Abstract
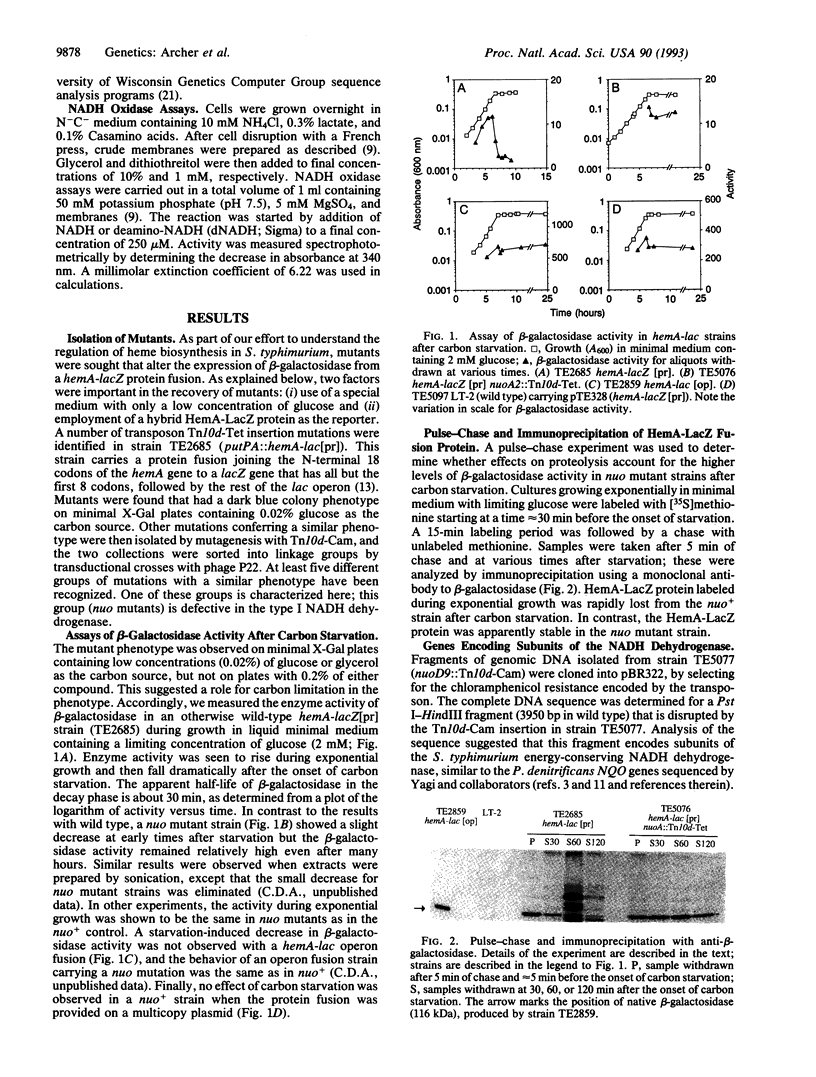
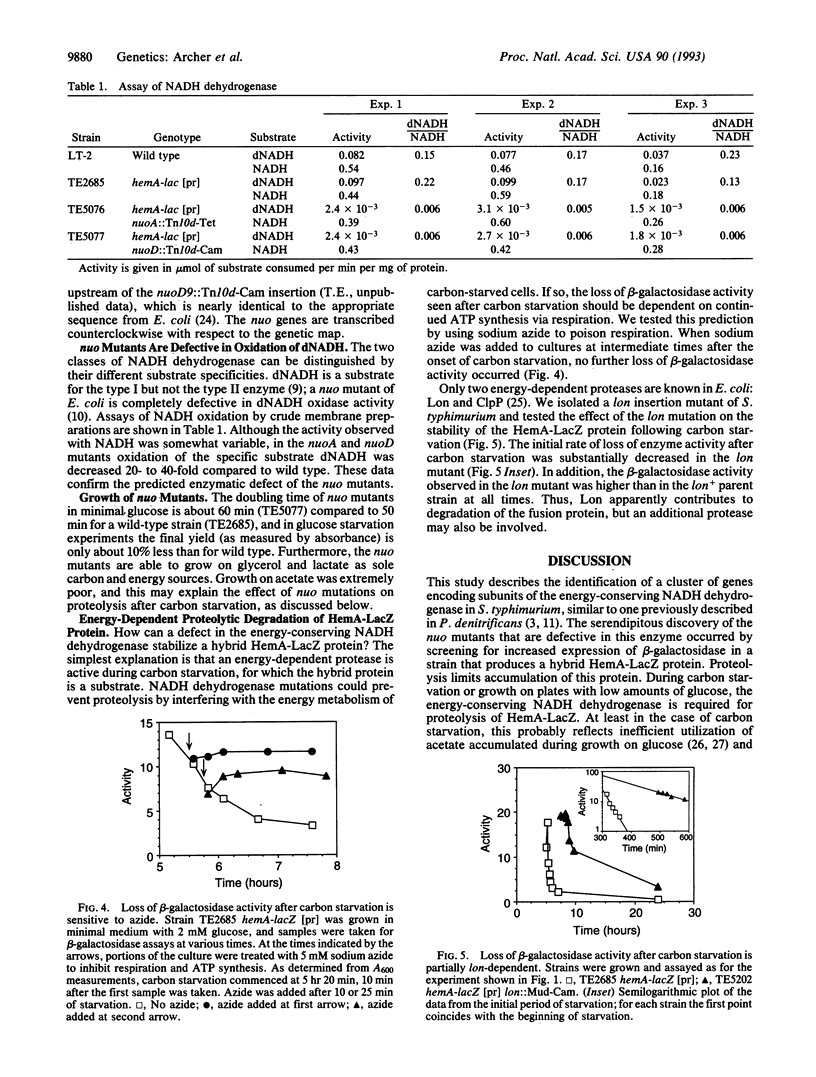
NADH dehydrogenase is the first component of the respiratory chain. It transfers electrons from NADH to ubiquinone and concomitantly establishes a proton motive force across the membrane. Salmonella typhimurium mutants defective in this enzyme were isolated in a screen for strains with increased expression of beta-galactosidase from a hemA-lacZ protein fusion. This unexpected phenotype results from stabilization of the hybrid protein during carbon starvation and is apparently due to an energy requirement for proteolytic attack. Sequence analysis of DNA fragments cloned from an insertion mutant indicates that S. typhimurium has a large cluster of genes encoding the energy-conserving NADH dehydrogenase, similar to one recently described in Paracoccus denitrificans. These findings establish the potential for genetic analysis of a complex enzyme whose function, especially in proton efflux, is poorly understood.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun M. W., Gennis R. B. Demonstration of separate genetic loci encoding distinct membrane-bound respiratory NADH dehydrogenases in Escherichia coli. J Bacteriol. 1993 May;175(10):3013–3019. doi: 10.1128/jb.175.10.3013-3019.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J Bacteriol. 1992 Jan;174(1):245–253. doi: 10.1128/jb.174.1.245-253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. Transport of 5-aminolevulinic acid by the dipeptide permease in Salmonella typhimurium. J Bacteriol. 1993 Jan;175(2):325–331. doi: 10.1128/jb.175.2.325-331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Maurizi M. R. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol Rev. 1992 Dec;56(4):592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Jaworowski A., Campbell H. D., Poulis M. I., Young I. G. Genetic identification and purification of the respiratory NADH dehydrogenase of Escherichia coli. Biochemistry. 1981 Mar 31;20(7):2041–2047. doi: 10.1021/bi00510a047. [DOI] [PubMed] [Google Scholar]
- Jaworowski A., Mayo G., Shaw D. C., Campbell H. D., Young I. G. Characterization of the respiratory NADH dehydrogenase of Escherichia coli and reconstitution of NADH oxidase in ndh mutant membrane vesicles. Biochemistry. 1981 Jun 9;20(12):3621–3628. doi: 10.1021/bi00515a049. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Kaback H. R. D-lactate oxidation and generation of the proton electrochemical gradient in membrane vesicles from Escherichia coli GR19N and in proteoliposomes reconstituted with purified D-lactate dehydrogenase and cytochrome o oxidase. Biochemistry. 