Abstract
Background
Previous studies documented racial/ethnic and socioeconomic disparities in survival after Hodgkin lymphoma (HL) among adolescents and young adults (AYAs), but did not consider the influence of combined-modality treatment and health insurance.
Methods
Data for 9,353 AYA patients aged 15–39 when diagnosed with HL during 1988–2011 were obtained from the California Cancer Registry. Using multivariate Cox proportional hazards regression, we examined the impact of socio-demographic characteristics (race/ethnicity, neighborhood socioeconomic status (SES), and health insurance), initial combined-modality treatment, and subsequent cancers on survival.
Results
Over the 24-year study period, we observed improvements in HL-specific survival by diagnostic period and differences in survival by race/ethnicity, neighborhood SES and health insurance for a subset of more recently diagnosed patients (2001–2011). In multivariable analyses, HL-specific survival was worse for Blacks than Whites with early-stage (Hazard Ratio (HR): 1.68; 95% Confidence Interval (CI): 1.14, 2.49) and late-stage disease (HR: 1.68; 95% CI: 1.17, 2.41) and for Hispanics than Whites with late-stage disease (HR: 1.58; 95% CI: 1.22, 2.04). AYAs diagnosed with early-stage disease experienced worse survival if they also resided in lower SES neighborhoods (HR: 2.06; 95% CI: 1.59, 2.68). Furthermore, more recently diagnosed AYAs with public health insurance or who were uninsured experienced worse HL-specific survival (HR: 2.08; 95% CI: 1.52, 2.84).
Conclusion
Our findings identify several subgroups of HL patients at higher risk for HL mortality.
Impact
Identifying and reducing barriers to recommended treatment and surveillance in these AYAs at much higher risk of mortality is essential to ameliorating these survival disparities.
Keywords: Hodgkin lymphoma, insurance, adolescent, race/ethnicity, survival
Introduction
For Hodgkin lymphoma (HL), one of the most common cancers of adolescents and young adults (AYAs) 15 to 39 years of age (1), treatment with combined-modality (radiation plus chemotherapy) regimens has led to substantial improvements in survival over time and has been commonly recommended for patients with limited stage disease and those with bulky, advanced stage disease (2, 3). However, these impressive survival gains have not been shared uniformly across the AYA population, as worse outcomes have been documented for 15–44 year-olds of lower neighborhood socioeconomic status (SES) (4) and non-White race/ethnicity (4, 5).
Among explanations for these disparities, the similarity of disparities for overall and HL-specific survival (4) and the persistent difference in relative survival by neighborhood SES over time (4), implicate variations in initial treatment and management more than variations in the late complications sometimes resulting from HL (6). We previously found that Blacks (52%) and Hispanics (47%) were more likely to receive chemotherapy alone (as compared with combined-modality) than non-Hispanic Whites (38%) or Asian/Pacific Islanders (APIs) (40%) 15–44 years of age (4). Recent (1995–2010) population-based data on AYAs with early-stage HL also showed that Blacks and Hispanics, and patients residing in lower SES neighborhoods had lower utilization of radiation therapy, and Blacks and Hispanics, and AYAs not receiving radiation had higher mortality (7).
In addition, inadequate health insurance is associated with later stage at diagnosis, under-treatment and worse survival (8–12). Adult (≥18 years) HL patients with early stage disease who were uninsured were less likely to receive chemotherapy and radiation (12), and AYAs who were uninsured, had public health insurance or resided in lower SES neighborhoods were more likely to be diagnosed with advanced-stage HL (11).
In efforts to date to understand HL survival disparities, no prior studies have considered the influence of both receipt of initial combined-modality treatment and insurance on the racial/ethnic and socioeconomic disparities in survival among AYAs diagnosed with all stages of HL. Therefore, to identify socio-demographic patient subgroups that have not benefited from well-established and effective treatments, we evaluated the impact of socio-demographic characteristics (race/ethnicity, neighborhood SES and health insurance), initial combined-modality treatment, and subsequent cancers on survival among AYAs diagnosed with early and late stage HL.
Materials and Methods
Patients
Patients eligible for the study were all persons who resided in California when newly diagnosed at ages 15 through 39 years with classical HL (International Classification of Diseases—Oncology, 3rd edition (13) morphology codes 9650–9655, 9663–9667) during the period January 1, 1988 through December 31, 2011 and reported to the California Cancer Registry (CCR). From the CCR, which operates under a state cancer reporting law and comprises three National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) registries, we obtained information routinely recorded in the medical record at diagnosis for each patient on age, sex, race/ethnicity (non-Hispanic White (hereafter called “White”), Hispanic, Black, and API), summary stage (localized (Ann Arbor stage I), regional (stage II), advanced (stage III/IV)), extent of disease, tumor histologic subtype (nodular sclerosis, mixed cellularity, lymphocyte depletion, lymphocyte rich, or not otherwise specified), marital status (never married, married, previously married, unknown), hospital providing initial care, and census-block group of residence. With information on extent of disease, we classified patients by the presence of B symptoms (weight loss, night sweats, and fever) and human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS). In addition, we obtained registry data on initial treatment modality (radiation (yes, no, unknown) and chemotherapy (yes, no, unknown)), from which we created a combined modality measure; subsequent primary cancer(s) reported during the study period; vital status (routinely determined by the CCR through hospital follow-up and linkages to state and national vital status and other databases) as of December 2012; and, for the deceased, the underlying cause of death as routinely coded by state vital statistics personnel.
We also obtained information on the primary source of payment at initial diagnosis and/or treatment (health insurance), which was reportable to the CCR for patients diagnosed from 2001 forward. Health insurance was grouped into public (Medicaid and other government-assisted programs), private (health maintenance organizations, preferred provider organizations, managed care not otherwise specified, and military care), none and unknown (11). Consistent with prior observations that the small percentage of AYA cancer patients who were uninsured likely reflect retroactive enrollment in Medicaid at the time of cancer diagnosis (9), we considered publicly insured and uninsured together in the survival analyses. Hospitals were classified according to whether or not they were NCI-designated cancer centers.
