Abstract
Objective
Pregnancies in women with the antiphospholipid syndrome (APS) are frequently complicated by fetal loss and intrauterine growth restriction (IUGR). How circulating antiphospholipid antibodies (aPL) cause pregnancy complications in APS is poorly understood. We sought to determine if the LDL receptor family member apoE receptor 2 (apoER2) mediates trophoblast dysfunction and pregnancy complications induced by aPL.
Methods
Placental and trophoblast apoER2 expression was evaluated by immunohistochemistry and immunoblotting. Normal human IgG (NHIgG) and aPL were purified from healthy individuals and APS patients, respectively. The role of apoER2 in aPL-induced changes in trophoblast proliferation, migration and kinase activation was assessed using RNA interference in HTR-8/SVneo cells. The participation of apoER2 in aPL-induced pregnancy loss and IUGR was evaluated in pregnant apoER2+/+ and apoER2−/− mice injected with aPL or NHIgG.
Results
We found that apoER2 is abundant in human and mouse placental trophoblasts, and in multiple trophoblast-derived cell lines including HTR-8/SVneo cells. ApoER2 and its interaction with the cell surface protein β2-glycoprotein I were required for aPL-induced inhibition of cultured trophoblast proliferation and migration. In parallel, aPL antagonism of Akt kinase activation by EGF in trophoblasts was mediated by apoER2. Furthermore, in a murine passive transfer model of pregnancy complications of APS, apoER2−/− mice were protected from both aPL-induced fetal loss and aPL-induced IUGR.
Conclusion
ApoER2 plays a major role in the attenuation of trophoblast function by aPL, and the receptor mediates aPL-induced pregnancy complications in vivo in mice. ApoER2-directed interventions can now potentially be developed to combat the pregnancy complications associated with APS.
Introduction
The antiphospholipid syndrome (APS) is an autoimmune disease characterized by the production of antiphospholipid antibodies (aPL) that promote arterial and venous thrombosis and pregnancy complications (1-4). In up to 5-7% of all pregnancies APS threatens the well-being of both the fetus and the mother (1-3). APS particularly afflicts women with systemic lupus erythematosus (SLE), with as many as 40% having circulating aPL (3;5). Women with aPL are at increased risk of pregnancy loss, as well as disorders associated with poor placental development such as preeclampsia and intrauterine growth restriction (IUGR) (1;6). Despite treatment with aspirin and heparin used to prevent presumed placental thrombosis in APS, the incidence of pregnancy complications remains high (7). Outcome studies have shown that the rate of fetal loss is up to 18% during APS pregnancies compared with a rate of early fetal loss of 1% and a rate of second and third trimester fetal demise of 5% in the general population (3;8;9). The reported frequency of IUGR in APS pregnancies ranges from 13 to 33% (8;10;11), and aPL are detected in 25% of women whose infants suffer from IUGR (3). Premature delivery occurs in 16 to 50% of APS pregnancies despite maternal therapy, with an average gestational age at birth of 31 weeks in one study (8;11;12). Thus, APS during pregnancy is an important health problem that places both the fetus and the mother in danger.
Because circulating aPL are strongly associated with increased risk for thrombosis in nonpregnant individuals, pregnancy failure in APS patients was initially thought to arise from thrombotic events occurring at the maternal-fetal interface. However, histological studies have revealed that intravascular or intervillous thrombi are rarely found in the placentas of APS patients (13). Instead, APS is characterized by attenuated placentation, with reduced decidual and vascular trophoblast invasion and less spiral artery transformation (13;14). These observations indicate that aPL likely alter trophoblast function rather than provoking thrombotic occlusion of the placental vasculature. Consistent with these in vivo findings, aPL attenuate the migration, invasion and differentiation of cultured trophoblasts and choriocarcinoma cell lines (6;15-19). The alterations in trophoblast function induced by the antibodies entail aPL binding to the cell surface protein β2-glycoprotein I (β2GPI). Antibodies directed against β2GPI are believed to be the major pathogenic antibodies in APS, and elevated levels of circulating anti-β2GPI antibodies are associated with reproductive failure in APS patients (20-22). β2GPI binds to exposed phosphatidylserine on trophoblasts and may provide the target for pathogenic aPL, which have been shown to localize to the placenta (23). Signaling pathways by which aPL and β2GPI invoke responses in trophoblasts such as IL-1β secretion are becoming more apparent (24;25). A critically important unknown is how the event of aPL recognition of β2GPI on the cell surface is transmitted across the trophoblast plasma membrane to alter intracellular processes in a manner that disturbs normal trophoblast function.
We previously demonstrated that aPL recognition of β2GPI on endothelial cells and resulting interactions of β2GPI with the LDL receptor family member apolipoprotein E receptor 2 (apoER2) underlie APS-related vascular thrombosis (26-28). In another recent study we discovered that apoER2 in endothelium also mediate responses to apolipoprotein E3 that are beneficial to cardiovascular health (29), highlighting the complexities of apoER2 function even in a single cell type. The biology of apoER2 is best understood in neurons in which it is critically involved in central nervous system development and Alzheimer’s disease (30). In the current work, using cell culture and mouse models we tested the hypotheses that apoER2 is expressed in trophoblasts, and that apoER2 mediates trophoblast dysfunction and pregnancy complications induced by aPL. We additionally determined how aPL and apoER2 disturb trophoblast function.
Methods
Isolation of Control IgG and aPL
NHIgG was obtained from healthy non-autoimmune individuals. Polyclonal aPL were isolated from patients with APS having high-titer aPL Ab (>80 GPL U), thromboses, and/or pregnancy complications (26;31). The relevant laboratory and clinical features of the patients who provided aPL are the following: Patient 1; age 50, female, aCL (>80 LA PGA) positive, anti-β2GPI positive, with clinical features of arterial thrombosis, pregnancy losses, catastrophic APS, and myocardial infarction. Patient 2; age 57, male, aCL (>80 LA PGA) positive, anti-β2GPI positive, with clinical features of arterial thrombosis, recurrent pulmonary hemorrhage, and catastrophic APS. Patient 3; age 36, female, aCL (>80 LA PGA) positive, anti-β2GPI positive with clinical features of deep venous thrombosis, renal microthromotic angiopathy and stroke. Individuals gave informed consent before participating in these studies, and all protocols were approved by the Institutional Review Boards of the Hospital for Special Surgery. The IgGs were purified by affinity chromatography using protein G–Sepharose chromatography columns (Amersham Biosciences). Endotoxin was removed using Centriprep ultracentrifugation devices (Millipore), and lack of endotoxin was confirmed using the Limulus amebocyte lysate assay (26;31). Mouse monoclonal antibodies directed to β2GPI (designated FC1 and 3F8) and their isotype-matched controls were prepared as previously described (26).
