Abstract
Purpose
To evaluate the reliability of the Autoimmune Bullous Diseases Quality of Life (ABQOL) questionnaire in a North American patient cohort.
Methods
Patients attending the Dermatology clinics of the University of Pennsylvania with a histological diagnosis of an autoimmune bullous disease (AIBD) and self-reported proficiency in English were recruited to participate in the study. Patients completed the ABQOL questionnaire at Day 0 and Day 3. Internal consistency was calculated through Cronbach’s alpha. Test-retest reliability was determined by the intraclass correlation coefficient.
Results
Of the 45 patients enrolled in the study, 39 patients (87%) participated to completion. The mean age was 60.7 years with an equal sex distribution observed. Patients had a range of AIBD including pemphigus vulgaris, bullous pemphigoid, pemphigus foliaceus, epidermolysis bullosa aquisita, mucous membrane pemphigoid and linear IgA disease. Cronbach’s alpha was calculated to be 0.90. The intraclass correlation coefficient was calculated to be 0.93 (95% confidence interval: 0.88–0.94).
Conclusion
The ABQOL was found to be reliable tested by internal consistency and test-retest reliability in an American patient cohort. It represents a promising disease-specific outcome measure for patients with AIBD.
Keywords: quality of life, vesiculobullous skin diseases, pemphigus, bullous pemphigoid
Introduction
Quality of life (QOL) is being increasingly recognized as an important clinical outcome within the field of dermatology. Many skin conditions do not exert a deleterious effect on mortality, but do significantly affect a patient’s QOL. This is secondary to the disfiguring nature of cutaneous pathology, with its adverse impact on body image and social function that may be independent of clinical severity. Physical symptoms such as itch and pain can also affect QOL and auxiliary considerations such as functional limitations, financial burden and side effects of treatment all exert a detrimental effect upon QOL [1].
Disease-specific QOL instruments are more sensitive to changes in clinical status than general QOL measures [2]. The Autoimmune Bullous Disease Quality of Life (ABQOL) questionnaire was developed in Australia as a patient-based measure to determine quality of life in patients with autoimmune bullous disease (AIBD) [3]. AIBD refer to a group of diseases characterized by the development of bullae and blistering as a result of autoimmunity to adhesion structures within the skin [4]. These diseases were previously associated with a guarded prognosis with secondary infection of bullae culminating in sepsis, however in the modern era with the advent of immunosuppressive therapies, AIBD tend to be chronic conditions with significant morbidity, and optimization of QOL is a more pertinent outcome of clinical care [5].
In the initial study reporting the construction and validation of the ABQOL, it was found to be a valid and reliable patient reported outcome measure. A comprehensive item generation process involving interviews with 26 AIBD patients and experts in the field was conducted to construct a pilot questionnaire. This was then administered to 70 patients and psychometric testing of the results was applied to refine the ABQOL to a 17 item questionnaire. The face and content validity were established through a comprehensive item generation process and expert review. In terms of convergent validity, the ABQOL was found to have a moderate correlation with more generalised QOL instruments such as the General Health subscale of the 36-Item Short Form Health Survey (R = 0.69) and the Dermatology Life Quality Index (R = 0.63). It was found to have a low correlation with the objective measures of disease activity such as the Pemphigus Disease Area Index (R = 0.42) and Autoimmune Bullous Disease Skin Disorder Intensity Score (R = 0.48). These findings are not unexpected given the specificity of the ABQOL in evaluating the QOL issues specific to AIBD which may not be represented in the more generic QOL instruments and which be independent of objective disease burden. Notably, in terms of discriminant validity, the more generic instruments had a significantly higher proportion of insensitive items. The ABQOL was found to be more sensitive than both the 36-Item Short Form Health Survey (p=0.01) and the Dermatology Life Quality Index (P = .02), reflecting the precision of the instrument in this setting. It was also found to be a reliable instrument evaluated by internal consistency with a Cronbach α coefficient of 0.84 [3].
The importance of validating patient reported outcome measures across different patient cohorts and different cultures has previously been foregrounded in the literature [6]. We sought further evaluate the reliability of the ABQOL in a different patient cohort of North American patients.
