ABSTRACT
The present study aimed to measure the levels of coagulation factors in stored whole blood of pregnant women and to determine their usefulness in treating pregnant women who developed coagulopathy. A prospective study to measure coagulation factors in stored donated whole blood from pregnant and non-pregnant women was conducted. Fibrinogen, FV, FVII, FVIII, FXIII, and von Willebrand factor were measured in blood stored at 4°C for 0, 1, 3, and 5 weeks. All coagulation factors except for factor XIII decreased during storage. Fibrinogen and factor VII in the blood collected from pregnant women gradually decreased over time and their levels were significantly higher after 5 weeks of storage than those of non-pregnant women at week 0. Whole blood donated by pregnant women for autologous blood transfusion and stored at 4°C may be expected being effectively for the prevention of coagulopathy and the treatment of circulatory blood volume loss.
Key Words: whole blood, autologous blood transfusion, coagulopathy, fibrinogen, obstetric hemorrhage
INTRODUCTION
Postpartum hemorrhage (PPH) is currently recognized as the major cause of maternal death due to obstetric factors in Japan.1) PPH is generally caused by unpredictable events,2) although predictable disorders include placental previa or accreta. These complications have made it imperative to promptly administer blood products. Alexander et al. reported that the risk of complications is significantly reduced in women with PPH when they undergo stored whole blood transfusions.3) They speculated that whole blood facilitates in the restoration of fibrinogen as well as the circulatory blood volume.
As neither maternal adverse effects nor significant fetal distress was noted during blood collection from pregnant women,4) pregnancy is therefore not a contraindication to the collection of autologous blood for transfusion.5) In addition, various pregnant women hope to collect autologous blood to avoid complications relating to allogeneic blood transfusions. Therefore, antepartum autologous blood collection from high-risk women has been widely employed among perinatal care centers in Japan.
Donated blood is usually stored as whole blood at 4°C. It is well known that higher levels of coagulation factors are present in maternal circulation blood. If these factors are preserved at sufficient levels during storage, it will be possible for us to transfuse whole blood as a mixture of both coagulation factors and plasma volume expander.
In this investigation, we evaluated the levels of coagulation factors in whole blood stored at 4°C for as long as 5 weeks.
MATERIALS AND METHODS
Study design and population
Ten pregnant female patients and who were planning to undergo Caesarean section having high risks for massive bleeding, e.g. placenta previa, huge myoma, or multifetal pregnancies were enrolled in this study. And 10 non-pregnant female patients undergoing hysterectomy due to myoma or endometriosis were also enrolled as a control. The institutional review board of Saitama Medical University Hospital approved this study prior to its initiation. The characteristics of the patients of both groups are presented in Table 1. After obtaining a written informed consent from every study participant, whole blood (200 mL) was collected into a 200-mL blood collection bag system containing acid citrate-dextrose and adenine (ACD-A) liquid as anticoagulant (Kawasumi Separate Bag PO; Kawasumi Laboratories, Tokyo, Japan). Using the same collection line, an additional 50 mL of whole blood was collected into a 50-mL syringe, then transferred to a small bag containing 7-mL of ACD-A liquid. This is the standard ratio of the ACD-A volume to a 50-mL whole blood collection in the commercial blood collection bags.
Table 1.
Characteristics of the donors in each group
| Pregnant | Non-pregnant | |
|---|---|---|
| N | 10 | 10 |
| Age | 35.8 ± 4.2 | 42.3 ± 6.2 |
| Weight (kg) | 68.5 ± 11.3 | 56.8 ± 10.9 |
| Weeks of gestation | 34.2 ± 1.5 | N.A. |
| Hemoglobin (g/dL) | 11.3 ± 1.6 | 12.4 ± 1.8 |
| Hematocrit (%) | 33.9 ± 2.0 | 40.0 ± 3.6 |
| Total protein (g/dL) | 6.2 ± 0.8 | 6.8 ± 1.8 |
N.A., not available
The small bags were stored at 4°C for 5 weeks. A 10-mL aliquot of the blood sample was drawn 0, 1, 3, and 5 weeks later to analyze a set of coagulating factors.
