Abstract
Nogo-B receptor (NgBR) was identified as a receptor specific for Nogo-B. Our previous work has shown that Nogo-B and its receptor (NgBR) are essential for chemotaxis and morphogenesis of endothelial cells in vitro and intersomitic vessel formation via Akt pathway in zebrafish. Here, we further demonstrated the roles of NgBR in regulating vasculature development in mouse embryo and primitive blood vessel formation in embryoid body culture systems, respectively. Our results showed that NgBR homozygous knockout mice are embryonically lethal at E7.5 or earlier, and Tie2Cre-mediated endothelial cell-specific NgBR knockout (NgBR ecKO) mice die at E11.5 and have severe blood vessel assembly defects in embryo. In addition, mutant embryos exhibit dilation of cerebral blood vessel, resulting in thin-walled endothelial caverns. The similar vascular defects also were detected in Cdh5(PAC)-CreERT2 NgBR inducible ecKO mice. Murine NgBR gene-targeting embryonic stem cells (ESC) were generated by homologous recombination approaches. Homozygous knockout of NgBR in ESC results in cell apoptosis. Heterozygous knockout of NgBR does not affect ESC cell survival, but reduces the formation and branching of primitive blood vessels in embryoid body culture systems. Mechanistically, NgBR knockdown not only decreases both Nogo-B and VEGF-stimulated endothelial cell migration by abolishing Akt phosphorylation, but also decreases the expression of CCM1 and CCM2 proteins. Furthermore, we performed immunofluorescence (IF) staining of NgBR in human cerebral cavernous malformation patient tissue sections. The quantitative analysis results showed that NgBR expression levels in CD31 positive endothelial cells is significantly decreased in patient tissue sections. These results suggest that NgBR may be one of important genes coordinating the cerebral vasculature development.
Introduction
The blood vessel (BV) formation and maturation is critical during embryogenesis (Jain, 2003). It involves the coordinated development of two cell types – endothelial cells (EC) and vascular smooth muscle cells (SMC) (Benjamin et al., 1999; Bergers and Song, 2005; Evensen et al., 2009). Blood vessels plexus is the first functioning system in a developing embryo, lending support to the developing organ systems by transporting nutrients, growth factors and gases (Herbert and Stainier, 2011). The BV development involves formation of an initial vascular plexus from EC, a process called vasculogenesis, followed by sprouting of new vessels from the existing ones, a process called angiogenesis (Geudens and Gerhardt, 2011; Herbert and Stainier, 2011; Kliche and Waltenberger, 2001; Patan, 2000; Risau, 1997). Migration and proliferation of EC play important roles during BV sprouting (Lamalice et al., 2007). The developing BVs are guided by local cues for migration, and prominent one among these factors is vascular endothelial growth factors (VEGF) (Coultas et al., 2005; Gerhardt, 2008; Kowanetz and Ferrara, 2006; Li et al., 2008). VEGF act as a chemoattractant, by interacting with VEGF receptors (VEGFR) on the tip of EC and initiates a signal cascade via activation of Akt and Erk kinases (Cleaver and Krieg, 1998; Evensen et al., 2009; Lin et al., 2007). The integrity of developing vascular structure is maintained by EC junctions (Dejana and Orsenigo, 2013), which are the points where EC contact with each other. There are two types of junctions, tight junctions and adherens junctions (Dejana and Orsenigo, 2013). Decreased junction stability may lead to hemorrhage and vascular lesions (Dejana and Orsenigo, 2013; Niessen et al., 2011; Vestweber et al., 2010; Vestweber et al., 2009). Abnormal vascularization in the first half of developmental period may results in lethality or ischemic diseases (Krebs et al., 2010). Cerebral cavernous malformations (CCMs) are abnormal clusters of thinner and dilated blood vessels called caverns (Draheim et al., 2014; Fischer et al., 2013; Zheng et al., 2012) that are filled with blood (He et al., 2010; Kleaveland et al., 2009; Whitehead et al., 2004). CCM affects up to 0.5% of the population (Al-Shahi Salman et al., 2012; Haasdijk et al., 2012), leading to seizures and stroke with symptoms, including hemorrhages and headache. Loss-of-function of three genes CCM1, CCM2 and CCM3 are associated with onset of this disease (Bergametti et al., 2005; Boulday et al., 2011; Dubovsky et al., 1995; Liquori et al., 2003). Several signaling pathways such as Erk, Akt, Rap1 and RhoA GTPases have been implicated in the pathogenesis of CCM (Bazzoni and Dejana, 2004; Dibble et al., 2010; Ma et al., 2007; Schleider et al., 2011; Wilhelm et al., 2006), but the exact link between these CCM proteins and their expression regulation still remains to be established. Here, we identify NgBR as a previously unidentified player in regulating CCM1 and CCM2 expression in endothelial cells as well as cerebral vasculature development.
Previous results show that Nogo-B is the major isoform of Nogo in blood vessels, and is highly expressed in both endothelial cells (EC) and smooth muscle cells (SMC) (Acevedo et al., 2004). Amino terminus of Nogo-B (AmNogo-B) promotes the migration of both EC and SMC (Acevedo et al., 2004). Mice deficient in Nogo-A/B show exaggerated neointimal proliferation, abnormal remodeling and a deficit in ischemia induced arteriogenesis and angiogenesis (Yu et al., 2009). Nogo-B receptor (NgBR) was identified as a receptor specific for AmNogo-B by an expression cloning approach. High affinity binding of AmNogo-B to NgBR is essential for AmNogo-B mediated chemotaxis and tube formation of EC (Miao et al., 2006). We have shown that NgBR is also essential for VEGF-induced chemotaxis and morphogenesis of EC cells in vitro, and BV formation in zebrafish via Akt pathway (Miao et al., 2006; Zhao et al., 2010). Here, we demonstrate that NgBR deficiency in EC decreases the expression of both CCM1 and CCM2 genes, and results in cerebral vascular defects in NgBR endothelial cell specific knockout (ecKO) mice. Our study suggests that NgBR plays an important role in regulating EC dynamics during cerebral blood vessel development.
Materials and Methods
Materials and reagents
Anti-NgBR rabbit polyclonal antibody was generated as described in our previous publication (Zhao et al., 2010). Anti-total ERK, Akt, phos-Akt, and phos-Erk antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-Hsp90 antibody was purchased from BD Biosciences (San Jose, CA). Anti-CCM1, CCM2 and CCM3 antibodies were purchased from Acris (San Diego, CA). Non-silencing (NS) control siRNA and siRNAs targeting NgBR were purchased from QIAGEN (Frederick, MD). Tek-Cre (Tie2-Cre) (Stock number 008863), ROSA26Sor-lacZ (stock number: 003474), and ROSA26Sor-EYFP (stock number: 006148) mice were purchased from Jackson laboratory (Bar harbor, Maine), and β-actin Cre mouse (Lewandoski et al., 1997) was a generous gift from Dr. Katherine Shim (Children’s Research Institute, MCW). Cdh5(PAC)-CreERT2 mice were transferred from Dr. Ralf Adams (Cancer Research UK).