Materials and methods
Following local Institutional Review Board approval, patients were recruited from the dermatology clinics of the University of Pennsylvania over a four-week period. Eligibility required a histological diagnosis of an AIBD and self-reported proficiency in English. Of 46 eligible patients, 45 were enrolled in the study with one patient declining participation. Patients were administered the ABQOL at the date of clinical review, and were provided with an additional copy of the questionnaire and asked to complete this three days afterwards. An interval of three days was selected as this would be long enough to mitigate recall bias but short enough that disease status would remain relatively stable.
Reliability was evaluated using standard psychometric indices employed in the evaluation of QOL instruments [7]. Internal consistency refers to the degree to which items within a given domain measure the same construct and was calculated through assessment of Cronbach’s alpha. Test-retest reliability refers to the consistency of scoring over time and was calculated by correlating the total ABQOL scores between Day 0 and Day 3 using the intraclass correlation coefficient (ICC). All statistical analyses were computed using IBM SPSS Statistics v22.
Results
Of the 45 patients enrolled, 39 patients (87%) executed it to completion. The mean age was 60.7 years with an equal sex distribution observed. Patients had a range of AIBD including pemphigus vulgaris (n=18), bullous pemphigoid (n=11), pemphigus foliaceus (n=7), epidermolysis bullosa aquisita (n=1), mucous membrane pemphigoid (n=1) and linear IgA disease (n=1). Patients had a range of clinical stages including partial remission on minimal therapy (n=16), relapse (n=9), complete remission off therapy (n=7), complete remission on therapy (n=2), at time to disease control (n=2), at end of consolidation phase (n=1), in partial remission off therapy (n=1), and at baseline (n=1) [8].
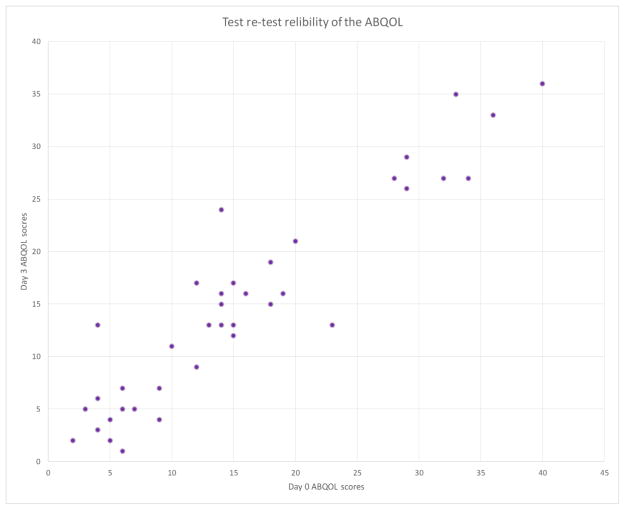
Cronbach’s alpha was calculated at 0.90 indicating a high internal consistency of the ABQOL in this patient group. In terms of test-retest reliability, the intraclass correlation coefficient was computed to be 0.93 (95% confidence interval: 0.88–0.94) indicating that the ABQOL was highly reliable (Fig. 1).
Figure 1.
Test-retest reliability of the ABQOL
Discussion
This study evaluated the ABQOL instrument for internal consistency and test-retest reliability and the ABQOL was found to be highly reliable by both indices. The setting was a tertiary referral center, as was the setting of the original study developing the ABQOL, and accordingly there is likely to be some selection bias in the patients included. The patients in this study cohort had a range of AIBD across a range of disease stages, though an important distinction from the Australian patient group is that a greater proportion of these patients had active disease. Mean ABQOL scores for the most common AIBD bullous pemphigoid (10.8 ± 2.5) and pemphigus vulgaris (16.4 ± 2.9), were slightly higher than those reported by our Australian group in the initial validation studies of the ABQOL (8.4 and 11.5 respectively).
When evaluating the reliability of patient reported measures for internal consistency, Cronbach’s alpha should ideally be above 0.70 [7]. In the original validation study in Australian patients Cronbach’s alpha was calculated at 0.84 and in this study it was calculated to be 0.90 indicating high internal consistency. This is the first time test-retest reliability has been formally evaluated for the ABQOL as only a small subset of patients were included in testing of this parameter in the Australian validation study. An ICC of 0.93 indicates the ABQOL will yield consistent results from patients under similar conditions.