Immunohistochemistry for apoER2 and Cytokeratin 7
The cellular localization of apoER2 expression in first trimester and term human placenta was performed as previously described (32). First trimester placentas were obtained from elective terminations of normal pregnancies (6 - 12 weeks of gestation) performed at Yale-New Haven Hospital, and placental tissue was also obtained from uncomplicated normal term pregnancies (37 - 41 weeks of gestation). The use of patient samples was approved under Yale University's Human Investigations Committee. Briefly, placental samples were fixed with 4% paraformaldehyde and then paraffin embedded. Sections of placenta (5μm) were then deparaffinized, rehydrated and subjected to an antigen retrieval step, before being blocked with 0.1% BSA and 1% goat serum for 1h at room temperature. Following three washes with PBS, samples were incubated overnight at 4°C with either the anti-apoER2 rabbit polyclonal antibody at 1μg/ml (LifeSpan Biosciences), anti-cytokeratin 7 mouse monoclonal antibody (Thermo Scientific), or non-specific rabbit IgG at 1μg/ml (Southern Biotechnology). After three washes with PBS, specific staining was detected by incubating sections for 1h at room temperature with a biotinylated goat anti-rabbit or anti-mouse antibody (1:500 dilution; Vector Laboratories; Burlingame, CA) followed by a 1h incubation with Vectastain ABC Elite reagent and then a 1-5min incubation with DAB substrate (Vector Laboratories). Tissue sections were then counterstained with haematoxylin (Sigma) before dehydration with ethanol and Histosolve (Shandon Inc., Pittsburg, PA). Slides were mounted with Permount (Fisher Scientific, Pittsburg, PA) and visualized by light microscopy.
The expression of apoER2 in mouse placenta at E11.5 was also evaluated by immunohistochemistry. Sections were de-paraffinized and rehydrated followed by antigen retrieval in Buffer A (Electron Microscopy Sciences, USA) using a 2100-Retriever (Electron Microscopy Science, USA). Sections were then treated with 3% H202 to quench endogenous peroxidase, and processed using the Vectastain ABC kit according to the manufacturer’s protocol (Vector Labs, USA). Anti-apoER2 antibody (Abcam, UK) or anti-cytokeratin (Dako, USA) was used at a dilution of 1:100 from stock concentration. For negative controls, slides were incubated in fresh block without primary antibody. Antibody binding was visualized using Impact DAB reagent according to the manufacturer’s protocol (Vector Labs, USA). Following counterstaining in hematoxylin (Gills #2, Sigma, USA) or Nuclear Fast Red (Vector Labs, USA), sections were dehydrated and mounted in xylene-based mounting medium.
Cell Culture and Transfection
HTR-8/SVneo cells were previously derived from extravillous trophoblasts in the first trimester of pregnancy and immortalized using simian virus 40 large T antigen (33). These cells exhibit a high proliferation index and they share numerous phenotypic similarities with the parental primary cells (33;34). HTR-8/SVneo cells were kindly provided by Dr Charles H. Graham (Queen’s University, Canada). The cells were maintained in RPMI 1640 containing 5% fetal bovine serum. In siRNA experiments, HTR8/SVneo cells were transfected with siRNAs using LipofectAMINE 2000 (Invitrogen) and studied 48 hours later (26;35). Double-stranded RNA with sequence 5′-GGACAGACCUGGAGAACGAUU-3′ (Antisense: 5′-UCGUUCUCCAGGUCUGUCCUU-3′) and 5′-CCUUGAAGAUGAUGGACUAUU-3′ (Antisense: 5′UAGUCCAUCAUCUUCAAGGUU-3′) designed to target the open reading frame of human apoER2 (accession number NM_00463) and control dsRNA were purchased from Dharmacon (catalog D-001210-01-20).
Immunoblot Analysis
To evaluate apoER2 expression by immunoblot analysis, cell lysates were prepared from primary trophoblasts isolated from human or mouse placentas or established human trophoblastic cell lines, and the protein expression was assessed using anti-apoER2 rabbit polyclonal antibody (26). Human first trimester and term trophoblast cells were isolated as previously described (32). The following mouse trophoblast cell line and human first-trimester trophoblast cell lines were also evaluated: mouse SM9-1 cell line, telomerase immortalized Sw.71 cells and HTR-8/SVneo cells (33;36). To evaluate aPL-induced changes in trophoblast intracellular signaling, HTR-8/SVneo cells were treated with EGF (100 ng/ml) for 0-10 min in the presence of NHIgG or aPL, and immunoblotting was performed evaluating phospho-Akt (S473), total Akt, phospho-MAPK, and total MAPK abundance. All findings in cell culture were replicated in 3 independent experiments.
Trophoblast Proliferation and Migration
Trophoblast proliferation was assessed by counting the number of viable cells per well following equal seeding at a density of 1 × 105 and growth in RPMI1640 + 10 % FCS using trypan blue exclusion. Twenty-four hours after seeding, cells were incubated in the presence of NHIgG or aPL (0-200 μg/ml) for 24h. In select studies the cells were also incubated with sBD1 (50 μg/ml) (28). Trophoblast migration was evaluated in scratch assays. Cells were grown to near-confluence in 6-well plates and treated with NHIgG or aPL (0-200 μg/ml) for 16 h in RPMI1640 containing 1% lipoprotein deficient serum (LPDS), and a defined region of cells was removed with a single-edged razor blade (37). In select studies cells were also incubated with sBD1 (50 μg/ml). The cells were incubated for additional 16h in the presence of NHIgG or aPL and fixed with 3% paraformaldehyde (Sigma-Aldrich), permeabilized in 0.2% Triton X-100 (Bio-Rad Laboratories), stained with hematoxylin (Fisher Scientific), and viewed under an inverted microscope (Zeiss Axiovert 100M). The number of cells migrated past the wound edge was quantified in a minimum of 3 (10x magnification) fields per well. The recombinant peptide sBD1 was expressed in HEK293–EBNA cells and purified as previously reported (28). All findings in cell culture were replicated in 3 independent experiments.