Analysis of coagulation factors
Blood samples were centrifuged at 2,270 ×g for 10 min to separate and collect the plasma, then the following assays were performed. Fibrinogen was measured using an automated coagulation analyzer STA-R Evolution (Diagnostica Stago) using commercially available reagents: Fibrinogen (Claus’s method) (thrombin reagent, STA-Fibrinogen, Diagnostica Stago). Factors V, VII, and VIII were all analyzed using a one-step clotting assay and factor-deficient plasma reagents (Instrumentation Laboratory, Japan) in the same analyzer. Von Willebrand factor ristocetin cofactor (vWF:RCo) was measured using a Siemens BCS using reagent (BCVW, Siemens). Factor XIII activity was determined using a clinical chemistry analyzer JCA-BM1650 (JEOL, Japan) using a reagent (Berichrom, Siemens).
Statistical analysis
The data were presented as medians and SD. A nonparametric U-test was used for comparisons between the two groups. ANOVA for repeated measures followed by Tukey’s honest significant difference test was used for comparisons of each group. Statistical analysis was performed using the SPSS version 9•1 (SPSS Inc., Chicago, IL, USA) software package.
RESULTS
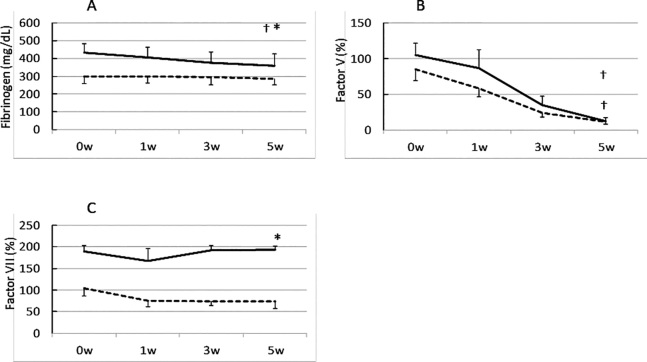
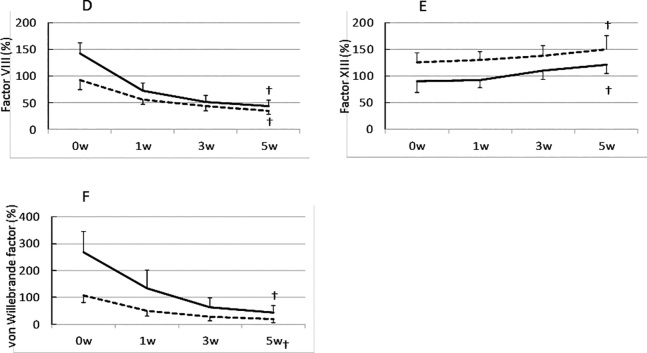
The plasma levels of coagulation factors before storage are presented in Table 2. We found significant differences in all coagulation factors between the two groups. Other than factor XIII, the remaining factors in the whole blood of pregnant women were significantly higher in levels than those of non-pregnant women. The trends in the coagulation factor activities during storage are demonstrated in Figure 1. All coagulation factors except for factor XIII decreased during storage. Majority of the coagulation factors showed an inverse correlation with storage period, whereas fibrinogen and factor VII levels in the blood of pregnant women after 5 weeks storage demonstrated significantly higher levels than those of non-pregnant women before storage, except for Factor XIII, which showed a small increase.
Table 2.
The values of coagulation factors at the time of blood collection
| Pregnant | Non-pregnant | Significance | |
|---|---|---|---|
| Fib (mg/dL) | 431.6 ± 52.7 | 302.7 ± 41.2 | <0.01 |
| Factor V (%) | 104.8 ± 16.9 | 82.0 ± 15.9 | <0.05 |
| Factor VII (%) | 179.8 ± 13.3 | 108.9 ± 18.4 | <0.01 |
| Factor VIII (%) | 141.9 ± 21.0 | 97.8 ± 17.9 | <0.01 |
| Factor XIII (%) | 89.4 ± 20.5 | 113.3 ± 18.0 | <0.01 |
| vWF (IU/dL) | 268.3 ± 77.9 | 134.4 ± 26.7 | <0.01 |
Fib, Fibrinogen; vWF, von Willebrand factor ristocetin cofactor, N.S., not significant;
Fig. 1.
Comparison of the activities of coagulation factors in stored whole blood collected from pregnant and non-pregnant women
Fibrinogen; B, Factor V; C, Factor VII; D, Factor VIII; E, Factor XIII; F, von Willebrand Factor. Solid line, pregnant women; dashed line, non-pregnant women
A nonparametric U-test was used for the comparison between groups.