We used a multi-component index of neighborhood SES based on patients’ residential census-block group at diagnosis. The index is derived from data from the 2000 U.S. Census (for cases diagnosed through 2005) and the 2006–2010 American Community Survey (for cases diagnosed in 2006 forward) on education, occupation, unemployment, household income, poverty, rent, and house values (14). The indices are grouped into quintiles, based on the distribution of SES across all census block groups in California, and, as done previoulsy (4), into one of two categories for models stratified by stage at diagnosis: lower SES (quintiles 1, 2 and 3) and higher SES (quintiles 4 and 5). Each cancer case was assigned to his/her neighborhood SES category. Based upon the population density of census blocks (15, 16), we defined urbanization level as metropolitan (metropolitan urban and metropolitan suburban blocks), non-metropolitan (city, town and rural blocks) or unknown.
The final study population included 9,353 AYA HL patients after exclusion, in a hierarchical manner, of those with: 1) unknown race/ethnicity (n=166); 2) cancer registry or death certificate evidence of HIV or AIDS (17) (n=262), because of the substantially poorer outcome of HIV-associated HL during the study period (18, 19); 3) HL diagnosis at autopsy only, by death certificate only, or with zero/invalid survival time (n=40). All study protocols were overseen by the Institutional Review Board of the Cancer Prevention Institute of California.
Statistical analyses
Outcomes of interest included overall survival, which considers death from all causes, and HL-specific survival, which considers only death from HL. For deceased patients, survival time was measured in days from the date of diagnosis to the date of death from any cause for overall survival, and to the date of death from HL for HL-specific survival. Patients who died from other causes were censored at the time of death in analyses of HL-specific survival. Patients alive at the study end date (12/31/2012) were censored at this time or at the date of last known contact. Ninety-four percent of censored patients had a follow-up date within two years of the study end date but this number was slightly higher for Whites (95%), Blacks (96%), and APIs (95%) than for Hispanics (89%).
To evaluate associations with survival (overall and HL-specific) controlling for known prognostic factors, we used multivariable Cox proportional hazards regression to estimate hazard ratios (HRs) and associated 95% confidence intervals (CIs). Models included variables with a priori reasons for inclusion (e.g., age, race/ethnicity, gender, year of diagnosis, marital status, B symptoms, histologic subtype, hospital type, neighborhood SES and urbanization, and insurance status) and included stage at diagnosis, combined-modality therapy, and subsequent cancer as stratifying variables to allow for differing baseline hazards associated with these variables. Additionally, separate Cox proportional hazards models were conducted by stage at diagnosis. Effect modification was assessed between SES and stage at diagnosis, health insurance, and gender; between race/ethnicity and SES, health insurance, and stage at diagnosis; and between age and marital status, by including interaction terms in the multivariable models. No interaction terms were statistically significant at p<0.05. In all models, the proportional hazards assumption was assessed numerically based on cumulative sums of Martingale residuals (20) and visually based on inspection of the survival curves (log (−log) of the survival distribution function by log (months)). There was evidence of a violation of this assumption with stage at diagnosis, combined-modality therapy and subsequent cancers; therefore, stratified Cox proportional hazards regression models are presented. Regression analyses were conducted using SAS version 9.3 software (SAS institute Inc., Cary, NC, USA).
Results