Animal Model of APS in Pregnancy
Mice were employed to evaluate processes occurring during pregnancies complicated by APS. Although there are differences in human and mouse placenta, both are hemochorial and placental development in both species entails syncytiotrophoblast and trophoblast invasion of decidual stroma and arteries, such that mice can provide valuable insights into human pregnancy disorders involving these cell types and processes (31;38-41). In vivo studies were performed in apoER2+/+ and apoER2−/− littermates on identical 129SvEv × C57BL/6J background (26). All experiments were approved by the Institutional Animal Care and Utilization Committees at the University of Texas Southwestern Medical Center and the Hospital for Special Surgery. We employed a passive transfer model that has been previously used by us and other investigators to dissect the mechanisms underlying mid- and late-pregnancy complications in APS (31;39). Virgin female apoER2+/+ or apoER2−/− mice (8-12 weeks old) were mated with apoER2+/+ or apoER2+/− males, respectively. ApoER2 homozygous null males are infertile due to a defect in sperm development (42). The two study groups are referred to by the genotype of the dam. The pregnant females were injected IP (10 mg/mouse) with NHIgG or aPL on days 8 and 12 of pregnancy, and on day 15 fetal resorption rates and fetal weights were evaluated (fetal resorption rate = numbers of resorption sites/numbers of surviving fetuses + numbers of resorption sites) and the genotypes of the surviving fetuses were determined. The dose of NHIgG or aPL used was determined in prior kinetic and dose-response studies, and the fetal survival and IUGR endpoints have also been previously established (31;39;43;44).
Results
ApoER2 expression in trophoblasts
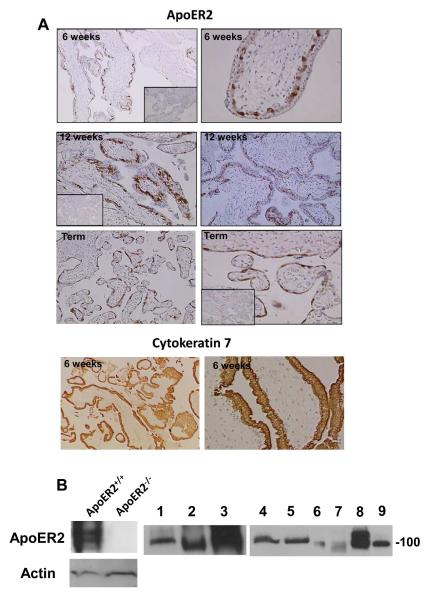
We first evaluated whether apoER2 is expressed in trophoblasts. Immunohistochemical analysis of human placental villous tissue obtained at 6 weeks gestation revealed that apoER2 is abundant in the inner layer of villous trophoblasts, which are identified by positive staining for cytokeratin 7 (Fig. 1A) (45). A similar staining pattern for apoER2 was observed at 12 weeks gestation, and villous trophoblasts at term were also positive for apoER2. The expression of apoER2 in both human and mouse trophoblasts was further demonstrated by immunoblotting of placental lysates and cell lysates prepared from primary trophoblasts and trophoblast cell lines (Fig. 1B). The disparities in the size of the detected apoER2 protein likely represent differences in splice variants or in receptor glycosylation (46).
Figure 1.
ApoER2 is expressed in human and mouse placental trophoblasts.
A. Human placental villous tissue from first trimester (6-12 weeks gestation) and normal term deliveries (37-41 weeks gestation) was immunostained for ApoER2 or cytokeratin 7. Inserts show negative immunostaining using a rabbit IgG control. Images are at 10X (left panels) and at 40X magnification (right panels). B. ApoER2 expression in human placental tissue, primary trophoblasts and trophoblast cell lines was evaluated by immunoblot analysis. Brain lysates from apoER2−/− and apoER2+/+ mice (5 μg total protein loaded) were used as controls. Lanes: 1; mouse placenta (25 μg), 2; mouse SM9-1 cells (mouse trophoblast cell line, 25 μg), 3; BeWo cells (human choriocarcinoma cell line, 25 μg), 4,5; human primary trophoblasts (term delivery, 25 μg), 6,7; human primary trophoblasts (first trimester) (6 μg), 8; Sw.71 cells (first trimester trophoblast cell line, 20 μg), 9; HTR-8/SVneo cells (first trimester trophoblast cell line, 20 μg).
ApoER2 and APL attenuation of trophoblast proliferation and migration
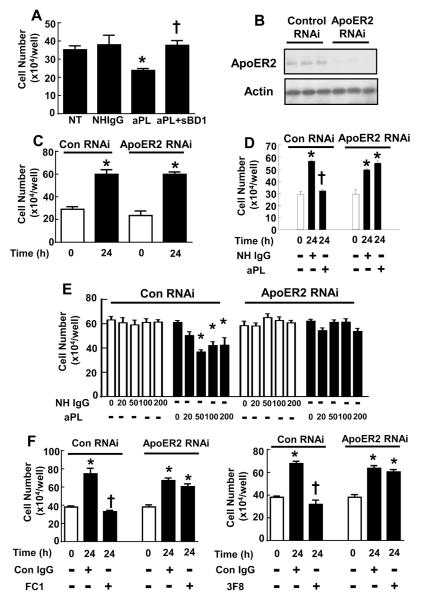
The primary defect in obstetrical APS is likely an impairment in placental trophoblast migration and invasion (13;14), and aPL inhibit trophoblast migration through binding to β2GPI on the cell surface (17;19;47). We and others previously showed that the interaction of β2GPI with apoER2 is required for aPL-induced changes in endothelial cell function (26;48). To better understand aPL actions on trophoblasts, we first evaluated whether β2GPI-apoER2 interaction is required for aPL-induced inhibition of trophoblast proliferation. This was accomplished using the soluble binding domain 1 of ApoER2 (sBD1), which is a peptide that competes for β2GPI and thereby blocks β2GPI binding to apoER2 (28). Cell proliferation was evaluated over 24h in HTR-8/SVNeo cells treated with IgG isolated from healthy human subjects (normal human IgG, or NHIgG), aPL isolated from APS patients, or aPL+sBD1 (Fig. 2A). In contrast to NHIgG, aPL decreased cell proliferation by 33%. However, aPL did not inhibit proliferation in the presence of sBD1. A requirement for apoER2 in aPL-induced inhibition of cell proliferation was also evaluated using RNA knockdown. HTR-8/SVneo cells were transfected with double-stranded RNA targeting human apoER2 sequence (ApoER2 RNAi) or scrambled sequence (Control RNAi), and effective apoER2 knockdown was confirmed by immunoblot analysis (Fig. 2B). Basal cell proliferation was evaluated by quantifying cell number at time 0 and 24h post-transfection, and it was found to be comparable in control and apoER2 knockdown cells (Fig. 2C). The impact of aPL was then assessed. APL treatment decreased trophoblast proliferation in control RNAi-transfected trophoblasts in a dose-dependent manner (Fig. 2D, E); in contrast, in cells deficient in apoER2, aPL did not inhibit cell proliferation. Furthermore, in contrast to isotype-matched control IgG, monoclonal antibodies against β2GPI (designated FC1 or 3F8) inhibited trophoblast proliferation, and they did so in an apoER2-dependent manner (Fig. 2F).
