [DOI] [PubMed] [Google Scholar]
- 10.Pickering T. Cardiovascular pathways: socioeconomic status and stress effects on hypertension and cardiovascular function. Ann N Y Acad Sci. 1999;896:262–77. doi: 10.1111/j.1749-6632.1999.tb08121.x. [DOI] [PubMed] [Google Scholar]
- 11.Vargas CM, Ingram DD, Gillum RF. Incidence of hypertension and educational attainment: the NHANES I epidemiologic followup study. First national health and nutrition examination survey. Am J Epidemiol. 2000;152:272–8. doi: 10.1093/aje/152.3.272. [DOI] [PubMed] [Google Scholar]
- 12.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 13.Heath AC, Berg K. Effects of social policy on the heritability of educational achievement. Prog Clin Biol Res. 1985;177:489–507. [PubMed] [Google Scholar]
- 14.Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam era twin (VET) registry: method of construction. Acta geneticae medicae et gemellologiae. 1987;36:61–6. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam era twin (VET) registry: ascertainment bias. Acta geneticae medicae et gemellologiae. 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- 16.Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam era twin registry: a resource for medical research. Public Health Rep. 1990;105:368–73. [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas C, Burt V, Gillum R, Pamuk E. Validity of self-reported hypertension in the national health and nutrition examination survey III. Prev Med. 1997;26:678–85. doi: 10.1006/pmed.1997.0190. [DOI] [PubMed] [Google Scholar]
- 18.Evans A, Van Baal GC, McCarron P, DeLange M, Soerensen TI, De Geus EJ, Kyvik K, Pedersen NL, Spector TD, Andrew T, Patterson C, Whitfield JB, Zhu G, Martin NG, Kaprio J, Boomsma DI. The genetics of coronary heart disease: the contribution of twin studies. Twin Res. 2003;6:432–41. doi: 10.1375/136905203770326439. [DOI] [PubMed] [Google Scholar]
- 19.Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- 20.Neale M, Boker S, Xie G, Maes H. VCU Box 900126. 6. Richmond, VA: Department of Psychiatry; 2002. Statistical Modeling. [Google Scholar]
- 21.Self S, Liang K-Y. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. Journal of the American Statistical Association. 1987;82:605–10. [Google Scholar]
- 22.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–9. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]