In this cohort of 9,353 AYA HL patients, 32% were followed for 15 years or more, with the mean follow-up time of 11.0 years (std=7.1). Socio-demographic and clinical characteristics of AYA HL patients varied by race/ethnicity, with a predominance of Whites (63%) and, to a lesser extent, Hispanics (24%), and an increasing proportion of Asians over time (Table 1). Black (40%) and Hispanic (37%) AYAs were more likely to be diagnosed at an advanced stage than Whites (31%) or APIs (34%), and higher percentages of Black (54%) and Hispanic (49%) AYAs received chemotherapy alone than Whites (40%) or APIs (42%). More than 73% of Blacks and Hispanics resided in the lowest three categories of neighborhood SES compared to fewer than 47% of Whites and APIs. Blacks (35%) and Hispanics (39%) were much more likely to have public or no insurance than Whites (17%) or APIs (16%). Nearly 6% of AYA HL patients were diagnosed with a subsequent primary cancer. As of December 2012, more than 13% of AYA HL patients had died, predominantly from cancer (72%).
Table 1.
Characteristics of adolescent and young adult Hodgkin lymphoma (HL) patients 15 to 39 years of age at diagnosis (N=9,353) by race/ethnicity, California, 1988–2011.
| Characteristics | Race/ethnicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients | Non-Hispanic White |
Black | Hispanic | Asian/ Pacific Islander |
p- value |
||||||
| n=9,353 | % | n=5,919 | % | n=643 | % | n=2,200 | % | n=591 | % | ||
| Sex | |||||||||||
| Male | 4,786 | 51.2 | 3,029 | 51.2 | 316 | 49.1 | 1,140 | 51.8 | 301 | 50.9 | |
| Female | 4,567 | 48.8 | 2,890 | 48.8 | 327 | 50.9 | 1,060 | 48.2 | 290 | 49.1 | 0.70 |
| Age at diagnosis (years) | |||||||||||
| 15–19 | 1,478 | 15.8 | 820 | 13.9 | 91 | 14.2 | 459 | 20.9 | 108 | 18.3 | |
| 20–24 | 2,224 | 23.8 | 1,374 | 23.2 | 139 | 21.6 | 541 | 24.6 | 170 | 28.8 | |
| 25–29 | 2,201 | 23.5 | 1,421 | 24.0 | 154 | 24.0 | 500 | 22.7 | 126 | 21.3 | |
| 30–34 | 1,937 | 20.7 | 1,279 | 21.6 | 140 | 21.8 | 413 | 18.8 | 105 | 17.8 | |
| 30–39 | 1,513 | 16.2 | 1,025 | 17.3 | 119 | 18.5 | 287 | 13.0 | 82 | 13.9 | <0.001 |
| Year of diagnosis | |||||||||||
| 1988–1992 | 2,052 | 21.9 | 1,528 | 25.8 | 135 | 21.0 | 317 | 14.4 | 72 | 12.2 | |
| 1993–1997 | 1,865 | 19.9 | 1,297 | 21.9 | 125 | 19.4 | 369 | 16.8 | 74 | 12.5 | |
| 1998–2002 | 1,800 | 19.2 | 1,138 | 19.2 | 100 | 15.6 | 443 | 20.1 | 119 | 20.1 | |
| 2003–2006 | 1,559 | 16.7 | 878 | 14.8 | 121 | 18.8 | 429 | 19.5 | 131 | 22.2 | |
| 2007–2011 | 2,077 | 22.2 | 1,078 | 18.2 | 162 | 25.2 | 642 | 29.2 | 195 | 33.0 | <0.001 |
| Marital status at diagnosis | |||||||||||
| Married | 3,265 | 34.9 | 2,147 | 36.3 | 161 | 25.0 | 753 | 34.2 | 204 | 34.5 | |
| Not married | 5,823 | 62.3 | 3,622 | 61.2 | 458 | 71.2 | 1,372 | 62.4 | 371 | 62.8 | |
| Unknown | 265 | 2.8 | 150 | 2.5 | 24 | 3.7 | 75 | 3.4 | 16 | 2.7 | <0.001 |
| Stage at diagnosis | |||||||||||
| I – localized | 1,200 | 12.8 | 774 | 13.1 | 87 | 13.5 | 263 | 12.0 | 76 | 12.9 | |
| II – regional | 4,507 | 48.2 | 2,960 | 50.0 | 264 | 41.1 | 986 | 44.8 | 297 | 50.3 | |
| III/IV – advanced | 3,128 | 33.4 | 1,852 | 31.3 | 258 | 40.1 | 817 | 37.1 | 201 | 34.0 | |
| Missing | 518 | 5.5 | 333 | 5.6 | 34 | 5.3 | 134 | 6.1 | 17 | 2.9 | <0.001 |
| B-symptoms | |||||||||||
| No | 3,530 | 37.7 | 2,252 | 38.0 | 221 | 34.4 | 800 | 36.4 | 257 | 43.5 | |
| Yes | 3,343 | 35.7 | 1,947 | 32.9 | 254 | 39.5 | 923 | 42.0 | 219 | 37.1 | |
| Missing/Unknown | 2,480 | 26.5 | 1,720 | 29.1 | 168 | 26.1 | 477 | 21.7 | 115 | 19.5 | <0.001 |
| Histologic subtype | |||||||||||
| Nodular sclerosis | 7,088 | 75.8 | 4,630 | 78.2 | 460 | 71.5 | 1,548 | 70.4 | 450 | 76.1 | |
| Mixed cellularity | 939 | 10.0 | 528 | 8.9 | 78 | 12.1 | 286 | 13.0 | 47 | 8.0 | |
| Lymphocyte depletion | 96 | 1.0 | 53 | 0.9 | 5 | 0.8 | 32 | 1.5 | 6 | 1.0 | |
| Lymphocyte rich | 212 | 2.3 | 114 | 1.9 | 26 | 4.0 | 59 | 2.7 | 13 | 2.2 | |
| Not otherwise specified | 1,018 | 10.9 | 594 | 10.0 | 74 | 11.5 | 275 | 12.5 | 75 | 12.7 | <0.001 |
| Combined-modality therapy | |||||||||||
| Chemotherapy and radiation | 3,421 | 36.6 | 2,267 | 38.3 | 171 | 26.6 | 718 | 32.6 | 265 | 44.8 | |
| Chemotherapy only | 4,019 | 43.0 | 2,341 | 39.6 | 348 | 54.1 | 1,082 | 49.2 | 248 | 42.0 | |
| Radiation only | 993 | 10.6 | 748 | 12.6 | 52 | 8.1 | 147 | 6.7 | 46 | 7.8 | |
| None/unknown | 920 | 9.8 | 563 | 9.5 | 72 | 11.2 | 253 | 11.5 | 32 | 5.4 | <0.001 |
| Subsequent cancer | |||||||||||
| No | 8,802 | 94.1 | 5,542 | 93.6 | 599 | 93.2 | 2,105 | 95.7 | 556 | 94.1 | |
| Yes* | 551 | 5.9 | 92 | 6.4 | 14 | 6.8 | 11 | 4.3 | 4 | 5.9 | |
| Breast | 121 | 22.0 | |||||||||
| Lymphoma | 62 | 11.3 | |||||||||
| Thyroid | 48 | 8.7 | |||||||||