* p < 0.05
ANOVA for repeated measures followed by Tukey’s honest significant difference test was used and data are expressed as medians (± SD).
† p < 0.05 compared to baseline values.
DISCUSSION
The activity levels of the coagulation factors in the initial blood samples from pregnant women except for factor XIII were significantly higher than those from non-pregnant women, which was consistent with the findings of a previous study.6) The levels of the coagulation factors fibrinogen and factor VII in the whole blood collected from pregnant women and stored at 4°C remained sufficiently after 5 weeks of collection. Although majority of coagulation factors decreased their activities, the levels of fibrinogen and factor VII in the blood samples collected from pregnant women and stored for 5 weeks remained significantly higher than those in the initial samples obtained from non-pregnant women. Thus, by using refrigeration storage, whole blood from pregnant women may be utilized as a red cell product to provide sufficient amounts of fibrinogen and factor VII.
The level of factor XIII in the whole blood slightly increased during storage. Previous studies have shown that factor XIII is stored in platelets.7-9) During whole blood storage, platelets lose their viability and subsequently release their cellular contents, including factor XIII. The increase in the level of factor XIII in the stored blood samples could therefore be due to its release from platelets. Factor V, VIII, and vWF in the blood of pregnant women seem to decline more promptly than those of non-pregnant women. However, these trends illustrate parallel in a linear manner when these values are described in a graph of a logarithmic scale (data not shown). So we consider that the degradation speed of coagulation factors demonstrates no significant difference between pregnant and non-pregnant women.
Massive PPH is often associated with uterine bleeding or microvascular bleeding due to coagulopathy, which is also known as obstetrical DIC.10) Recent studies have demonstrated that women who developed massive PPH may benefit from early fibrinogen administration.11,12 Reports have shown that early administration of sufficient amounts of fresh frozen plasma to women experiencing massive PPH is an effective transfusion strategy. The recommended ratio of red cell concentrates to fresh frozen plasma is 1:1.13,14) Factor VII and fibrinogen in stored whole blood could contribute to achieving hemostasis. In case of emergency, the time taken to thaw fresh frozen plasma, which varies from 10 to 20 min, is relatively long and could cost the life of a patient. If stored whole blood can be immediately used to supply plasma that is rich in coagulation factors, transfusion could be directly performed without delay.
Our previous study has shown that 2–4 g of fibrinogen concentrate is effective in achieving hemostasis in PPH patients whose plasma fibrinogen levels are <150 mg/dL.15) Calculations from the data shown in Table 2 indicate that 1,000 mL of stored whole blood from pregnant women may contain 2.2–3.1 g of fibrinogen after 5 weeks storage. The amount of fibrinogen in stored whole blood may be equivalent to the commercially available fibrinogen product used in preventing hypofibrinogenemia.
To maintain the highest activity levels of the coagulation factors, plasma that has been isolated by centrifugation is usually frozen and stored at a temperature below −20°C. This autologous fresh frozen plasma can be utilized as an alternative coagulation factor mixture. Frozen plasma could be slowly thawed and the cryoprecipitate that comprises mainly fibrinogen and coagulation factor VIII is administered to a patient having uncontrollable bleeding.16) However, the utilization rate of autologous blood in the obstetrical clinical setting is relatively low, and manufacturing these specific blood products could be highly cost-ineffective. Based on these obstacles, preservation of whole blood by refrigeration is therefore practical.
Another strategy for the treatment of blood loss during an operation is intraoperative cell salvage system, which has been widely employed in various surgical settings.2) However, the use of intraoperative cell salvage system during a Caesarean delivery is also rare and applied only in cases when a massive hemorrhage could be predicted. However, when it is indeed performed, coagulopathy may be a potential complication associated with cell salvage, particularly when a large volume of blood is used during re-transfusion, because the recovered blood cells are washed and coagulation factors in the plasma are removed.17) From this standpoint, autologous and stored whole blood could be utilized in rescuing coagulopathy as well as in replacing the significant amount of blood lost during a cesarean operation.
The present study has limitations that need to be acknowledged and addressed. The present study demonstrated the quality of stored autologous blood but did not show any related in vivo effects particularly relating to the correction of coagulopathy after transfusion. Further studies are thus required to assess the efficacy of this procedure, possibly involving a randomized controlled clinical trial. Despite its partial correcting effect, autologous whole blood is a safe and cost-effective approach; therefore, this blood product may be initially used in transfusions involving women with PPH.