Mouse experiments
The mice were housed in the Medical College of Wisconsin Biological Resource Center. The Institutional Animal Care and Use Committee at the Medical College of Wisconsin approved the procedures performed here. For generating NgBR global knockout, we used β-actin Cre line (Lewandoski et al., 1997). For timed pregnancies, female mouse (NgBR flox/flox, NgBRfl/fl) was caged with male (Cre positive NgBR flox/WT, NgBRfl/WT:Cre) in the evening; following morning pregnancy was established by the presence of vaginal plug (embryonic stage 0.5). Embryos were collected at E6.5, 7.5, 8.5 for β-actin Cre experiments. For the endothelial-specific loss of NgBR, embryos were collected from NgBR flox/flox females mated to Tie2Cre positive (Jackson laboratory, USA) NgBR flox/WT males. Embryos were collected at E8.5, E9.0, E9.5, E10.5, E11.5 and E13.5. For temporal loss of NgBR in endothelial cells specifically, we used Cdh5(PAC)-CreERT2-positive NgBR flox/WT males to mate with NgBR flox/flox females. NgBR knockout was activated by injecting pregnant females with 2mg of tamoxifen (T5648, Sigma Aldrich, St. Louis, MO) at E8.5 and harvested embryos at E10.5. To confirm Cre efficiency, we used NgBR flox/flox:LacZ/LacZ (NgBRfl/fl:LacZ/LacZ) females to mate with heterozygous males (NgBRfl/fl:Cre). Littermate embryos were used as controls for each animal experiment. Mixed backcrossed animals were used β-actin Cre and Tie2Cre experiments, and C57BL/6 backcrossed animals were used for Cdh5(PAC)- CreERT2 experiments.
Whole mount immunofluorescence staining
Embryos were harvested at E10.0 dpc and fixed in 4% (wt/vol) paraformaldehyde overnight. The following day, embryos were washed with PBST (0.1% tween-20 in PBS) and dehydrated in ascending series of methanol in PBST for 10 minutes each. The embryos were bleached with Dent’s bleach (64% Methanol and 16% DMSO) for 3 hours and were rehydrated via graded methanol series. Embryos were blocked overnight with 5% (wt/vol) normal serum in PBST (PBSST) at 4 °C. Following day, embryos were incubated with PECAM-1 antibody (BD Biosciences, San Jose, CA) in PBSST for 2 days. Embryos were washed and incubated with secondary antibody overnight at 4 °C, followed by overnight PBST washes. Embryos were fixed with 4% (wt/vol) paraformaldehyde for 2 hours before imaging. Confocal images were taken with Carl Zeiss LSM 510 microscope using AIM4.2 software and processed using automeasure plus module of AxioVision 4.8 ver (Zeiss).
3D embryoid body culture
NgBR floxed E14Tg2a 129/Ola embryonic stem cells (ESCs) were generated by homologous recombination of a targeting vector in which LoxP sites flank NgBR exons 2 and 4. Correct gene targeting was confirmed by Southern blot analysis of genomic DNA isolated from established ES cells. E14Tg2a 129/Ola ESCs are feeder-independent, and cultured in GMEM medium (Sigma Aldrich, St. Louis, MO) supplemented with 2mM glutamine (Invitrogen, Carlsbad, CA), 1 mM sodium pyruvate (Invitrogen), 1x nonessential amino acids, 10% (v/v) fetal bovine serum (characterized, Hyclone, Canada), beta-mercaptoethanol (Sigma Aldrich), and 1000 units per ml of leukocyte inhibitor factor (LIF) (Chemicon, Temecula, CA). Heterozygous knockout of NgBR in ESCs was generated by transfecting Cre-GFP plasmid DNA and sorted based on positive GFP expression. To generate embryoid bodies, 300 ES cells in 10 μl of ES cell growth medium without LIF were added to the lid of Petri dish and cultured in hanging drops for 2 days. Subsequently, the aggregates were cultured in suspension in Petri-dish containing ES medium without LIF for 7 days and then seed in 1mg/ml Collagen type I gel (about 2 mm thick) in 24 well plate without LIF and in the presence of VEGF (50 ng/ml) for another 11 days. The differentiated EB were fixed using 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100 in TBS (TBS-T). After blocking in 5% horse serum plus 1% BSA, the fixed EB was incubated with primary antibodies against murine PECAM-1 (BD Biosciences, San Jose, CA) at 4°C overnight and developed with corresponding Alexa-Fluor 594 fluorescence-conjugated second antibody (Molecular Probes, Eugene, OR). Cell nuclei were counterstained with 20 g/mL Hoechst 33342 (Sigma Aldrich). Extent of blood vessel sprouting was analyzed by a confocal laser-scanning microscope (Zeiss LSM 510 META) and analyzed by axiovison software aided 3D rendering.
LacZ staining
As a control for LacZ stained KO embryos, Tie2Cre transgenic mice were bred with ROSA26Sor-LacZ female. β–galactosidase (LacZ) staining was performed according to previous reports (Loughna and Henderson, 2007). Briefly, at E10.5, embryos were harvested and fixed with 4% (wt/vol) paraformaldehyde and 0.2% (vol/vol) glutaraldehyde for 15 mins at 4°C. After washing for 5 minutes, embryos were stained with 1% (wt/vol) X-gal (Invitrogen) solution for 6 hours at 37°C. To generate endothelial-restricted, LacZ positive NgBR KO animals, Tie2Cre-NgBRfl/wt males were bred with LacZ/LacZ:NgBRfl/fl females. At E10.5, embryos were harvested and processed as described above. Olympus stereomicroscope with DP-72 camera and cellsens 1.8 software was used for capturing and processing images, respectively. After staining, embryos were fixed with 4% (wt/vol) paraformaldehyde overnight and were processed for paraffin sectioning.
Human tissue samples
Human infantile hemangioma and human cerebral cavernous malformation tissue specimens from Pediatric BioBank at the Children’s Hospital of Wisconsin Research Institute are anonymous. Research on human patient samples were performed according to the Medical College of Wisconsin-approved institutional review Board protocols, and informed consent was obtained in accordance with the declaration of Helsinki.
Data analysis
Data are presented as mean ± the standard error of the mean (SEM). The statistical significance of differences was evaluated with the ANOVA analysis. Significance was defined as P < 0.05.