| Melanoma | 39 | 7.1 | |||||||||
| Acute myeloid leukemia | 34 | 6.2 | |||||||||
| Head and neck | 28 | 5.1 | |||||||||
| Lung | 26 | 4.7 | |||||||||
| Uterus | 24 | 4.4 | |||||||||
| Colorectal | 22 | 4.0 | |||||||||
| Other Leukemia | 22 | 4.0 | |||||||||
| Soft tissue | 17 | 3.1 | |||||||||
| Kidney | 13 | 2.4 | |||||||||
| Vulva | 11 | 1.0 | |||||||||
| Pancreas | 8 | 1.5 | |||||||||
| Acute lymphocytic leukemia | 7 | 1.3 | |||||||||
| Anus | 6 | 1.1 | |||||||||
| Prostate | 6 | 1.1 | |||||||||
| Esophagus | 5 | 0.9 | |||||||||
| Testis | 5 | 0.9 | |||||||||
| Other | 47 | 8.5 | |||||||||
| Received care at an NCI-designated cancer center | |||||||||||
| No/Missing | 8,312 | 88.9 | 5,245 | 88.6 | 605 | 94.1 | 1,956 | 88.9 | 506 | 85.6 | |
| Yes | 1,041 | 11.1 | 674 | 11.4 | 38 | 5.9 | 244 | 11.1 | 85 | 14.4 | <0.001 |
| Neighborhood socioeconomic status (quintiles) | |||||||||||
| 1 (Lowest) | 1,310 | 14.0 | 431 | 7.3 | 177 | 27.5 | 660 | 30.0 | 42 | 7.1 | |
| 2 | 1,805 | 19.3 | 961 | 16.2 | 157 | 24.4 | 605 | 27.5 | 82 | 13.9 | |
| 3 | 2,036 | 21.8 | 1,349 | 22.8 | 141 | 21.9 | 420 | 19.1 | 126 | 21.3 | |
| 4 | 2,161 | 23.1 | 1,570 | 26.5 | 115 | 17.9 | 317 | 14.4 | 159 | 26.9 | |
| 5 (Highest) | 2,041 | 21.8 | 1,608 | 27.2 | 53 | 8.2 | 198 | 9.0 | 182 | 30.8 | <0.001 |
| Urbanization level | |||||||||||
| Metropolitan | 6,130 | 65.5 | 3,662 | 61.9 | 497 | 77.3 | 1,493 | 67.9 | 478 | 80.9 | |
| Non-metropolitan | 3,072 | 32.8 | 2,173 | 36.7 | 133 | 20.7 | 662 | 30.1 | 104 | 17.6 | |
| Unknown | 151 | 1.6 | 84 | 1.4 | 13 | 2.0 | 45 | 2.0 | 9 | 1.5 | <0.001 |
| Health insurance status, limited to patients diagnosed from 2001 to 2011 (n=4,406) | |||||||||||
| Private/military insurance | 3,113 | 70.7 | 1,908 | 78.2 | 199 | 61.6 | 697 | 55.5 | 309 | 79.4 | |
| Public insurance | 881 | 20.0 | 335 | 13.7 | 97 | 30.0 | 391 | 31.2 | 58 | 14.9 | |
| No insurance | 200 | 4.5 | 75 | 3.1 | 16 | 5.0 | 103 | 8.2 | 6 | 1.5 | |
| Unknown | 212 | 4.8 | 121 | 5.0 | 11 | 3.4 | 64 | 5.1 | 16 | 4.1 | <0.001 |
| Vital status | |||||||||||
| Alive | 8,108 | 86.7 | 5,148 | 87.0 | 518 | 80.6 | 1,910 | 86.8 | 532 | 90.0 | |
| Death from HL | 678 | 7.2 | 388 | 6.6 | 72 | 11.2 | 183 | 8.3 | 35 | 5.9 | |
| Death from non-Hodgkin lymphoma | 113 | 1.2 | 64 | 1.1 | 14 | 2.2 | 29 | 1.3 | 6 | 1.0 | |
| Death from other cancer | 108 | 1.2 | 80 | 1.4 | 11 | 1.7 | 12 | 0.5 | 5 | 0.8 | |
| Death from heart/cerebrovascular | 102 | 1.1 | 75 | 1.3 | 5 | 0.8 | 17 | 0.8 | 5 | 0.8 | |
| Death from other cause | 188 | 2.0 | 127 | 2.1 | 18 | 2.8 | 35 | 1.6 | 8 | 1.4 | |
| Death from unknown cause | 56 | 0.6 | 37 | 0.6 | 5 | 0.8 | 14 | 0.6 | 0 | 0 | <0.001 |
Data on type of subsequent cancer are presented for all patients only in order to protect confidentiality, as many cancer types included <5 adolescent and young adults when presented by race/ethnicity.
In multivariable analyses, worse HL-specific survival was associated with male sex (borderline significance), earlier year of diagnosis, and presence of B symptoms (Table 2). Black AYAs experienced a 62% higher risk of HL death (hereafter referred to as mortality) (HR: 1.62; 95% CI: 1.24, 2.11) and Hispanics experienced a 35% higher risk of HL mortality (HR: 1.35; 95% CI: 1.12, 1.64) than Whites. In addition, AYAs residing in lower SES neighborhoods experienced a 52% to 77% greater risk of HL mortality than those residing in the highest SES neighborhood categories. Worse overall survival (Table 2) was associated with many of the same factors as HL-specific survival, except that earlier age at diagnosis was associated with better all-cause survival.
Table 2.
Multivariable hazard ratios (HR) and 95% confidence interval (95% CI) estimates for death from all causes (overall survival) and death from Hodgkin lymphoma (HL-specific survival) in adolescent and young adult HL patients, California, 1988–2011.
| Characteristic | Overall Survival* | HL-Specific Survival* | ||||
|---|---|---|---|---|---|---|
| Deaths | HR | (95% CI) | Deaths | HR | (95% CI) | |
| Sex | ||||||
| Female | 516 | Reference | 290 | Reference | ||
| Male | 729 | 1.31 | (1.16, 1.47) | 388 | 1.16 | (0.99, 1.35) |
| Age at diagnosis (years) | ||||||
| 15–19 | 152 | 0.59 | (0.48, 0.74) | 108 | 0.87 | (0.65, 1.16) |
| 20–24 | 273 | 0.72 | (0.60, 0.86) | 155 | 0.85 | (0.66, 1.10) |