This is also the first application of the ABQOL outside an Australian population with the instrument still found to be a reliable measure amongst American patients. Cross-cultural validation of patient reported outcomes is important even when language is a consistent variable. It is known, for instance, that normative data regarding QOL in Australian patients is distinct from American or British patients, despite English being the primary language in all three populations [9]. Further validation studies are required across different languages and cultures before the ABQOL can be used more broadly. Previous work has shown that the specificity of the ABQOL enables it to overcome some of the disadvantages of more generic QOL instruments and represents a promising patient-based measure to quantify disease burden, monitor disease activity and evaluate response to therapeutic intervention [10]. The specificity of the instrument also allows clinicians to recognize which aspects of disease most affect a patient (eg. the symptoms themselves, social dysfunction etc.) and tailor management accordingly, facilitating the provision of personalized care. Importantly, AIBD are extremely rare diseases and there is paucity of research in this field to guide physicians in terms of evidence based care. Studies in AIBD patient often have low participant numbers and employ arbitrary outcome measures. The construction of standardized disease specific outcome measures such as the ABQOL is important to allow comparisons between different research studies and for meta-analysis [11]. This study further demonstrates the reliability of this patient reported outcome and it is hoped the ABQOL may be used as both a clinical tool and outcome measure for clinical trials in the future.
Acknowledgments
The authors are indebted to the Medical Dermatology Society for awarding Deshan Sebaratnam a mentorship with Victoria Werth to complete this research and a poster prize at the Medical Dermatology Society Annual Meeting 2014; and to John Stanley, Ioannis Koutroulis and Nicole Fett for their assistance with patient recruitment. This material is supported by the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development).
Footnotes
Conflicts of Interest
None declared
References
- 1.Sebaratnam DF, McMillan JW, Werth VP, Murrell DF. Quality of life in patients with bullous dermatoses. Clinics in Dermatology. 2012;30(1):103–7. doi: 10.1016/j.clindermatol.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S. Dermatology Quality of Life Instruments: Sorting Out the Quagmire. Journal of Investigative Dermatology. 2007;127(12):2695–2696. doi: 10.1038/sj.jid.5701176. [DOI] [PubMed] [Google Scholar]
- 3.Sebaratnam DF, Hanna AM, Chee SN, et al. Development of a quality-of-life instrument for autoimmune bullous disease: the Autoimmune Bullous Disease Quality of Life questionnaire. JAMA Dermatology. 2013;149(1):1186–91. doi: 10.1001/jamadermatol.2013.4972. [DOI] [PubMed] [Google Scholar]
- 4.Karpouzis A, Vamvassakis E, Stavrianeas N, et al. Ultrastructural immunocytochemistry of autoimmune bullous diseases. Australasian Journal of Dermatology. 2002;43(2):113–119. doi: 10.1046/j.1440-0960.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 5.Sebaratnam DF, Murrell DF. Treatment of pemphigus vulgaris and pemphigus foliaceus. Expert Review of Dermatology. 2009;4(5):469–481. [Google Scholar]
- 6.Grob JJ. Why Are Quality of Life Instruments Not Recognized as Reference Measures in Therapeutic Trials of Chronic Skin Disorders? Journal of Investigative Dermatology. 2007;127(10):2299–2301. doi: 10.1038/sj.jid.5701081. [DOI] [PubMed] [Google Scholar]
- 7.Prinsen CAC, de Korte J, Augustin M, et al. Measurement of health-related quality of life in dermatological research and practice: outcome of the EADV Taskforce on Quality of Life. Journal of the European Academy of Dermatology and Venereology. 2013;27(10):1195–1203. doi: 10.1111/jdv.12090. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbach M, Murrell DF, Bystryn JC, et al. Reliability and convergent validity of two outcome instruments for pemphigus. Journal of Investigative Dermatology. 2009;129(10):2404–10. doi: 10.1038/jid.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens S, Begum N, Harper C, et al. A comparison of EQ-5D-3L population norms in Queensland, Australia, estimated using utility value sets from Australia, the UK and USA. Quality of Life Research. 2014;23(8):2375–2381. doi: 10.1007/s11136-014-0676-x. [DOI] [PubMed] [Google Scholar]
- 10.Sebaratnam DF, Frew JW, Davatchi F, et al. Quality-of-life measurement in blistering diseases. Dermatologic Clinics. 2012;30(2):301–7. doi: 10.1016/j.det.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Martin LK, Werth VP, Villaneuva EV, et al. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. Journal of the American Academy of Dermatology. 2011;64(5):903–8. doi: 10.1016/j.jaad.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]