Results
NgBR expression in adult mouse tissue
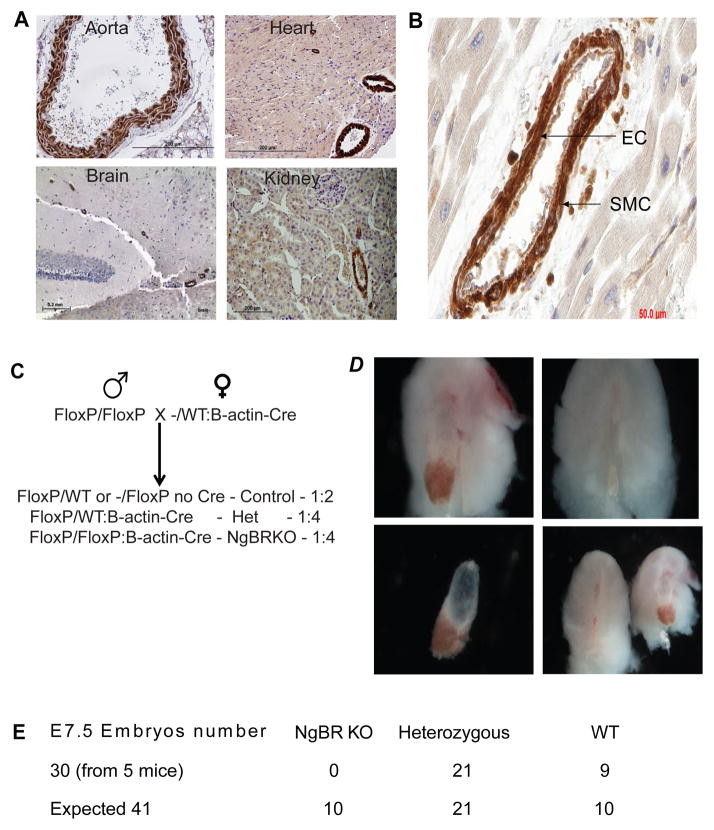
Previous studies have shown that Nogo-B, ligand to NgBR, is the only Nogo isoform that was expressed in the blood vessels (Acevedo et al., 2004; Miao et al., 2006). Hence, as an overture to the phenotypic studies of NgBR, we set forth to understand the expression of NgBR in murine tissues, with respect to Nogo-B. Immunohistochemical evaluation of adult mouse tissues (4 week old) shows that NgBR is specifically expressed in both smooth muscle cells (SMC) and endothelial cells (EC) of aorta (Fig 1A). NgBR expression is negative in embryonic and adult hematopoietic cells except for megakaryocyte (data not shown). NgBR expression in brain is restricted to the blood vessels whereas in kidney, NgBR is expressed in both tubules and BV. Glomeruli in kidney are negative for NgBR expression. Specificity of NgBR expression in BV is consistent in heart as staining is positive in BV whereas cardiomyocytes are negative. NgBR Immunohistochemistry staining on human tissue section of a benign vascular neoplasm, infantile hemangioma that is abundant in vasculature showed that NgBR is specifically expressed in both SMC and EC (Fig. 1B). Localization of NgBR expression is highly consistent with its ligand, Nogo-B, (Fig. S1). The immunohistochemistry results confirmed that NgBR is highly expressed in both SMCs and ECs of blood vessels.
Figure 1.
NgBR expression in adult mouse tissues. A. Immunohistochemistry of NgBR on paraffin embedded adult mouse tissue. NgBR is specifically expressed in blood vessels in aorta, heart, brain and kidney. Scale bar=200μm. B. NgBR expression in blood vessel. NgBR expression in smooth muscle cells is stronger than endothelial cells. Scale bar = 50μm. C. NgBR was knocked out globally in the developing embryo by β-actin Cre. Homozygous floxed female was bred with heteroygous male (flox/WT:Cre/WT). D. At E7.5, no NgBR KO embryo was harvested because the decidua is empty in NgBR KO embryos (b,d). E. The statistical analysis of embryo lethality exhibits that no live KO embryos were available beyond E7.5.
NgBR global knockout results in early embryonic lethality
To determine the role of NgBR in mammalian embryogenesis, we bred NgBRfl/fl mice with β-actin Cre mice that ubiquitously expresses Cre gene (Mahoney Rogers et al., 2011) (Fig. 1C). After breeding heterozygous Cre male (NgBRfl/WT:β-actin Cre) with homozygous females (NgBRfl/fl), the embryos were harvested at E7.5. Genotyping results revealed that heterozygous (NgBRfl/WT:β-actin Cre) and control (NgBRfl/fl: no β-actin Cre) embryos were viable whereas knockout embryos (NgBRfl/fl:β-actin-Cre) did not survive, as indicated by empty decidua (Fig. 1D). The disparity from Mendelian ratios of 1:2:1 (25%:50%:25%) at E7.5 further supports our conclusion that global loss of NgBR results in the embryonic lethality (Fig. 1E). The results indicate that NgBR is vital for early embryonic development (gastrulation) and could be a key molecule in later stages of embryonic development.
NgBR knockout in endothelial cells induces early embryo lethality
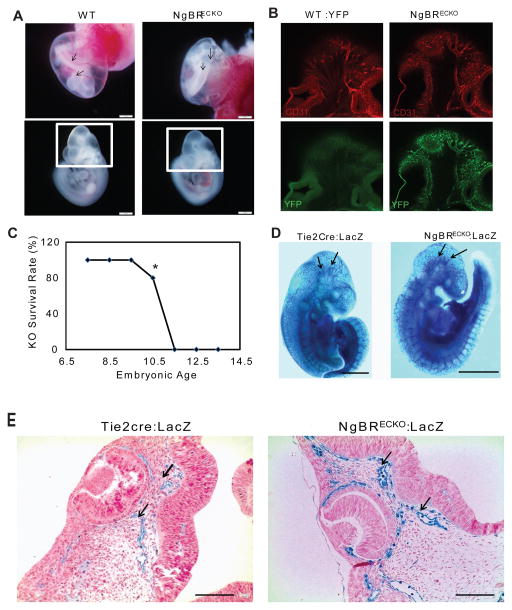
Because of robust expression of NgBR in the vasculature (Fig. 1), and embryonic lethality associated with NgBR global knockout mice, we investigated the effect of NgBR loss selectively in the ECs of the vasculature during embryonic development. We bred NgBRfl/fl mice with Tie2Cre mice. Tie2Cre mice are endothelial and hematopoietic cells-specific Cre mice, where the tie2 promoter drives Cre expression, which begins around E7.5 (Kisanuki et al., 2001). The specificity of Cre activity was confirmed by using YFP reporter mice (ROSA26Sor-EYFP), where NgBRfl/fl:YFP/YFP female mice was bred with heterozygous Tie2Cre male mice. Positive YFP signal specifically in BV confirms that Cre is highly expressed in endothelial cells (Fig. 2B, showing brain region as indicated in Fig. 2A box), which supports the conclusion of endothelial specific loss of NgBR because NgBR is not expressed in embryonic blood cells. The NgBR Tie2Cre endothelial cell specific knockout mice (ecKO) exhibit embryonic lethality at E10.5-11.5, whereas heterozygous NgBR ecKO embryos are viable (Fig. 2A, 2C). Although no change in somite count was observed among mutant and control littermate embryos (Fig. S3), homozygous ecKO embryos are smaller in size as compared to the littermate control (Fig. 2A).
Figure 2.
NgBR ecKO in developing embryo exhibits lethality at E11.5. A. NgBR ecKO embryo at E10.0. As pointed by arrows, Tie2Cre-NgBRfl/fl (NgBRECKO) embryo yolk sac (c) showed minimal BV development as compared to NgBRfl/fl (WT) (a). The box around the cerebral region highlights the region shown in panel B. B. Confocal images of whole mount PECAM-1 staining shows blood vessel patterning in control and mutant E10.5 embryos. Positive YFP staining in mutant embryo (NgBRECKO:YFP) shows that the Cre is active and specific in blood vessels. Control embryos (WT:YFP) show negative YFP signal. C. Statistical analysis of embryo lethality. NgBR ecKO embryo start to die around E10.5 and no alive KO embryo was recovered post E11.5. Scale bar = 1mm. D. LacZ staining confirms Cre activity in E10.5 embryo. Tie2Cre-LacZ (Control) embryo shows well-developed cerebral BV; inversely NgBRECKO: LacZ embryo exhibits truncated and dilated BV in the brain. Arrow points at the developing BV in WT brain and aberrant BV in KO brain. E. Paraffin sections (4 μm) of LacZ stained embryos at E10.5. Blue LacZ staining indicates that Cre activity is specific for endothelium. Arrows mark dilated vessels in KO. Scale bar= 100μm.