| 25–29 | 281 | 0.72 | (0.61, 0.86) | 153 | 0.87 | (0.68, 1.12) |
| 30–34 | 292 | 0.93 | (0.78, 1.10) | 152 | 1.09 | (0.85, 1.39) |
| 35–39 | 247 | Reference | 110 | Reference | ||
| Year of diagnosis | ||||||
| 1988–1992 | 520 | 2.42 | (1.88, 3.12) | 246 | 2.79 | (2.05, 3.80) |
| 1993–1997 | 317 | 1.83 | (1.43, 2.34) | 160 | 1.73 | (1.28, 2.33) |
| 1998–2002 | 187 | 1.27 | (0.98, 1.65) | 118 | 1.29 | (0.95, 1.77) |
| 2003–2006 | 129 | 1.15 | (0.88, 1.51) | 88 | 1.15 | (0.83, 1.59) |
| 2007–2011 | 92 | Reference | 66 | Reference | ||
| Marital status at diagnosis | ||||||
| Married | 453 | Reference | 223 | Reference | ||
| Not married | 764 | 1.11 | (0.98, 1.26) | 434 | 1.14 | (0.95, 1.36) |
| Unknown | 28 | 0.95 | (0.64, 1.41) | 21 | 1.30 | (0.82, 2.07) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 771 | Reference | 388 | Reference | ||
| Black | 125 | 1.40 | (1.14, 1.71) | 72 | 1.62 | (1.24, 2.11) |
| Hispanic | 290 | 1.16 | (1.00, 1.34) | 183 | 1.35 | (1.12, 1.64) |
| Asian/Pacific Islander | 59 | 1.11 | (0.85, 1.46) | 35 | 1.21 | (0.85, 1.72) |
| B Symptoms | ||||||
| No | 277 | Reference | 138 | Reference | ||
| Yes | 499 | 1.56 | (1.34, 1.81) | 316 | 1.87 | (1.52, 2.29) |
| Missing/unknown | 469 | 1.26 | (1.07, 1.49) | 224 | 1.42 | (1.12, 1.80) |
| Histologic subtype | ||||||
| Nodular sclerosis | 907 | Reference | 514 | Reference | ||
| Mixed cellularity | 153 | 0.98 | (0.82, 1.17) | 75 | 0.86 | (0.67, 1.10) |
| Lymphocyte depletion | 21 | 1.34 | (0.86, 2.07) | 14 | 1.41 | (0.82, 2.41) |
| Lymphocyte rich | 25 | 0.88 | (0.58, 1.33) | 6 | 0.48 | (0.21, 1.08) |
| Not otherwise specified | 139 | 1.30 | (1.08, 1.57) | 69 | 1.06 | (0.82, 1.37) |
| Received care at an NCI-designated cancer center | ||||||
| No/Missing | 1,114 | Reference | 606 | Reference | ||
| Yes | 131 | 0.99 | (0.83, 1.20) | 72 | 0.98 | (0.76, 1.25) |
| Neighborhood socioeconomic status, quintiles | ||||||
| 1 (lowest) | 243 | 1.88 | (1.53, 2.30) | 137 | 1.77 | (1.34, 2.33) |
| 2 | 278 | 1.53 | (1.26, 1.85) | 154 | 1.52 | (1.17, 1.97) |
| 3 | 278 | 1.44 | (1.20, 1.74) | 157 | 1.54 | (1.20, 1.99) |
| 4 | 250 | 1.16 | (0.96, 1.41) | 130 | 1.17 | (0.90, 1.51) |
| 5 (highest) | 196 | Reference | 100 | Reference | ||
| Urbanization level | ||||||
| Metropolitan | 809 | Reference | 436 | Reference | ||
| Non-metropolitan | 412 | 1.04 | (0.91, 1.17) | 225 | 1.08 | (0.91, 1.27) |
| Unknown | 24 | 1.50 | (0.99, 2.27) | 17 | 1.90 | (1.15, 3.13) |
| Health insurance status, limited to patients diagnosed from 2001 to 2011 (n=4,406)* | ||||||
| Private/military insurance | 160 | Reference | 103 | Reference | ||
| Public insurance/ no insurance | 125 | 2.05 | (1.58, 2.66) | 86 | 2.08 | (1.52, 2.84) |
| Unknown | 13 | 1.25 | (0.70, 2.24) | 9 | 1.25 | (0.62, 2.51) |
Stratified by stage at diagnosis, combined modality therapy, and subsequent cancer; adjusted for all variables in the table
AYAs with public or no insurance experienced much worse HL-specific survival (HR: 2.08; 95% CI: 1.52, 2.84) than those with private or military insurance (Table 2). The addition of health insurance to the multivariable models attenuated the hazard ratios for race/ethnicity by less than 9%, but attenuated the hazard ratios for neighborhood SES by up to 22%. Nevertheless, the hazard ratio for HL-specific survival comparing the highest neighborhood SES to the lowest was still evident, although of borderline significance (HR: 1.67; 95% CI: 0.97, 2.86; data not shown in tables).
In separate models for stage at diagnosis (Table 3), Blacks with early- and late-stage disease experienced a 68% greater risk of HL mortality, while Hispanics with late-stage, but not early-stage, disease experienced a greater risk of HL mortality (HR: 1.58; 95% CI: 1.22, 2.04). In addition, the association between lower neighborhood SES and HL-specific survival only was apparent among AYAs diagnosed with early-stage disease (HR: 2.06; 95% CI: 1.59, 2.68). AYAs with early or late-stage disease experienced greater than a two-fold increased risk of HL mortality if they had public or no insurance. Worse overall survival by stage at diagnosis (Table 3) was associated with many of the same factors as HL-specific survival.
Table 3.