Figure 2.
ApoER2 and β2GPI-apoER2 interaction are required for the suppression of trophoblast proliferation by aPL.
A. HTR-8/SVneo cells were plated at 10 × 104cells/well and incubated with PBS (NT), NHIgG or aPL (100 μg/ml) in the presence or absence of sBD1 (50 μg/ml). Cell number was counted after 24h incubation (N=9, mean±SEM, *p<0.05 vs. NT, †p<0.05 vs. aPL alone). B. HTR-8/SVneo cells were transfected with control siRNA or double-stranded siRNA targeting apoER2, and whole cell lysates were immunoblotted for apoER2 and actin. Results for 3 samples for each condition are shown. C. HTR-8/SVneo cells were transfected with control siRNA or double-stranded siRNA targeting apoER2, and following transfection the increase in cell number over 24 h was evaluated. N=3, Mean±SEM, *p<0.05 vs. 0h. D, E. Following transfection with control siRNA or siRNA targeting apoER2, the increase in cell number over 24 h was evaluated in cells treated with various concentrations of NHIgG or aPL. (0-200 μg/ml, N=3, Mean±SEM, *p<0.05 vs. 0h, †p<0.05 vs. NHIgG). F. Following transfection with control siRNA or siRNA targeting apoER2, the increase in cell number over 24 h was evaluated in cells treated with anti-β2GPI monoclonal antibodies (FC1 or 3F8) or isotype-matched control IgG (Con IgG). (N=3, Mean±SEM, *p<0.05 vs. 0h, †p<0.05 vs. Con IgG).
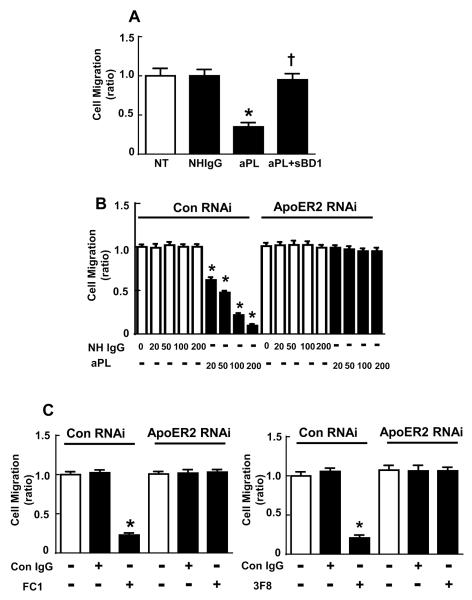
To determine if β2GPI-apoER2 interaction is required for aPL inhibition of trophoblast migration, HTR-8/SVneo cells were treated with NHIgG, aPL or aPL+sBD1, and trophoblast migration was evaluated over 16h using a scratch assay (Fig. 3A). In the presence of aPL, migration was inhibited by 65%, and the addition of sBD1 fully attenuated the effect of aPL. A requirement for apoER2 in aPL-induced inhibition of cell migration was further evaluated by knockdown of the receptor by RNAi (Fig. 3B). Twenty-four hours after transfection, the cells were treated with aPL or NHIgG for 16h and migration assays were performed during additional 16h in the presence of the antibodies. In cells with normal levels of apoER2, dose-dependent inhibition of trophoblast migration by aPL was demonstrable. In contrast, in cells depleted of apoER2, aPL did not inhibit migration. Furthermore, trophoblast migration was inhibited by monoclonal antibodies against β2GPI (FC1 or 3F8) but not by isotype-matched control IgG, and the inhibition required apoER2 (Fig. 3C). Taken together, these results indicate that apoER2 and interaction between β2GPI and apoER2 are necessary for aPL-induced inhibition of trophoblast proliferation and migration.
Figure 3.
ApoER2 and β2GPI-apoER2 interaction are required for the attenuation of trophoblast migration by aPL.
A. HTR-8/SVneo cells were treated with PBS (NT), NHIgG (100 μg/ml), aPL (100 μg/ml), or aPL+sBD1 (50 μg/ml) for 24 h during scratch assays. Migration was expressed relative to migration with NT (N=4, Mean±SEM, *p<0.05 vs. NT, †p<0.05 vs. aPL alone). B. Scratch assays were performed in HTR-8/SVneo cells transfected with control siRNA or apoER2 siRNA in the presence of various concentrations of NHIgG of aPL (0-200 μg/ml) for 24 h. Migration was expressed relative to migration of NHIgG-treated cells (N=10, Mean±SEM, *p<0.05 vs. NHIgG). C. Scratch assays were performed in HTR-8/SVneo cells transfected with control siRNA or apoER2 siRNA in the presence of control IgG (Con IgG) or anti-β2 GPI monoclonal antibodies (FC1 or 3F8) Migration was expressed relative to migration of Control IgG-treated cells (N=10, Mean±SEM, *p<0.05 vs. NHIgG).