We monitored the Cre specificity and BV patterning in ecKO by generating NgBR conditional gene targeting mice with Rosa26-LacZ reporter gene (flox/flox:LacZ/LacZ). When bred with Tie2cre mice, β-galactosidase staining of the embryos, at E10.5, confirmed Cre activity specifically in BV. As a negative control for comparing the effect of EC-specific NgBR KO on BV assembly, we also bred homozygous LacZ female mice with Tie2Cre male. At E10.5, the embryos harvested from this breeding were stained for β-galactosidase as described in the Methods. In Figure 2D, LacZ staining results show that a well-patterned (tree-like) major cerebral arteries branching and spreading evenly in the developing brain (black arrows) were presented in control embryos at E10.5. However, LacZ stained BV in E10.5 mutant embryos show an aberrant patterning. Instead of tree-like branching pattern of BV as shown in control mice, cerebral BV of NgBR ecKO mice are irregular and truncated. To further characterize these vessel defects, we closely examined transverse paraffin sections of LacZ stained embryos that were counterstained with nuclear fast red staining. Figure 2E shows cerebral blood vessels near eye region in NgBR ecKO embryo are dilated and exhibit disrupted organization. These results indicate that NgBR expression in EC is essential for cerebral blood vessel assembly and embryonic viability during development.
NgBR endothelial cell specific KO causes defective cerebral BV patterning and dilation
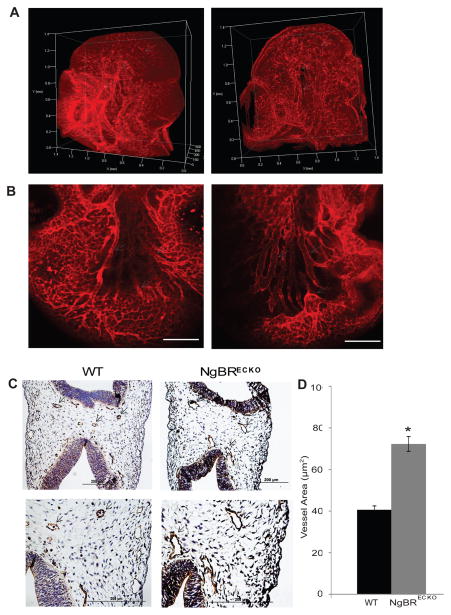
To investigate the majority of the embryonic vasculature, we performed whole-mount immunofluorescent staining using PECAM-1 antibody, an EC marker. After staining, embryos were cleared with BBB solution (benzyl benzoate/benzyl alcohol) and were imaged using confocal microscopy. Reconstructed 3D images show that unlike a well-organized tree-like patterning presented in littermate control, cerebral blood vessel assembly of NgBR ecKO mice is consistently truncated and exhibit disrupted organization (Fig 3A). As shown in littermate WT control, a communicating artery connects the two major middle cerebral arteries (pointed by arrow) (Fig. 3A left panel, movie 1). In NgBR ecKO heterozygous embryo (movie 2), the communicating artery is lost but surprisingly little patterning defect of the middle cerebral arteries was observed. In NgBR ecKO homozygous embryos, the communicating artery was also lost but in addition, disruption of major artery patterning was observed (Fig. 3A right panel, movie 3). At higher magnification, cerebral blood vessels in mutant embryos are highly dilated (pointed by arrows) as compared to their littermate controls (Fig. 3B). We also performed PECAM-1 immunohistochemical (IHC) staining on serial transverse paraffin sections of mutant and control littermate embryos (E10.5). PECAM-1 IHC staining results revealed that BV in NgBR ecKO mice is dilated whereas BV in control WT mice is smaller in diameter (Fig. 3C). Image J was used for quantification of BV area. The results (Fig. 3D) further confirmed that the blood vessels in mutant embryos are dilated as compared to control embryos. In order to characterize the mutant vessels closely, we performed PECAM-1 immunofluorescence staining on E8.5 and E9.5 embryos (Fig. S4). Results show that no BV differences in mutant and control embryos were appreciated at E8.5, whereas by E9.5 we start observing the dilation of mutant vessels (Fig. S4). Further, as compared to control, mutant embryos are smaller in size, but have the identical somite numbers. Cardiac development of mutant and control embryos was also examined at different stages of development (E9.0, E9.5, E10.5) and no obvious defect was observed (Fig. S5). In addition, no obvious bronchial arch artery defects were observed in mutant embryos (Fig. S5). During these stages, mice with loss of NgBR expression in EC have completed cardiac looping, and show appropriate myocardial trabeculation. In addition, we assessed BV proliferation by double immunofluorescent (IF) staining of Isolectin B4, a glycoprotein that stains EC, and phosphorylated histone 3 (phos-H3). As shown in Figure S6, there is no difference in EC proliferation in mutant and control embryos.
Figure 3.
Whole mount PECAM-1 IF staining shows aberrant BV assembly in the brain of NgBR ecKO embryo. A. Whole embryo (E10.0) was stained with PECAM-1 antibody to observe BV assembly in the embryo. NgBRECKO brain exhibited a dilated and defective-patterned cerebral arteries (pointed by white arrows) and sprouting BVs. B. Brain vasculature of WT and NgBRECKO at a higher magnification showing dilation of vessels in NgBRECKO. Scale bar = 100μm. C. Immunohistochemical staining of PECAM-1 on paraffin sections of E10.5 embryo shows dilated and poorly developed BV in the brain of NgBR ecKO (b) as compared to control (a). (c) and (d) shows aberrant BV in higher magnification. D. Quantification of blood vessel area of mutant and control embryos at E10.5 using image J software. * P< 0.05 (n=15). Increased vessel area in mutant embryo confirms vasodilation.
NgBR endothelial cell specific KO exhibits yolk sac vasculature defect later than E9.0
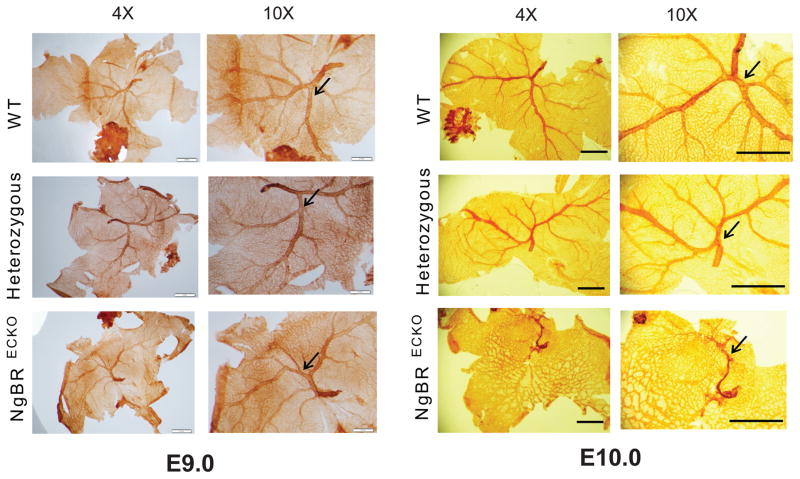
Yolk sac is a major embryonic vascularization site in the developing embryo. In fact, the first BVs observed in yolk sac are important for providing nutrients to the growing embryo (Garcia and Larina, 2014). To exclude the possibility that BV dilations in the brain are not secondary to defective yolk sac development, we performed whole mount PECAM-1 staining on yolk sac from E9.0 and E10.0 embryos. E9.0 control, heterozygous and homozygous NgBR ecKO yolk sac does not exhibit any disruption in BV assembly (Fig. 4A). On the contrary, at E10.0, mutant yolk sac displays aberrant BV development as opposed to control and heterozygous, which still maintains a well-organized BV structure (Fig. 4B). These results consolidate the fact that the cerebral BV assembly defect in mutant embryo is a direct consequence of NgBR deficiency in EC
Figure 4.