Multivariable adjusted* hazard ratios (HR) and 95% confidence interval (95% CI) estimates for death from all causes (overall survival) and death from Hodgkin lymphoma (HL-specific survival) in adolescent and young HL patients, by stage at diagnosis, California, 1988–2011.
| Characteristic | Stage I/II | Stage III/IV | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall Survival* | HL-Specific Survival* |
Overall Survival* | HL-Specific Survival* |
|||||
| Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | |
| Sex | ||||||||
| Female | 277 | Reference | 142 | Reference | 208 | Reference | 134 | Reference |
| Male | 318 | 1.33 (1.12, 1.57) | 150 | 1.19 (0.94, 1.50) | 368 | 1.28 (1.07, 1.52) | 221 | 1.13 (0.90, 1.40) |
| Age at diagnosis (years) | ||||||||
| 15–19 | 78 | 0.67 (0.49, 0.91) | 55 | 1.15 (0.74, 1.81) | 67 | 0.55 (0.40, 0.75) | 48 | 0.68 (0.46, 1.01) |
| 20–24 | 132 | 0.76 (0.58, 0.99) | 64 | 0.91 (0.60, 1.39) | 128 | 0.72 (0.55, 0.93) | 86 | 0.87 (0.62, 1.21) |
| 25–29 | 132 | 0.73 (0.57, 0.95) | 62 | 0.93 (0.62, 1.41) | 126 | 0.72 (0.56, 0.92) | 85 | 0.89 (0.64, 1.23) |
| 30–34 | 147 | 0.94 (0.73, 1.21) | 74 | 1.32 (0.89, 1.97) | 130 | 0.92 (0.72, 1.17) | 69 | 0.91 (0.65, 1.28) |
| 35–39 | 106 | Reference | 37 | Reference | 125 | Reference | 67 | Reference |
| Year of diagnosis | ||||||||
| 1988–1992 | 250 | 2.49 (1.70, 3.67) | 98 | 2.87 (1.79, 4.60) | 239 | 2.35 (1.64, 3.37) | 135 | 2.64 (1.72, 4.05) |
| 1993–1997 | 160 | 1.86 (1.28, 2.69) | 72 | 1.62 (1.03, 2.55) | 130 | 1.68 (1.18, 2.39) | 75 | 1.65 (1.08, 2.51) |
| 1998–2002 | 89 | 1.24 (0.84, 1.83) | 57 | 1.28 (0.80, 2.03) | 92 | 1.42 (0.99, 2.04) | 60 | 1.43 (0.93, 2.20) |
| 2003–2006 | 56 | 1.09 (0.72, 1.64) | 36 | 1.02 (0.62, 1.69) | 67 | 1.27 (0.87, 1.85) | 50 | 1.33 (0.85, 2.07) |
| 2007–2011 | 40 | Reference | 29 | Reference | 48 | Reference | 35 | Reference |
| Marital status at diagnosis | ||||||||
| Married | 221 | Reference | 92 | Reference | 210 | Reference | 122 | Reference |
| Not married | 364 | 1.16 (0.97, 1.40) | 193 | 1.31 (0.99, 1.72) | 358 | 1.02 (0.85, 1.23) | 227 | 1.01 (0.80, 1.29) |
| Unknown | 10 | 1.07 (0.57, 2.04) | 7 | 1.57 (0.72, 3.42) | 8 | 0.56 (0.27, 1.14) | 6 | 0.68 (0.29, 1.57) |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 391 | Reference | 177 | Reference | 335 | Reference | 192 | Reference |
| Black | 56 | 1.39 (1.04, 1.86) | 31 | 1.68 (1.14, 2.49) | 62 | 1.55 (1.17, 2.06) | 38 | 1.68 (1.17, 2.41) |
| Hispanic | 120 | 1.04 (0.84, 1.29) | 68 | 1.10 (0.82, 1.48) | 148 | 1.31 (1.07, 1.62) | 106 | 1.58 (1.22, 2.04) |
| Asian/Pacific Islander | 28 | 1.09 (0.74, 1.61) | 16 | 1.22 (0.72, 2.05) | 31 | 1.19 (0.82, 1.74) | 19 | 1.22 (0.76, 1.97) |
| B Symptoms | ||||||||
| No | 183 | Reference | 90 | Reference | 90 | Reference | 48 | Reference |
| Yes | 208 | 1.66 (1.35, 2.03) | 121 | 1.89 (1.44, 2.50) | 282 | 1.54 (1.21, 1.97) | 191 | 1.95 (1.42, 2.69) |
| Missing/unknown | 204 | 1.08 (0.86, 1.35) | 81 | 1.07 (0.76, 1.50) | 204 | 1.50 (1.14, 1.97) | 116 | 1.83 (1.27, 2.65) |
| Histologic subtype | ||||||||
| Nodular sclerosis | 472 | Reference | 242 | Reference | 390 | Reference | 252 | Reference |
| Mixed cellularity | 56 | 0.83 (0.63, 1.10) | 24 | 0.76 (0.50, 1.17) | 89 | 1.07 (0.84, 1.35) | 47 | 0.87 (0.63, 1.20) |
| Lymphocyte depletion | 6 | 1.23 (0.55, 2.77) | <5 | ~ | 13 | 1.40 (0.80, 2.45) | 10 | 1.56 (0.82, 2.98) |
| Lymphocyte rich | 17 | 0.92 (0.55, 1.51) | <5 | ~ | 8 | 1.07 (0.53, 2.18) | <5 | ~ |
| Not otherwise specified | 44 | 1.06 (0.77, 1.45) | 20 | 0.87 (0.55, 1.38) | 76 | 1.47 (1.14, 1.89) | 43 | 1.19 (0.85, 1.66) |
| Received care at an NCI-designated cancer center | ||||||||
| No/Missing | 536 | Reference | 265 | Reference | 511 | Reference | 312 | Reference |
| Yes | 59 | 1.02 (0.78, 1.35) | 27 | 0.94 (0.63, 1.41) | 65 | 0.94 (0.72, 1.22) | 43 | 1.00 (0.73, 1.39) |
| Neighborhood socioeconomic status | ||||||||
| Low (Quintiles 1–3) | 389 | 1.77 (1.48, 2.11) | 204 | 2.06 (1.59, 2.68) | 366 | 1.20 (1.00, 1.44) | 228 | 1.15 (0.92, 1.45) |
| High (Quintile 4–5) | 206 | Reference | 88 | Reference | 210 | Reference | 127 | Reference |
| Urbanization level | ||||||||
| Metropolitan | 374 | Reference | 179 | Reference | 385 | Reference | 238 | Reference |
| Non-metropolitan | 212 | 1.10 (0.93, 1.31) | 106 | 1.12 (0.87, 1.44) | 178 | 1.04 (0.86, 1.24) | 108 | 1.06 (0.84, 1.34) |
| Unknown | 9 | 1.32 (0.68, 2.59) | 7 | 1.97 (0.91, 4.25) | 13 | 2.01 (1.14, 3.55) | 9 | 2.27 (1.14, 4.52) |