In conclusion, the transfusion of stored autologous whole blood to pregnant women may be expected as an effective approach for the prevention of coagulopathy as well as in the restoration of circulatory blood volume.
DISCLOSURE
The authors declare that they have no conflict of interest.
REFERENCES
- 1).Mothers’ & Children’s Health & Welfare Association, Japan. Maternal death in Maternal and child health statistics of Japan. Tokyo: Kamiya K, Mothers’ & Children’s Health Organization, 2012.
- 2).Tevet A, Grisaru-Granovsky S, Samueloff A, Ioscovich A. Peripartum use of cell salvage: a university practice audit and literature review. Arch Gynecol Obstet, 2012; 285: 281–284. [DOI] [PubMed]
- 3).Alexander JM, Sarode R, McIntire DD, Burner JD, Leveno KJ. Whole blood in the management of hypovolemia due to obstetric hemorrhage. Obstet Gynecol, 2009; 113: 1320–1326. [DOI] [PubMed]
- 4).O’Dwyer G, Mylotte M, Sweeney M, Egan EL. Experience of autologous blood transfusion in an obstetrics and gynaecology department. Br J Obstet Gynaecol, 1993; 100: 571–574. [DOI] [PubMed]
- 5).Santoso JT, Lin DW, Miller DS. Transfusion medicine in obstetrics and gynecology. Obstet Gynecol Surv, 1995; 50: 470–481. [DOI] [PubMed]
- 6).Uchikova EH, Ledjev II. Changes in haemostasis during normal pregnancy. Eur J Obstet Gynecol Reprod Biol, 2005; 119: 185–188. [DOI] [PubMed]
- 7).Malara A, Gruppi C, Rebuzzini P, Visai L, Perotti C, Moratti R, Balduini C, Tira ME, Balduini A. Megakaryocyte-matrix interaction within bone marrow: new roles for fibronectin and factor XIII-A. Blood, 2011; 117: 2476–2483. [DOI] [PubMed]
- 8).Jayo A, Conde I, Lastres P, Jiménez-Yuste V, González-Manchón C. New insights into the expression and role of platelet factor XIII-A. J Thromb Haemost, 2009; 7: 1184–1191. [DOI] [PubMed]
- 9).Inbal A, Muszbek L, Lubetsky A, Katona E, Levi I, Kárpáti L, Nagler A. Platelets but not monocytes contribute to the plasma levels of factor XIII subunit A in patients undergoing autologous peripheral blood stem cell transplantation. Blood Coagul Fibrinolysis, 2004; 15: 249–253. [DOI] [PubMed]
- 10).Kobayashi T. Obstetrical disseminated intravascular coagulation score. J Obstet Gynaecol Res, 2014; 40: 1500–1506. [DOI] [PubMed]
- 11).Charbit B, Mandelbrot L, Samain E, Baron G, Haddaoui B, Keita H, Sibony O, Mahieu-Caputo D, Hurtaud-Roux MF, Huisse MG, Denninger MH, de Prost D; PPH Study Group. The decrease of fibrinogen is an early predictor of the severity of postpartum haemorrhage. J Thromb Haemost, 2007; 5: 266–273. [DOI] [PubMed]
- 12).Onwuemene O, Green D, Keith L. Postpartum hemorrhage management in 2012: predicting the future. Int J Gynaecol Obstet, 2012; 119: 3–5. [DOI] [PubMed]
- 13).McLintock C, James AH. Obstetric hemorrhage. J Thromb Haemost, 2011; 9: 1441–1451. [DOI] [PubMed]
- 14).Fuller AJ, Bucklin BA. Blood product replacement for postpartum hemorrhage. Clin Obstet Gynecol, 2010; 53: 196–208. [DOI] [PubMed]
- 15).Kikuchi M, Itakura A, Miki A, Nishibayashi M, Ikebuchi K, Ishihara O. Fibrinogen concentrate substitution therapy for obstetric hemorrhage complicated by coagulopathy. J Obstet Gynaecol Res, 2013; 39: 770–776. [DOI] [PubMed]
- 16).Carless PA, Anthony DM, Henry DA. Systematic review of the use of fibrin sealant to minimize perioperative allogeneic blood transfusion. Br J Surg, 2002; 89: 695–703. [DOI] [PubMed]
- 17).Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth, 2010; 105: 401–416. [DOI] [PubMed]