NgBR ecKO exhibits vasculature defect in yolk sac later than E9.0. A. Whole mount PECAM-1 staining of yolk sac from E 9.0 embryo shows no defect in NgBRECKO BV development. B. Whole mount PECAM-1 staining of yolk sac from E10.0 embryo. Control and heterozygous exhibits well-define BV development, but NgBRECKO exhibits aberrant BV formation.
BV defects in NgBR inducible endothelial cell specific knockout mice
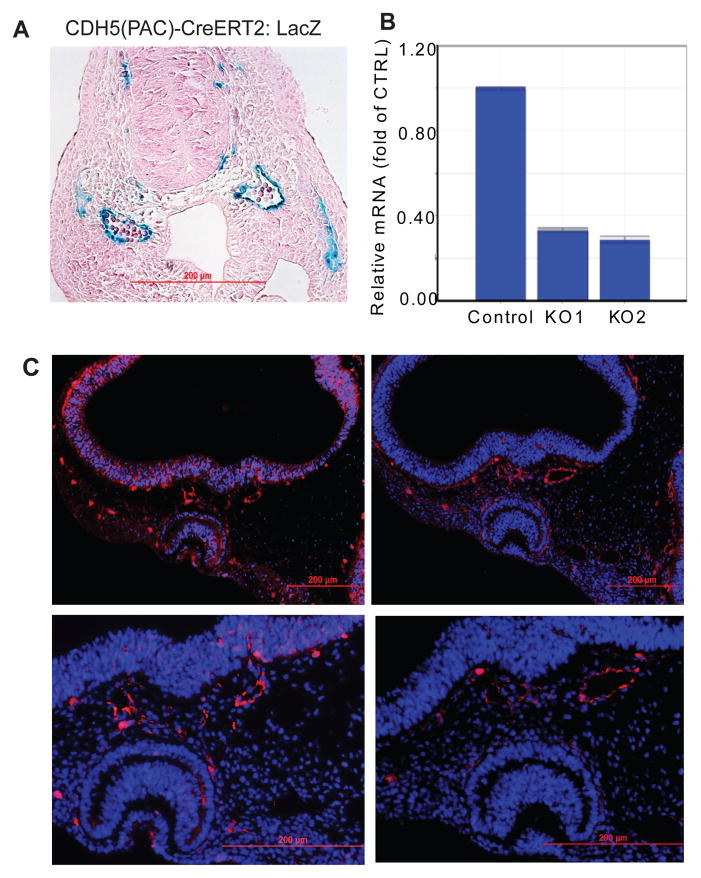
Since EC and hematopoietic cells have common origin, one of the concerns for using Tie2Cre model is the fact that Tie2 is also expressed in hematopoietic cells (Kisanuki et al., 2001). Although NgBR is not expressed in embryonic blood cells, we decided to complement our Tie2Cre with Cdh5(PAC)-CreERT2 line, where Cre is under the control of the VE-cadherin (cdh5) promoter (Wang et al., 2010). The CreERT2 also allows for temporal regulation of the Cre enzyme thereby achieving both temporal and spatial depletion of NgBR. We bred NgBRfl/fl mice with Cdh5(PAC)-CreERT2mice from Dr. Ralf Adams, Cancer Research UK (Wang et al., 2010). The Cre was activated in embryos via intraperitoneal injection of tamoxifen (2mg) to pregnant mothers at different gestational stages. Figure 5A shows that when pregnant mother (LacZ/LacZ female mated with Cdh5(PAC)-CreERT2 male) is injected with tamoxifen at E8.5, the Cre is specifically activated in the endothelial cells of BV, as shown by positive LacZ staining. Lack of positive LacZ staining in blood cells confirmed that Cdh5(PAC)- CreERT2 is not expressed in hematopoietic cells.
Figure 5.
Inducible Cdh5-Cre confirms BV defect due to loss of NgBR in EC. A. LacZ stained E9.5 embryo paraffin transverse section. Tamoxifen injection was given to pregnant mother at E 8.0. Positive blue staining around blood vessel confirms that the Cre is specific for EC. Blood cells are negative for blue LacZ staining. B. Real-time PCR array analysis of E9.5 yolk sac after tamoxifen injection at E8.0. The transcripts of NgBR decreases in NgBR inducible ecKO yolk sac. Data are presented as change fold as normalized to housekeeping gene GAPDH. The result confirms that the tamoxifen injection is efficient in depleting NgBR in mutant embryos (NgBRiECKO). C. PECAM-1 immunofluorescence staining of E10.5 embryo after tamoxifen injection at E8.5. The PECAM-1 staining shows that unlike control, cerebral blood vessels in mutant embryos are dilated and defective-patterned. Scale bar = 100μm.
NgBRfl/fl females were bred with NgBRfl/fl:Cdh5(PAC)-CreERT2 males. The pregnant females were injected with tamoxifen once at E8.5 and embryos were harvested at E10.5. NgBR gene knockout was confirmed by real-time PCR (Fig. 5B). Unlike littermate control and heterozygous embryos, mutant embryos are smaller size and exhibit aberrant cerebral BV patterning. Results of PECAM-1 IF staining on serial transverse sections (Fig. 5C) show that mutant embryos have dilated and disrupted organization of BV as opposed to a well-organized BV in control littermate, which are consistent with defects observed in Tie2Cre model (Fig. 3C). In addition, we have included 3D image of transverse section of cerebral BV (Fig. S7A). We also used Cdh5-Cre model, an endothelial cell specific Cre, to generate NgBR mutant embryos at E12.5. The 3D image of whole mount PECAM-1 IF staining (Fig. S7C) shows dilated cerebral BV in the brain of NgBR mutant embryos. Results from two different endothelial cell specific Cre models demonstrate that NgBR deficiency in EC results in dilated and poorly organized BV assembly in the developing brain, which are reminiscent of dilated blood vessels presented in cerebral cavernous malformation (CCM) (Whitehead et al., 2004).
NgBR deficiency impairs the primitive blood vessel formation in embryoid bodies
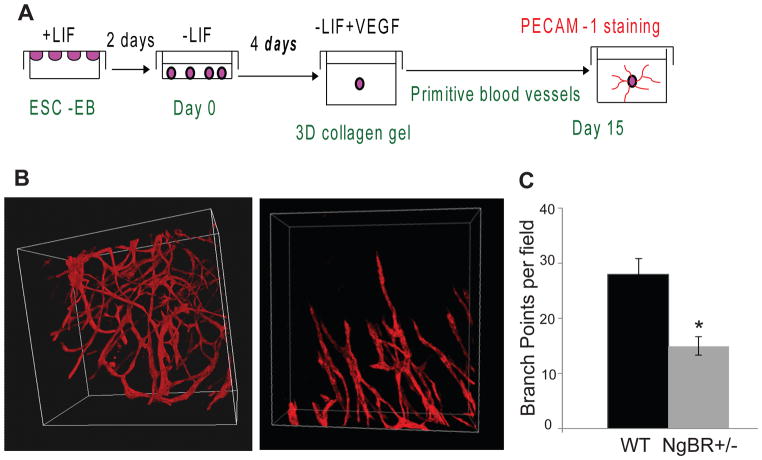
Interestingly, similar vascular defects have been recapitulated in vitro 3D embryonic culture system. Embryoid bodies were generated by culturing embryonic stem cells (ESCs) in hanging drops for 2 days, subsequently, the aggregates were cultured in suspension in ES medium without leukemia inhibitory factor (LIF) for 4 days and then seed in 1mg/ml Collagen type I gel (about 2 mm thick) without LIF and in the presence of VEGF (50ng/mL) for another 5–8 days as shown in Figure 6A. PECAM-1 whole mount staining was used to determine blood vessel formation. Homozygous NgBR knockout embryonic stem cells (ESC) do not survive because NgBR plays a vital role in early embryonic development. The genotyping results from ESC culture shows that homozygous knockout ESC cells were sorted and cultured but by day 3 these cells were floating in the media (Fig. S8). EB derived from heterozygous NgBR knockout ESC exhibit defective BV development, supporting our in vivo observation that NgBR could regulate EC dynamics and is required for BV formation. Figure 6B shows that unlike wild-type control (Fig. 6B, left panel, movie 4), heterozygous NgBR knockout EB (Fig. 6B, right panel, movie 5) exhibits dilated and truncated EC tubes, which have much less branching and fail to develop into a tree-like structure. The quantitative results of branching points from both wild-type control and heterozygous NgBR knockout EB are shown in Figure 6C. This result highlights that NgBR has a critical role in regulating blood vessel formation during embryonic development.