| Health insurance status, limited to patients diagnosed from 2001 to 2011 (n=4,406)* | ||||||||
| Private/military insurance | 78 | Reference | 50 | Reference | 75 | Reference | 50 | Reference |
| Public insurance/ no insurance | 53 | 2.19 (1.49, 3.21) | 37 | 2.07 (1.30, 3.30) | 69 | 2.09 (1.46, 2.99) | 49 | 2.16 (1.41, 3.32) |
| Unknown | 5 | 1.05 (0.42, 2.64) | <5 | ~ | 6 | 1.36 (0.58, 3.20) | 5 | 1.63 (0.63, 4.21) |
Stratified by combined modality therapy and subsequent cancer; adjusted for all variables in the table
Data not shown
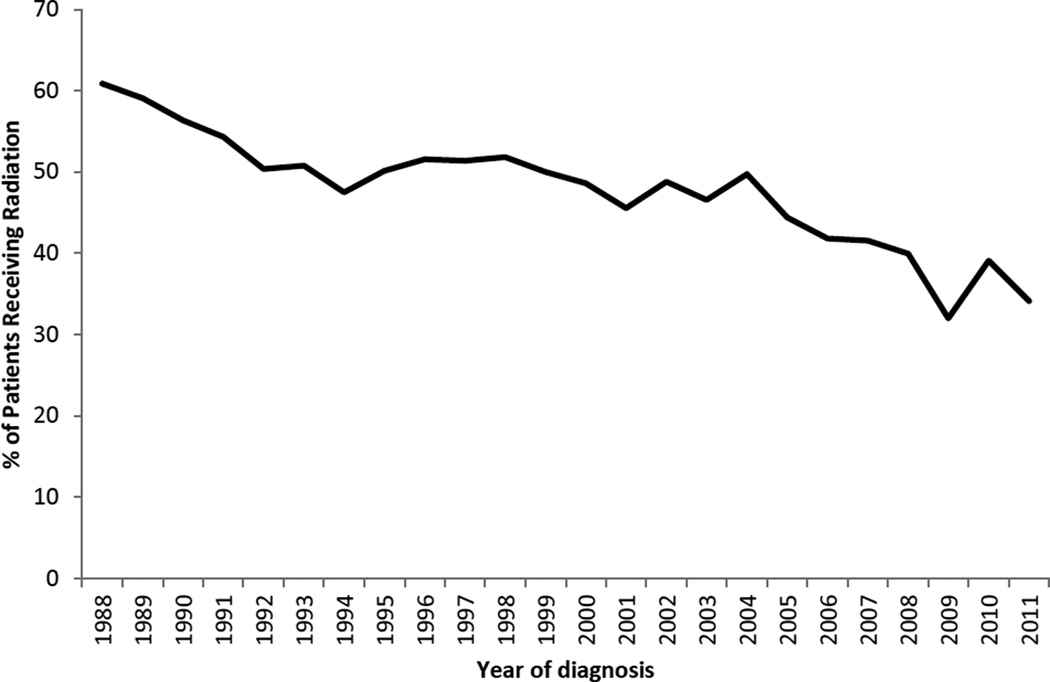
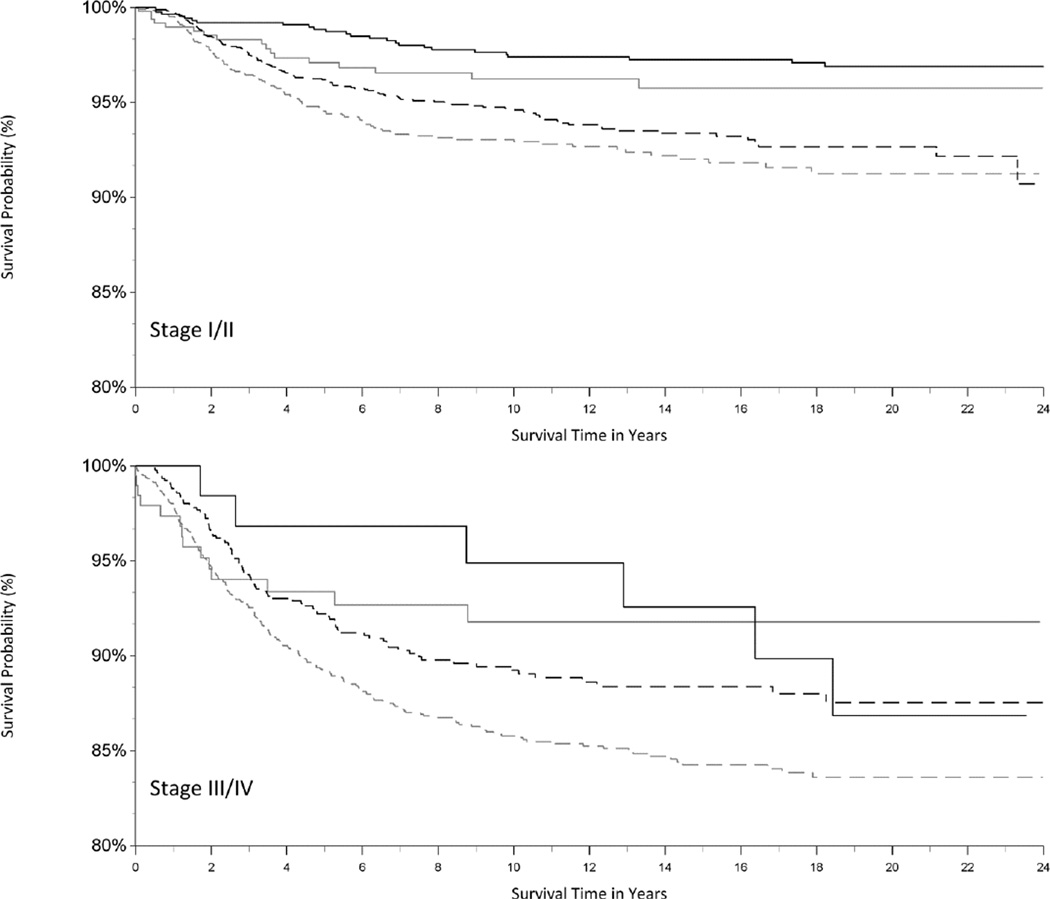
The use of radiation in HL treatment decreased over time (Figure 1). To compare our results to those in prior studies (7, 12), we also examined the impact of initial combined-modality therapy on survival as an adjustment variable (rather than a stratifying variable due the violation of the proportional hazards assumption). We found that initial treatment with radiation was associated with better HL-specific survival (HR for chemotherapy and radiation (vs. chemotherapy only): 0.85; 95% CI: 0.71, 1.02; HR for radiation only (vs. chemotherapy only): 0.34; 95% CI: 0.23, 0.51; data not shown in tables). These associations were similar for early- and late-stage disease (Figure 2).
Figure 1.
Percentage (%) of adolescent and young adult Hodgkin lymphoma patients undergoing radiation therapy by year of diagnosis, California, 1988–2011.
Figure 2.
Kaplan-Meier curve of Hodgkin lymphoma (HL)-specific survival in adolescent and young adult patients, by combined-modality therapy and stage of disease (stage I/II, stage III/IV), California, 1988–2011. The vertical axis represents survival probability; the horizontal axis represents survival time in years. Combined-modality therapy (dotted black line), radiation therapy only (solid black line), chemotherapy only (dotted grey line), no or unknown therapy (solid grey line).
Discussion
In this population-based study of more than 9,000 AYAs diagnosed with HL over a 24-year period, we observed improvements in survival over time, but also striking disparities in survival by race/ethnicity, neighborhood SES and health insurance for a subset of more recently diagnosed patients. In particular, Blacks (regardless of disease stage) and Hispanics with late-stage disease experienced worse survival than Whites. In addition, AYAs diagnosed with early-stage disease experienced worse survival if they also resided in lower SES neighborhoods. Furthermore, more recently diagnosed AYAs with public health insurance or no insurance experienced greater than a two-fold increased risk of HL mortality. Together, these findings identify vulnerable subgroups of HL patients at higher risk for HL mortality and point to disparities in treatment delivery and follow-up care as likely contributing factors.
Our previous work in outcomes for young adult HL patients suggested that survival disparities by race/ethnicity or neighborhood SES may be due to variations in initial treatment and management (4). Consistent with studies that found initial treatment to vary by race/ethnicity (4, 7), neighborhood SES (7), and health insurance (12), we also observed that Blacks and Hispanics, AYAs residing in lower SES neighborhoods, and AYAs with public or no insurance were more likely to receive chemotherapy alone as initial treatment. Our group and others (7, 12) also found that these disparities in treatment were associated with worse survival. Despite the survival benefits of radiation, this therapy has been associated with subsequent primary malignancies (6, 7, 21), an outcome that we were able to consider in our analyses. Specifically, we found that a somewhat higher proportion of patients who received radiation for their HL had subsequent cancer (4.9% versus 6.9%), which was associated with worse overall survival (data not shown); modern treatments with lower doses of radiation (7) may result in fewer subsequent cancers (21) and should continue to be monitored. However, even after controlling for initial treatment and subsequent cancers, we still observed differences in survival by race/ethnicity, neighborhood SES and health insurance, suggesting that other factors are influencing these associations.