ApoER2 and aPL attenuation of EGF signaling in trophoblasts
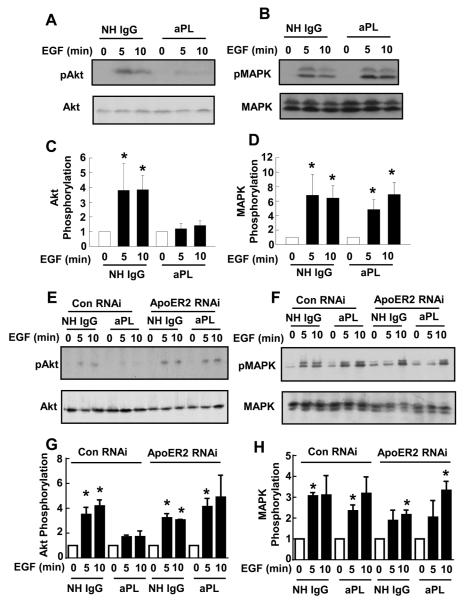
In processes critical to placental development, epidermal growth factor (EGF), Wnt-3A and chorionic gonadotropin upregulate matrix metalloproteins in trophoblasts and stimulate their migration via Akt kinase and MAP kinase (MAPK) activation (35;49;50). To determine if aPL actions via apoER2 interfere with these key signaling pathways in trophoblasts, we examined the effects of aPL on EGF-induced activation of Akt kinase or MAPK (Fig. 4A-D). In HTR-8/SVneo treated with NHIgG, EGF stimulated Ser473 phosphorylation of Akt (pAkt) and Thr202/Tyr204 phosphorylation of MAPK (pMAPK) indicative of their activation. In contrast, EGF did not activate Akt in aPL-treated cells. The inhibitory effect of aPL was specific to Akt activation since MAPK phosphorylation (pMAPK) was not affected. To determine if apoER2 is required for aPL-induced alterations in kinase activation, RNAi-based knockdown was employed (Fig. 4E-H). Whereas MAPK activation by EGF was not affected by aPL, Akt kinase activation was inhibited by aPL in cells with normal apoER2 expression; in contrast, in cells with diminished apoER2 expression, aPL had no impact on EGF-induced Akt activation. Thus, apoER2 is required for aPL inhibition of Akt activation by EGF.
Figure 4.
APL inhibit EGF-induced Akt activation via apoER2.
A-D. HTR-8/SVneo cells were incubated with NHIgG or aPL (100 μg/ml) for 16 h, then treated with EGF (100 ng/ml) for 0, 5, or 10 min. Cell lysates were immunoblotted for phospho-Akt (S473), total Akt, phospho-MAPK, and total MAPK A, B. Representative immunoblots are shown. C, D. Summary data for 3 experiments, mean±SEM, *p<0.05 vs. time 0. E-H. HTR-8/SVneo cells were transfected with control siRNA or double-stranded siRNA targeting apoER2. Twenty-four hours following transfection, the cells were treated and immunoblot analyses were done as in A-D. E, F. Representative immunoblots are shown. G, H. Summary data for 3 experiments, mean±SEM, *p<0.05 vs.time 0.
ApoER2 and aPL-induced fetal loss and IUGR
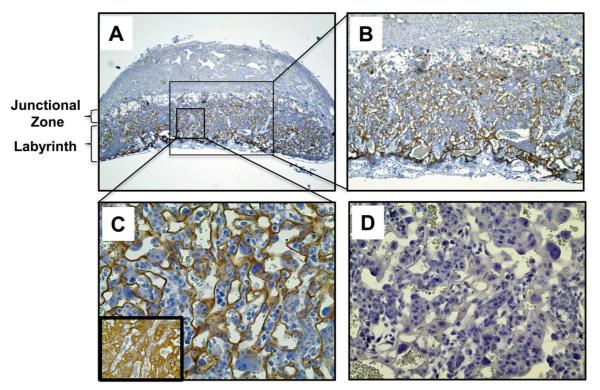
Having demonstrated that apoER2 is expressed in trophoblasts and critically involved in the attenuation of their proliferation and migration by aPL, we determined whether apoER2 is required for aPL-induced pregnancy complications in vivo. In preparation for studies in mice, we performed immunohistochemical analysis of apoER2 in the mouse placenta (Fig. 5). We found that apoER2 is abundantly expressed in the labyrinth of the mouse placenta. Staining was observed in cells that also express cytokeratin 7 and are associated with maternal and fetal blood spaces, which are characteristics of trophoblasts.
Figure 5.
ApoER2 is expressed in mouse placental trophoblasts in vivo.
A-C. Immunostaining of ApoER2 was performed in wild-type mouse placenta (E11.5). ApoER2 protein was detected in trophoblasts in the labyrinth. Images are at 4X (A), 10X (B) and 40X magnification (C). Inset in C shows cytokeratin 7 staining in an adjacent placenta section. D. Control staining without primary antibody.
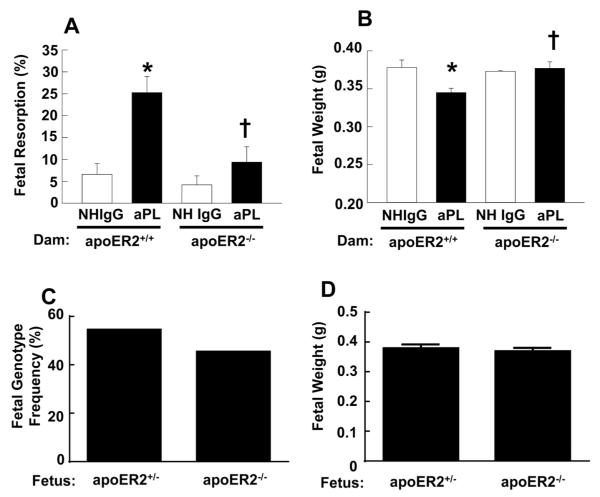
We then studied a mouse model of APS in pregnancy in which the passive transfer of aPL induces fetal loss and IUGR, and compared pregnancy outcomes in apoER2+/+ and apoER2−/− females (31;39). Virgin female apoER2+/+ and apoER2−/− mice were mated with apoER2+/+ and apoER2+/− males, respectively. Mating with apoER2−/− males was not feasible due to sterility related to abnormal sperm development (42). NHIgG or aPL was then administered IP on days 8 and 12 of pregnancy, and on day 15 fetal resorption rates and fetal weights were evaluated. The fetal resorption rate in apoER2+/+ pregnancies was increased 4.2-fold with aPL compared to NHIgG control treatment (Fig. 6A). In contrast, aPL did not increase the rate of fetal loss in apoER2−/− pregnancies. Furthermore, compared with NHIgG, aPL caused a decrease in the weights of the surviving fetuses in apoER2+/+ pregnancies (Fig. 6B). However, normal fetal weights were preserved despite aPL treatment in apoER2−/− pregnancies. In apoER2−/− dams treated with aPL, there was no difference in survival rate (Fig. 6C) or fetal weight (Fig. 6D) between apoER2−/− and apoER2+/− fetuses. This indicates that the lack of one or both alleles of the receptor in the fetus, which is the source of placental trophoblasts, conferred similar protection against aPL-induced fetal loss and IUGR. These observations in the mouse model demonstrate for the first time that apoER2 is critically involved in the fetal loss and IUGR that complicate APS during pregnancy.