Figure 6.
Effects of NgBR deficiency on the primitive blood vessel formation in embryoid body. A. Experimental diagram of embryoid body (EB) formation. ESCs were grown in stem cell media containing LIF, which prevents the differentiation of ESCs. EB were formed by hanging drop culture. After 4-day suspension culture, EB was implanted in collagen gel and was allowed to develop blood vessel structure in 15 days by removing LIF and adding VEGF (50 ng/ml). Established blood vessels were determined by PECAM-1 IF staining. B. Immunofluorescence PECAM-1 staining of NgBR WT and heterozygous EB blood vessel structure. WT exhibit tree-like well-developed BV network whereas heterozygous exhibits truncated and stout BV structure. D. Quantification of branching points of wild-type and heterozygous knockout blood vessels. * P< 0.05 (n=3). Decreased branching point in heterozygous knockout highlights poorly formed BV structure.
NgBR deficiency inhibits migration of HBMVECs in vitro
BV development relies heavily on ability of EC to respond to local cues (chemoattractant such as VEGF) and perform functions such as migration and proliferation. To understand how NgBR deficiency can affect brain EC functions i.e. migration and activation in response to chemoattractant such as VEGF and AmNogo-B, an NgBR specific ligand (Miao et al., 2006), we chose human brain microvascular endothelial cells (HBMVEC). In order to determine the effects of NgBR deficiency on HBMVECs migration, we conducted modified Boyden chamber migration experiments. HBMVECs cells were transfected with non-silencing control siRNA (NS) and validated siRNA targeting NgBR (siNgBR) (Miao et al., 2006). 1X105 number of cells of NS and siNgBR HBMVECs were added in each transwell coated with 1% collagen. The transwells were placed in the chambers containing basal media (control), or basal media containing either VEGF (100ng/mL) or 100nM AmNogo-B. As shown in Figure S9A, both VEGF and AmNogo-B induced the migration of HBMVECs. NgBR deficiency reduced either VEGF or AmNogo-B-induced migration of HBMVECs as compared to respective controls. The result implicates that NgBR deficiency prevents EC migrating towards local cues and results in defective tube like structure and/or BV patterning during blood vessel development.
Given the observed migration defects in NgBR deficient ECs, we further determined the effects of NgBR deficiency on the EC activation by detecting the phosphorylation of Akt and ERK, which are major signaling pathways attributed to the migration of ECs (Shiojima and Walsh, 2002). Figure S9B and S9C shows that both VEGF and AmNogo-B stimulate the phosphorylation of Akt and Erk in quiescent HBMVECs. NgBR deficiency diminishes the VEGF and AmNogo-B-stimulated phosphorylation of Akt and Erk as compared to the respective controls (Fig. S9B and S9C). These results suggest that NgBR is an essential player in EC response to VEGF and AmNogo-B.
Discussion
NgBR was discovered as a receptor for Nogo-B (Miao et al., 2006). Earlier studies have revealed that Nogo-B is the only isoform of Nogo-family that is expressed in blood vessels, and regulated EC and SMC adhesion and migration (Acevedo et al., 2004; Miao et al., 2006). Several lines of evidence suggest that NgBR is required for chemotactic actions of Nogo-B and VEGF, and is required for migration of EC (Miao et al., 2006). Zebrafish studies have shown that NgBR is required for intersomitic vessel (ISV) formation (Zhao et al., 2010), which further emphasizes the vital role of NgBR in vascular development. However, the roles of NgBR in mammalian development are currently unknown. In this study, we demonstrate the essential role of NgBR in mouse vasculature development.
The earliest defects in mice lacking NgBR results in lethality during gastrulation, implicating that NgBR could be a key regulator of pathways that are building blocks of life such as stem cell differentiation and angioblast migration. The vascular defects are observed in mice lacking NgBR specifically in EC, in both Tie2-Cre and inducible Cdh5-Cre models. The mutant embryo fails to survive beyond E11.0 and exhibits impaired cerebral BV assembly as well as dilation of cerebral blood vessels, resulting in thin-walled, endothelial caverns that are redolent of cerebral cavernous malformation. These vascular defects in NgBR ecKO mice indicate that NgBR could be the key to unravel the pathogenesis of vascular anomalies. The vascular defects are associated with the inability of NgBR deficient EC to migrate towards chemoattractants such as VEGF and/or AmNogo-B (Miao et al., 2006; Zhao et al., 2010). We also demonstrated that NgBR-mediated EC migration is dependent on Akt activation, which is vital for VEGF signaling pathway (Zhao et al., 2010). Constitutively activated Akt can rescue the vascular anomaly in NgBR deficient zebrafish (Zhao et al., 2010). We observed similar effect in HBMVECs, where NgBR deficiency impairs the phosphorylation of Erk and Akt. The defects of cerebral BV assembly could possibly be due to reliability of brain on angioblast migration for building up BV (Schmidt et al., 2007). Brain could be specifically affected since it lacks resident angioblast and relies heavily on VEGF signaling for angioblast migration and cerebral blood vessel development (Bautch, 2011). Therefore, these defects of BV assembly in the brain of NgBR ecKO mice could be due to the disruption of angioblast or EC migration in a developing embryo because NgBR is essential for both VEGF and AmNogo-B-stimulated phosphorylation of Erk and Akt. Our results indicate that NgBR plays a pivotal role during cerebral blood vessel assembly and could be a key player in governing EC patterning and tube formation.