1986 May 6;25(9):2321–2327. doi: 10.1021/bi00357a004. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Ohnishi T., Kaback H. R. NADH-ubiquinone oxidoreductases of the Escherichia coli aerobic respiratory chain. Biochemistry. 1987 Dec 1;26(24):7732–7737. doi: 10.1021/bi00398a029. [DOI] [PubMed] [Google Scholar]
- Strathmann M., Hamilton B. A., Mayeda C. A., Simon M. I., Meyerowitz E. M., Palazzolo M. J. Transposon-facilitated DNA sequencing. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1247–1250. doi: 10.1073/pnas.88.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E. The NADH:ubiquinone oxidoreductase (complex I) of respiratory chains. Q Rev Biophys. 1992 Aug;25(3):253–324. doi: 10.1017/s003358350000425x. [DOI] [PubMed] [Google Scholar]
- Weidner U., Nehls U., Schneider R., Fecke W., Leif H., Schmiede A., Friedrich T., Zensen R., Schulte U., Ohnishi T. Molecular genetic studies of complex I in Neurospora crassa, Aspergillus niger and Escherichia coli. Biochim Biophys Acta. 1992 Jul 17;1101(2):177–180. [PubMed] [Google Scholar]
- Weiss H., Friedrich T., Hofhaus G., Preis D. The respiratory-chain NADH dehydrogenase (complex I) of mitochondria. Eur J Biochem. 1991 May 8;197(3):563–576. doi: 10.1111/j.1432-1033.1991.tb15945.x. [DOI] [PubMed] [Google Scholar]
- Yagi T. The bacterial energy-transducing NADH-quinone oxidoreductases. Biochim Biophys Acta. 1993 Feb 8;1141(1):1–17. doi: 10.1016/0005-2728(93)90182-f. [DOI] [PubMed] [Google Scholar]
- Yagi T., Xu X., Matsuno-Yagi A. The energy-transducing NADH-quinone oxidoreductase (NDH-1) of Paracoccus denitrificans. Biochim Biophys Acta. 1992 Jul 17;1101(2):181–183. [PubMed] [Google Scholar]
- Young I. G., Rogers B. L., Campbell H. D., Jaworowski A., Shaw D. C. Nucleotide sequence coding for the respiratory NADH dehydrogenase of Escherichia coli. UUG initiation codon. Eur J Biochem. 1981 May;116(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Young I. G., Wallace B. J. Mutations affecting the reduced nicotinamide adenine dinucleotide dehydrogenase complex of Escherichia coli. Biochim Biophys Acta. 1976 Dec 6;449(3):376–385. doi: 10.1016/0005-2728(76)90149-3. [DOI] [PubMed] [Google Scholar]
- Zuber U., Schumann W. The eighth copy of IS1 in Escherichia coli W3110 maps at 49.6 minutes. J Bacteriol. 1993 Mar;175(5):1552–1552. doi: 10.1128/jb.175.5.1552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