Figure 6.
ApoER2−/− mice are protected from aPL-induced fetal resorption and IUGR. Following mating of apoER2+/+ or apoER2+/− males with apoER2+/+ or apoER2−/− females respectively, the females were injected with NHIgG or aPL (10 mg/mouse IP) on day 8 and 12 of pregnancy. Mice were euthanized on day 15 of pregnancy, uteri were dissected, embryos were weighed, and fetal resorption rates were calculated (number of resorptions/number of live fetuses + number of resorptions). A. Fetal resorption frequency. N=7814, Mean±SEM. *p<0.05 vs. NHIgG, †p<0.05 vs. apoER2+/+. B. Fetal weights. N=55896, Mean±SEM. *p<0.05 vs. NHIgG, †p<0.05 vs. apoER2+/+. C, D. Surviving fetuses from apoER2−/− pregnant mice treated with aPL were genotyped for apoER2 (C) and fetal weights were determined (D). In a total of 66 fetuses, 36 were apoER2+/− and 30 were apoER2−/−.
Discussion
In humans with APS and in experimental animal models of the disease, aPL trigger adverse pregnancy outcomes including fetal loss, IUGR, preterm birth and preeclampsia (1-4). Proposed mechanisms by which aPL induce pregnancy complications involve multiple interacting mechanisms: the activation of innate immune receptors, the production of inflammatory effectors, and alterations in trophoblast function (2;51;52). Our previous work indicated that aPL binding to trophoblasts activates complement to invoke placental injury and cause fetal loss and IUGR (31;39). In the present studies we identified apoER2 as a key plasma membrane protein on the trophoblast mediating the adverse actions of aPL. We further revealed that apoER2 provides the critical mechanistic link between β2GPI recognition by aPL on the cell surface and the resulting alterations in trophoblast function. Moreover, using an established mouse model of the pregnancy complications of APS, we provide the first evidence that apoER2 is required for aPL-induced fetal loss and IUGR. Taken together with previous work by us and others indicating that apoER2 mediates aPL-induced thrombosis remote from pregnancy (26;28;48), these findings reveal that apoER2 is the common linchpin in the pathogenesis of APS-related disease in various clinical paradigms.
Along with revealing the role of apoER2 in the pregnancy complications of APS, the present work interrogates apoER2 in the placenta for the first time. In that manner the current findings extend our understanding of the biology of apoER2 beyond its well-established role in CNS development and Alzheimer’s disease (30) and its recently revealed functions in endothelium (26;29). Immunohistochemical analysis of human placenta revealed that apoER2 is abundantly expressed in trophoblasts throughout gestation, and immunoblotting demonstrated that the receptor is also expressed in HTR-8/SVneo and SW.71 cells that retain the characteristics of trophoblasts in culture (33;34;36). We observed no reproduction-related abnormalities in the pregnancies in apoER2−/− mice treated with control NHIgG, with similar numbers of live fetuses and fetal weights in apoER2−/− versus apoER2+/+ dams. These results indicate that the receptor is not required for normal trophoblast function or other processes governing fetal well-being. However, in the presence of aPL, via β2GPI the receptor elicits changes in trophoblast function, and apoER2 deficiency affords protection from aPL-induced fetal loss and IUGR. These findings reveal that the blockade of receptor actions invoked by aPL and β2GPI may prevent APS-related fetal and/or maternal disease in at-risk pregnancies, with little risk of altering normal trophoblast function.
In the investigation of how aPL impact intracellular signaling in trophoblasts we found that aPL inhibit Akt phosphorylation at S473 in response to EGF, and that this requires apoER2. Our previous work showed that in endothelium aPL-β2GPI binding to apoER2 initiates intracellular signaling that leads to the activation of PP2A, which inhibits eNOS activation by dephosphorylating the enzyme at the critical serine residue S1177(26). In trophoblasts PP2A regulates the phosphorylation of the bHLH transcription factors HAND-1 and HAND-2, which are required for trophoblast differentiation (53). It is not yet known whether PP2A modulates Akt in trophoblasts. In neurons the apoER2 ligand Reelin binds to apoER2 and initiates intracellular signaling by recruiting the adaptor protein Dab-1 to the cytoplasmic domain of apoER2 (54). In mice, a knock-in mutation of the apoER2 cytoplasmic domain that disrupts Dab-1 interaction (alteration of the NFDNPVY motif to EIGNPVY) leads to neuronal developmental defects identical to those observed in Reelin−/− mice (54). Interestingly, in platelets aPL induces the binding of Dab-1 to apoER2 (28). Studies are now warranted to determine whether Dab-1 or other apoER2 adaptor molecules are required for aPL effects in trophoblasts.
Up to 5 to 7% of pregnancies are complicated by APS (1-3) and it is the only known cause of fetal death, preterm birth, IUGR and preeclampsia/eclampsia that can be diagnosed in the mother to actually prevent these disorders if effective therapy were available. Based on the thrombosis that occurs with APS remote from pregnancy and despite the lack of evidence that placental thrombosis occurs in APS, current interventions to prevent the obstetric complications of APS are limited to heparin and aspirin (1;3;7). Despite these treatments, in APS pregnancies the fetal loss rate is up to 18%, the IUGR rate is up to 33%, and the rate of premature births is up to 50%, and heparin therapy can cause maternal bleeding (1;3;7). Fortunately our understanding of APS pathogenesis during pregnancy now goes well beyond a possible involvement of thrombosis., which has been recently shown not to contribute to placental pathology (13). With the discovery of the pathogenetic role of apoER2, mechanism-directed therapies can now be developed that may offer far greater efficacy and fewer treatment-related side-effects than anticoagulation.