The cerebral blood vessel dilation observed in NgBR mutant embryos bears resemblance to defects observed in CCM gene mutant mice (Boulday et al., 2009; Boulday et al., 2011; Chan et al., 2011; He et al., 2010; Kleaveland et al., 2009; Whitehead et al., 2009; Whitehead et al., 2004). Given that CCM1/2/3 proteins have been identified as responsible for cerebral cavernous malformations (CCMs) (He et al., 2010; Kar et al., 2015), a brain blood vessel assembly defect, we investigated whether these proteins were attenuated in NgBR deficient endothelial cells. NgBR deficiency in EC decreases both transcriptional and translational levels of CCM1 and CCM2 in HBMEC determined by real-time PCR (Fig. S10A) and Western blot analysis (Fig. S10B). The quantification of western blot using image J (Fig. S10C) reiterates that lack of NgBR in HBMVECs results in loss of CCM1 and CCM2 proteins. These results were also confirmed in vivo where genetic depletion of NgBR in EC decreases the transcript of CCM1 and CCM2 in the yolk sac of NgBR inducible knockout mouse embryos (Fig. S10D). To address the role of NgBR in disease, and because we observed association with loss of NgBR and CCM proteins in vitro and in vivo, we performed NgBR immunohistochemical (IHC) staining on 11 cerebral cavernous malformation patient tissue samples and compared them to 6 control tissue samples from epilepsy patients. CCM lesions were confirmed by H&E staining showing the thin grape-like CCM lesions and decreased CCM2 IHC staining in CCM patient samples (Fig. S10E). Figure S10E shows that both NgBR and CCM2 IHC staining in cerebral cavernous malformation BV is weaker than the control patient BV, indicating that disease patient tissues exhibit loss of NgBR. To quantify the expression difference, we performed CD31 and NgBR co-immunofluorescence staining on these patient samples (Fig. S10F). The intensity of NgBR IF staining in endothelial cells was quantified using iCys software, where both CD31 and NgBR positive signals were quantified. Figure S10G shows that percentage of NgBR to CD31 ratio is decreased in CCM patient samples as compared to control patient samples. Fig S10H shows histogram from iCys analysis reiterating that cerebral cavernous malformation tissues show loss of NgBR in CD31 positive EC.
Our results identify an association between NgBR and CCM proteins in that loss of NgBR in endothelial cells shows less CCM1 and CCM2 proteins, which are involved in the pathogenesis of cerebral cavernous malformations (He et al., 2010; Kar et al., 2015). CCMs disease is a common form of vascular malformation with a prevalence of 0.1 to 0.5 % in the human population (Al-Shahi Salman et al., 2012; Draheim et al., 2014; Fischer et al., 2013; Haasdijk et al., 2012). CCMs happens primarily in the brain shown as thin-walled, dilated blood vessels (BV), which may cause seizures, headaches and stroke in midlife and are often associated with focal hemorrhage (He et al., 2010; Kleaveland et al., 2009; Whitehead et al., 2004). Although significant progress has been made by identifying CCM family proteins (CCM1/KRIT1, CCM2, CCM3/PDCD10), the molecular mechanism of the CCMs pathogenesis remains to be established (Haasdijk et al., 2012). Our data implies NgBR, a receptor for Nogo-B, as a putative new regulator in the pathogenesis of cerebral vascular anomalies because EC homeostasis and cerebral vascular plexus formation is disrupted in NgBR ecKO mice. In addition, recent studies have connected Erk and Akt phosphorylation signaling to CCM pathways (Dibble et al., 2010; Ma et al., 2007; Schleider et al., 2011; Wilhelm et al., 2006). Since in vitro results show that NgBR deficiency can hinder phosphorylation of Akt and Erk in HBMVECs (Fig. S9B and S9C), NgBR could be a potential player in CCM pathways. In addition, our in vitro and in vivo results (Fig. S10A–D) demonstrated that loss of NgBR expression in brain EC decreased the RNA and protein levels of CCM1 and CCM2. Based on a Knudson’s two-hit hypothesis in CCM pathogenesis (Gault et al., 2005) (Fischer et al., 2013), the initiation of cavernous malformation would require subsequent loss of both CCM gene alleles in individual cells. Loss of one allele in all cells (germline mutation, first hit) would be followed in some cells by a somatic mutation or expression loss in the other allele (second hit). The causes of the second hit remain are still unclear (Fischer et al., 2013). Our results in Figure S10E-H have shown that CCM disease patients may exhibit significant loss of NgBR in EC. Our findings implicate that loss of NgBR in EC may be the one of factors leading to the second hit of causing the decreased expression of CCM1 and CCM2 genes. However, the link between vascular lesions presented in NgBR ecKO mice to the CCM pathogenesis needs to be further investigated.
Conclusions
In summary, the cerebral vasculature defects observed in NgBR mutant embryos demonstrated the important roles of NgBR in regulating cerebral blood vessel assembly and pathophysiology of brain vasculature.
Supplementary Material
Highlights.
We demonstrated the role of NgBR in regulating blood vessel formation in mouse.
NgBR knockout in endothelial cells (ecKO) impairs cerebral blood vessel assembly.
NgBR is a novel factor regulating the expression of CCM1 and CCM2 genes.
NgBR expression levels in endothelial cells are significantly decreased in CCM patient tissue sections.
Loss of NgBR in endothelial cells is a risk factor for cerebral vascular anomalies.
Acknowledgments
Cdh5(PAC)-CreERT2 mice and β-actin Cre mice were transferred from Dr. Ralf Adams (Cancer Research UK) and Dr. Katherine Shim (Children’s Research Institute, MCW), respectively. VEGF is a gifted reagent from Genentech. Histology and imaging core facilities at the Children’s Research Institute of Medical College of Wisconsin provides service and technique support for histology staining and imaging. This work was supported in part by start-up funds from Divisions of Pediatric Surgery and Pediatric Pathology, Medical College of Wisconsin (MCW) and Advancing a Healthier Wisconsin endowment to MCW, NIH R01HL108938 to Q.R.M. Ziegler Family Chair for Research to H.W. S.M.C., M.B. and R.R. are supported in part by funds from NIH grants HL102745 and HL112639 and M.C.-W. by NIH grant HL111583.