Acknowledgements
We thank Mohamed Ahmed for his technical assistance.
Supported by grants from NIH 5R01HL109604 (to Dr. Mineo), R37 HL63762 (to Dr. Herz), K12 HD000849 (to Dr. Gelber), the Mary Kirkland Center for Lupus Research (to Dr. Salmon), the March of Dimes (to Drs. Gelber and Abrahams), the Bright Focus Foundation (to Dr. Herz), and the Hartwell Foundation (to Dr. Shaul).
Footnotes
Authorship contributions
All authors were involved in drafting the article critically for important intellectual content, and all authors approved the final version to be published. Drs. Salmon and Mineo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Ulrich, Natale, Abrahams, Shaul, Salmon, Mineo.
Acquisition of data. Ulrich, Gelber, Vukelic, Sacharidou, Natale, Harihara, Redecha, Abrahams, Salmon, Mineo
Analysis and interpretation of data. Herz, Urbanus, de Groot, Natale, Shaul, Salmon, Mineo.
Conflict of Interest Disclosures
All authors declare no conflict of interests.
Reference List
- (1).Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;(2):CD002859. doi: 10.1002/14651858.CD002859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011;7(6):330–9. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- (3).Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498–509. doi: 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- (4).Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- (5).Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346(10):752–63. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- (6).D'Ippolito S, Di SN, Di NF, Castellani R, Caruso A. Antiphospholipid antibodies: effects on trophoblast and endothelial cells. Am J Reprod Immunol. 2007;58(2):150–8. doi: 10.1111/j.1600-0897.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- (7).Deruelle P, Coulon C. The use of low-molecular-weight heparins in pregnancy--how safe are they? Curr Opin Obstet Gynecol. 2007;19(6):573–7. doi: 10.1097/GCO.0b013e3282f10e33. [DOI] [PubMed] [Google Scholar]
- (8).Canti V, Castiglioni MT, Rosa S, Franchini S, Sabbadini MG, Manfredi AA, et al. Pregnancy outcomes in patients with systemic autoimmunity. Autoimmunity. 2012;45(2):169–75. doi: 10.3109/08916934.2011.593600. [DOI] [PubMed] [Google Scholar]
- (9).Ruffatti A, Tonello M, Visentin MS, Bontadi A, Hoxha A, De CS, et al. Risk factors for pregnancy failure in patients with anti-phospholipid syndrome treated with conventional therapies: a multicentre, case-control study. Rheumatology (Oxford) 2011;50(9):1684–9. doi: 10.1093/rheumatology/ker139. [DOI] [PubMed] [Google Scholar]
- (10).De CS, Botta A, Santucci S, Garofalo S, Martino C, Perrelli A, et al. Predictors of pregnancy outcome in antiphospholipid syndrome: a review. Clin Rev Allergy Immunol. 2010;38(2-3):116–24. doi: 10.1007/s12016-009-8144-z. [DOI] [PubMed] [Google Scholar]
- (11).Motta M, Boffa MC, Tincani A, Avcin T, De CS, Lachassinne E. Follow-up of babies born to mothers with antiphospholipid syndrome: preliminary data from the European neonatal registry. Lupus. 2012;21(7):761–3. doi: 10.1177/0961203312446387. [DOI] [PubMed] [Google Scholar]
- (12).Geis W, Branch DW. Obstetric implications of antiphospholipid antibodies: pregnancy loss and other complications. Clin Obstet Gynecol. 2001;44(1):2–10. doi: 10.1097/00003081-200103000-00002. [DOI] [PubMed] [Google Scholar]
- (13).Sebire NJ, Fox H, Backos M, Rai R, Paterson C, Regan L. Defective endovascular trophoblast invasion in primary antiphospholipid antibody syndrome-associated early pregnancy failure. Hum Reprod. 2002;17(4):1067–71. doi: 10.1093/humrep/17.4.1067. [DOI] [PubMed] [Google Scholar]
- (14).Bose P, Kadyrov M, Goldin R, Hahn S, Backos M, Regan L, et al. Aberrations of early trophoblast differentiation predispose to pregnancy failure: lessons from the anti-phospholipid syndrome. Placenta. 2006;27(8):869–75. doi: 10.1016/j.placenta.2005.09.007. [DOI] [PubMed] [Google Scholar]
- (15).Di SN, Raschi E, Testoni C, Castellani R, D'Asta M, Shi T, et al. Pathogenic role of anti-beta 2-glycoprotein I antibodies in antiphospholipid associated fetal loss: characterisation of beta 2-glycoprotein I binding to trophoblast cells and functional effects of anti-beta 2-glycoprotein I antibodies in vitro. Ann Rheum Dis. 2005;64(3):462–7. doi: 10.1136/ard.2004.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Di SN, Luigi MP, Marco D, Fiorella DN, Silvia D, Clara DM, et al. Pregnancies complicated with antiphospholipid syndrome: the pathogenic mechanism of antiphospholipid antibodies: a review of the literature. Ann N Y Acad Sci. 2007;1108:505–14. doi: 10.1196/annals.1422.054. [DOI] [PubMed] [Google Scholar]
- (17).Di SN, Meroni PL, de PN, Raschi E, Caliandro D, De Carolis CS, et al. Antiphospholipid antibodies affect trophoblast gonadotropin secretion and invasiveness by binding directly and through adhered beta2-glycoprotein I. Arthritis Rheum. 2000;43(1):140–50. doi: 10.1002/1529-0131(200001)43:1<140::AID-ANR18>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Katsuragawa H, Kanzaki H, Inoue T, Hirano T, Mori T, Rote NS. Monoclonal antibody against phosphatidylserine inhibits in vitro human trophoblastic hormone production and invasion. Biol Reprod. 1997;56(1):50–8. doi: 10.1095/biolreprod56.1.50. [DOI] [PubMed] [Google Scholar]
- (19).Abrahams VM. Mechanisms of antiphospholipid antibody-associated pregnancy complications. Thromb Res. 2009;124(5):521–5. doi: 10.1016/j.thromres.2009.07.011. [DOI] [PubMed] [Google Scholar]
- (20).Blank M, Shoenfeld Y. Antiphospholipid antibody-mediated reproductive failure in antiphospholipid syndrome. Clin Rev Allergy Immunol. 2010;38(2-3):141–7. doi: 10.1007/s12016-009-8146-x. [DOI] [PubMed] [Google Scholar]
- (21).Carp HJ, Shoenfeld Y. Anti-phospholipid antibodies and infertility. Clin Rev Allergy Immunol. 2007;32(2):159–61. doi: 10.1007/s12016-007-0010-2. [DOI] [PubMed] [Google Scholar]
- (22).Lee RM, Emlen W, Scott JR, Branch DW, Silver RM. Anti-beta2-glycoprotein I antibodies in women with recurrent spontaneous abortion, unexplained fetal death, and antiphospholipid syndrome. Am J Obstet Gynecol. 1999;181(3):642–8. doi: 10.1016/s0002-9378(99)70507-7. [DOI] [PubMed] [Google Scholar]