Footnotes
Author contributions:
U.R., Z.L. R.R. and Q.R.M. designed experiments, interpreted results. U.R. and Q.R.M. wrote the manuscript. U.R., S.K., H.W., M.C.-W., R.R. and Q.R.M. edited the manuscript. U.R. and Z.L. characterized in vivo phenotypes. U.R., Z.L., S.K., B.Z., W.H., S.C., M.L.H. performed experiments. J.F. and H.W. generated NgBR mutant mice. U.R., Z.L. M.B. W.H. maintained mouse breeding. M.C-W., R.P.M., P.N. provided reagents and technique supports. S.S. and P.N. conducted the pathological examination.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM, Sessa WC. A new role for Nogo as a regulator of vascular remodeling. Nature medicine. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, Counsell CE, Murray GD, Papanastassiou V, Ritchie V, Roberts RC, Sellar RJ, Warlow CP Scottish Audit of Intracranial Vascular Malformations c. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. The Lancet Neurology. 2012;11:217–224. doi: 10.1016/S1474-4422(12)70004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17:1437–1443. doi: 10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiological reviews. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. The Journal of clinical investigation. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergametti F, Denier C, Labauge P, Arnoult M, Boetto S, Clanet M, Coubes P, Echenne B, Ibrahim R, Irthum B, Jacquet G, Lonjon M, Moreau JJ, Neau JP, Parker F, Tremoulet M, Tournier-Lasserve E Societe Francaise de N. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. American journal of human genetics. 2005;76:42–51. doi: 10.1086/426952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulday G, Blecon A, Petit N, Chareyre F, Garcia LA, Niwa-Kawakita M, Giovannini M, Tournier-Lasserve E. Tissue-specific conditional CCM2 knockout mice establish the essential role of endothelial CCM2 in angiogenesis: implications for human cerebral cavernous malformations. Disease models & mechanisms. 2009;2:168–177. doi: 10.1242/dmm.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulday G, Rudini N, Maddaluno L, Blecon A, Arnould M, Gaudric A, Chapon F, Adams RH, Dejana E, Tournier-Lasserve E. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. The Journal of experimental medicine. 2011;208:1835–1847. doi: 10.1084/jem.20110571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AC, Drakos SG, Ruiz OE, Smith AC, Gibson CC, Ling J, Passi SF, Stratman AN, Sacharidou A, Revelo MP, Grossmann AH, Diakos NA, Davis GE, Metzstein MM, Whitehead KJ, Li DY. Mutations in 2 distinct genetic pathways result in cerebral cavernous malformations in mice. The Journal of clinical investigation. 2011;121:1871–1881. doi: 10.1172/JCI44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. Journal of cell science. 2013;126:2545–2549. doi: 10.1242/jcs.124529. [DOI] [PubMed] [Google Scholar]
- Dibble CF, Horst JA, Malone MH, Park K, Temple B, Cheeseman H, Barbaro JR, Johnson GL, Bencharit S. Defining the functional domain of programmed cell death 10 through its interactions with phosphatidylinositol-3,4,5-trisphosphate. PloS one. 2010;5:e11740. doi: 10.1371/journal.pone.0011740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim KM, Fisher OS, Boggon TJ, Calderwood DA. Cerebral cavernous malformation proteins at a glance. Journal of cell science. 2014;127:701–707. doi: 10.1242/jcs.138388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky J, Zabramski JM, Kurth J, Spetzler RF, Rich SS, Orr HT, Weber JL. A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Human molecular genetics. 1995;4:453–458. doi: 10.1093/hmg/4.3.453. [DOI] [PubMed] [Google Scholar]
- Evensen L, Micklem DR, Blois A, Berge SV, Aarsaether N, Littlewood-Evans A, Wood J, Lorens JB. Mural cell associated VEGF is required for organotypic vessel formation. PloS one. 2009;4:e5798. doi: 10.1371/journal.pone.0005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Zalvide J, Faurobert E, Albiges-Rizo C, Tournier-Lasserve E. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends in molecular medicine. 2013;19:302–308. doi: 10.1016/j.molmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Garcia MD, Larina IV. Vascular development and hemodynamic force in the mouse yolk sac. Frontiers in physiology. 2014;5:308. doi: 10.3389/fphys.2014.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault J, Shenkar R, Recksiek P, Awad IA. Biallelic somatic and germ line CCM1 truncating mutations in a cerebral cavernous malformation lesion. Stroke; a journal of cerebral circulation. 2005;36:872–874. doi: 10.1161/01.STR.0000157586.20479.fd. [DOI] [PubMed] [Google Scholar]
- Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- Haasdijk RA, Cheng C, Maat-Kievit AJ, Duckers HJ. Cerebral cavernous malformations: from molecular pathogenesis to genetic counselling and clinical management. European journal of human genetics : EJHG. 2012;20:134–140. doi: 10.1038/ejhg.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang H, Yu L, Gunel M, Boggon TJ, Chen H, Min W. Stabilization of VEGFR2 signaling by cerebral cavernous malformation 3 is critical for vascular development. Science signaling. 2010;3:ra26. doi: 10.1126/scisignal.2000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nature reviews Molecular cell biology. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nature medicine. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Kar S, Samii A, Bertalanffy H. PTEN/PI3K/Akt/VEGF signaling and the cross talk to KRIT1, CCM2, and PDCD10 proteins in cerebral cavernous malformations. Neurosurgical review. 2015;38:229–236. doi: 10.1007/s10143-014-0597-8. discussion 236–227. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, Sweeney SM, Chen M, Guo L, Lu MM, Zhou D, Kitajewski J, Affolter M, Ginsberg MH, Kahn ML. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nature medicine. 2009;15:169–176. doi: 10.1038/nm.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliche S, Waltenberger J. VEGF receptor signaling and endothelial function. IUBMB life. 2001;52:61–66. doi: 10.1080/15216540252774784. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Starling C, Chervonsky AV, Gridley T. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis. 2010;48:146–150. doi: 10.1002/dvg.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circulation research. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harbor symposia on quantitative biology. 1997;62:159–168. [PubMed] [Google Scholar]
- Li X, Claesson-Welsh L, Shibuya M. VEGF receptor signal transduction. Methods in enzymology. 2008;443:261–284. doi: 10.1016/S0076-6879(08)02013-2. [DOI] [PubMed] [Google Scholar]
- Lin FJ, Tsai MJ, Tsai SY. Artery and vein formation: a tug of war between different forces. EMBO reports. 2007;8:920–924. doi: 10.1038/sj.embor.7401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liquori CL, Berg MJ, Siegel AM, Huang E, Zawistowski JS, Stoffer T, Verlaan D, Balogun F, Hughes L, Leedom TP, Plummer NW, Cannella M, Maglione V, Squitieri F, Johnson EW, Rouleau GA, Ptacek L, Marchuk DA. Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. American journal of human genetics. 2003;73:1459–1464. doi: 10.1086/380314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughna S, Henderson D. Methodologies for staining and visualisation of beta-galactosidase in mouse embryos and tissues. Methods in molecular biology. 2007;411:1–11. doi: 10.1007/978-1-59745-549-7_1. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhao H, Shan J, Long F, Chen Y, Chen Y, Zhang Y, Han X, Ma D. PDCD10 interacts with Ste20-related kinase MST4 to promote cell growth and transformation via modulation of the ERK pathway. Molecular biology of the cell. 2007;18:1965–1978. doi: 10.1091/mbc.E06-07-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney Rogers AA, Zhang J, Shim K. Sprouty1 and Sprouty2 limit both the size of the otic placode and hindbrain Wnt8a by antagonizing FGF signaling. Developmental biology. 2011;353:94–104. doi: 10.1016/j.ydbio.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao RQ, Gao Y, Harrison KD, Prendergast J, Acevedo LM, Yu J, Hu F, Strittmatter SM, Sessa WC. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10997–11002. doi: 10.1073/pnas.0602427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiological reviews. 2011;91:691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. Journal of neuro-oncology. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Schleider E, Stahl S, Wustehube J, Walter U, Fischer A, Felbor U. Evidence for anti-angiogenic and pro-survival functions of the cerebral cavernous malformation protein 3. Neurogenetics. 2011;12:83–86. doi: 10.1007/s10048-010-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Broermann A, Schulte D. Control of endothelial barrier function by regulating vascular endothelial-cadherin. Current opinion in hematology. 2010;17:230–236. doi: 10.1097/MOH.0b013e328338664b. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends in cell biology. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Jones CA, Zhu W, Marchuk DA, Davis GE, Li DY. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nature medicine. 2009;15:177–184. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131:1437–1448. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nature reviews Drug discovery. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- Yu J, Fernandez-Hernando C, Suarez Y, Schleicher M, Hao Z, Wright PL, DiLorenzo A, Kyriakides TR, Sessa WC. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17511–17516. doi: 10.1073/pnas.0907359106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Chun C, Liu Z, Horswill MA, Pramanik K, Wilkinson GA, Ramchandran R, Miao RQ. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood. 2010;116:5423–5433. doi: 10.1182/blood-2010-02-271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Xu C, Smith AO, Stratman AN, Zou Z, Kleaveland B, Yuan L, Didiku C, Sen A, Liu X, Skuli N, Zaslavsky A, Chen M, Cheng L, Davis GE, Kahn ML. Dynamic regulation of the cerebral cavernous malformation pathway controls vascular stability and growth. Developmental cell. 2012;23:342–355. doi: 10.1016/j.devcel.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.