- (23).Agostinis C, Biffi S, Garrovo C, Durigutto P, Lorenzon A, Bek A, et al. In vivo distribution of beta2 glycoprotein I under various pathophysiologic conditions. Blood. 2011;118(15):4231–8. doi: 10.1182/blood-2011-01-333617. [DOI] [PubMed] [Google Scholar]
- (24).Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, et al. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1beta production by human first trimester trophoblast. PLoS One. 2013;8(6):e65237. doi: 10.1371/journal.pone.0065237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Mulla MJ, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, et al. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009;62(2):96–111. doi: 10.1111/j.1600-0897.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J Clin Invest. 2011;121(1):120–31. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lutters BC, Derksen RH, Tekelenburg WL, Lenting PJ, Arnout J, de Groot PG. Dimers of beta 2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2'. J Biol Chem. 2003;278(36):33831–8. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- (28).Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2'. J Thromb Haemost. 2008;6(8):1405–12. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- (29).Ulrich V, Konaniah ES, Herz J, Gerard RD, Jung E, Yuhanna IS, et al. Genetic variants of ApoE and ApoER2 differentially modulate endothelial function. Proc Natl Acad Sci U S A. 2014;111(37):13493–8. doi: 10.1073/pnas.1402106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Lane-Donovan C, Philips GT, Herz J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014;83(4):771–87. doi: 10.1016/j.neuron.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112(11):1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cardenas I, Mulla MJ, Myrtolli K, Sfakianaki AK, Norwitz ER, Tadesse S, et al. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J Immunol. 2011;187(2):980–6. doi: 10.4049/jimmunol.1100578. [DOI] [PubMed] [Google Scholar]
- (33).Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204–11. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- (34).Irving JA, Lysiak JJ, Graham CH, Hearn S, Han VK, Lala PK. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta. 1995;16(5):413–33. doi: 10.1016/0143-4004(95)90100-0. [DOI] [PubMed] [Google Scholar]
- (35).Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128(3):355–63. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- (36).Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, et al. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30(11):939–48. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98(1):63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- (38).Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- (39).Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195(2):211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of anti-phospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal anti-cardiolipin antibodies. Proc Natl Acad Sci U S A. 1991;88(8):3069–73. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).de la Torre YM, Pregnolato F, D'Amelio F, Grossi C, Di SN, Pasqualini F, et al. Anti-phospholipid induced murine fetal loss: novel protective effect of a peptide targeting the beta2 glycoprotein I phospholipid-binding site. Implications for human fetal loss. J Autoimmun. 2012;38(2-3):J209–J215. doi: 10.1016/j.jaut.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, Erdmann B, et al. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem. 2003;278(26):23989–95. doi: 10.1074/jbc.M302157200. [DOI] [PubMed] [Google Scholar]
- (43).Qing X, Redecha PB, Burmeister MA, Tomlinson S, D'Agati VD, Davisson RL, et al. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011;79(3):331–9. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- (44).Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203(9):2165–75. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57(1):67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- (46).Kim DH, Magoori K, Inoue TR, Mao CC, Kim HJ, Suzuki H, et al. Exon/intron organization, chromosome localization, alternative splicing, and transcription units of the human apolipoprotein E receptor 2 gene. J Biol Chem. 1997;272(13):8498–504. doi: 10.1074/jbc.272.13.8498. [DOI] [PubMed] [Google Scholar]
- (47).Blank M, Anafi L, Zandman-Goddard G, Krause I, Goldman S, Shalev E, et al. The efficacy of specific IVIG anti-idiotypic antibodies in antiphospholipid syndrome (APS): trophoblast invasiveness and APS animal model. Int Immunol. 2007;19(7):857–65. doi: 10.1093/intimm/dxm052. [DOI] [PubMed] [Google Scholar]
- (48).Romay-Penabad Z, guilar-Valenzuela R, Urbanus RT, Derksen RH, Pennings MT, Papalardo E, et al. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117(4):1408–14. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Dilly M, Hambruch N, Haeger JD, Pfarrer C. Epidermal growth factor (EGF) induces motility and upregulates MMP-9 and TIMP-1 in bovine trophoblast cells. Mol Reprod Dev. 2010;77(7):622–9. doi: 10.1002/mrd.21197. [DOI] [PubMed] [Google Scholar]
- (50).LaMarca HL, Dash PR, Vishnuthevan K, Harvey E, Sullivan DE, Morris CA, et al. Epidermal growth factor-stimulated extravillous cytotrophoblast motility is mediated by the activation of PI3-K, Akt and both p38 and p42/44 mitogen-activated protein kinases. Hum Reprod. 2008;23(8):1733–41. doi: 10.1093/humrep/den178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Tong M, Viall CA, Chamley LW. Antiphospholipid antibodies and the placenta: a systematic review of their in vitro effects and modulation by treatment. Hum Reprod Update. 2014 doi: 10.1093/humupd/dmu049. [DOI] [PubMed] [Google Scholar]
- (52).Salem D, Subang R, Laplante P, Levine JS, Rauch J. The dual role of innate immunity in antiphospholipid syndrome and systemic lupus erythematosus. Lupus. 2014;23(12):1327–31. doi: 10.1177/0961203314548248. [DOI] [PubMed] [Google Scholar]
- (53).Firulli AB. A HANDful of questions: the molecular biology of the heart and neural crest derivatives (HAND)-subclass of basic helix-loop-helix transcription factors. Gene. 2003;312:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- (54).Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, et al. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci. 2006;26(7):2041–52. doi: 10.1523/JNEUROSCI.4566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]