Expanding on a prior study that found AYA HL patients who were uninsured or had public health insurance were more likely to be diagnosed with advanced-stage HL (11), we found that AYA HL patients with these types of insurance experienced worse HL-specific survival, whether they had early- or late-stage disease. In studies of AYA cancer survivors, health insurance rates have been found to decrease with time from diagnosis (22, 23), particularly for older AYAs and those with less education (23), and a lack of health insurance is a barrier to receiving any medical care (22, 24). Further, more than two-thirds of uninsured survivors have been found to have no personal provider or routine medical care and, even with health insurance, AYA cancer survivors are more likely to forgo medical care due to costs (24). Collectively, these results suggest that health insurance is a critical barrier to receiving cancer-related medical care that can impact prognosis. While the implementation of the measures from the Patient Protection and Affordable Care Act (ACA) of 2010 and 2014 (25, 26) should improve AYA cancer survivors’ access to health insurance, studies should continue to monitor barriers to health insurance enrollment and medical care in AYAs.
Our findings of worse survival among Blacks and Hispanics and AYAs with HL residing in lower SES neighborhoods are consistent with those of prior studies (4, 7). We observed some differences by stage at diagnosis: the impact of neighborhood SES was stronger for early-stage disease and Hispanics with late-stage disease experienced worse HL survival. Although we did not have health insurance information for AYAs throughout our study period, it is likely that factors beyond health insurance are influencing these race/ethnicity and neighborhood SES associations. Inadequate long-term follow-up in patients could result in a delay in diagnosing and treating complications (27), particularly for AYAs, who tend to lack knowledge about their higher risk for developing complications (28–30). Financial concerns, including lost wages, copayments, high deductibles, childcare and transportation costs (24, 31–33) can be burdensome and influence follow-up care, particularly for AYAs with financial limitations (e.g., debt from college or starting a career). In addition, if poor health behaviors and comorbid conditions are more prevalent in lower-SES and/or non-White HL patients, as found for patients with other cancers (34–38), these factors, too, could increase post-treatment complications and reduce survival.
Our study is subject to some limitations. We considered the first course of treatment, but lacked details (e.g., dosing) on chemotherapy, radiation and other treatment received after this period, and there is the potential for radiation (39, 40) and chemotherapy (40) to be under-ascertained; therefore, our findings could be subject to residual confounding from incomplete treatment data (41). While it is possible that our findings are partially influenced by indication bias (i.e., patients who received radiation alone had more favorable disease characteristics and thus more favorable outcomes), it is also possible that some patients received radiation alone because they had comorbidities that precluded use of chemotherapy. Given that combined-modality therapy was broadly recommended by guidelines across all stages of HL during the study period (2, 3), the most plausible explanation for improved survival among patients who received radiation alone is under-ascertainment of chemotherapy, particularly for patients with advanced-stage disease. We were unable to consider health insurance at diagnosis for patients diagnosed before 2001, and lacked information on changes to health insurance after initial treatment, treatment adherence or quality of care--factors that can influence subsequent care and outcomes. We also were unable to determine if patients were undocumented and ineligible for public health insurance. Because cancer registry data do not include potentially relevant clinical data such as prognostic serum measures (42), International Prognostic Index, or prognostic tumor characteristics (e.g., presence of Epstein-Barr virus in tumor cells (5)), or information on lifestyle behaviors or comorbid conditions, our analyses could not examine the impact of these factors on survival. Our study also lacked individual-level measures of SES to consider separately or with our neighborhood measure. While neighborhood and individual SES are associated, neighborhood SES has been found to underestimate associations observed with individual-level SES (43). However, our multifaceted measure of neighborhood SES at the block group-level incorporated several domains of education, income, employment, and cost of living that capture various elements of the socioeconomic environment that may augment individual-level SES. Our study may also be subject to potential misclassification of race/ethnicity, although we previously have detected excellent overall agreement with self-reported race/ethnicity for Whites and Blacks, and good agreement for Hispanics and Asians (44, 45).
Despite these limitations, this study was population-based and included a large diverse population of AYA HL cancer patients who received their care across all types of institutions, increasing the generalizability of these findings. In addition, our study is one of the first to consider the influence of initial combined-modality treatment and health insurance on previously noted racial/ethnic and socioeconomic disparities in survival among AYAs diagnosed with early-and late-stage HL.
This study found that AYA HL patients of Black or Hispanic race/ethnicity, those who resided in lower SES neighborhoods, and those who were uninsured or publicly insured experienced worse HL-specific survival. While prior studies have noted these racial/ethnic and SES survival disparities, our study extends these previous efforts by considering combined-modality treatment, subsequent primary cancers and health insurance. With uninsurance rates historically peaking in adolescence and young adulthood (46), AYA HL patients may be particularly vulnerable to failing to receive cancer survivor-focused medical care. The ACA has the potential to influence both access to insurance and use of necessary health care for AYAs and should be evaluated in future studies. In addition, identifying and reducing barriers to recommended treatment and surveillance in these AYAs at higher risk of mortality is essential to ameliorating these survival disparities.
Acknowledgments
Financial support: This work was supported by the Stanford Cancer Institute (T.H.M. Keegan), the Cancer Prevention Institute of California (S.L. Glaser), the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California (M.C. DeRouen, C.A. Clarke, D. Goldberg), and a National Cancer Institute Career Development Award (H.M. Parsons, K07CA175063). The funders did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
C.A. Clarke received research funding from Genentech unrelated to the submitted work.
Footnotes
Conflicts of interest: There are no other financial disclosures or conflicts of interest to report.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosery CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. http://seer.cancer.gov/csr/1975_2012/ based on November 2014 SEER data submission, posted to the SEER web site, April 2015 ed. [Google Scholar]
- 2.Das P, Ng A, Constine LS, Advani R, Flowers C, Friedberg J, et al. ACR Appropriateness Criteria(R) on Hodgkin's lymphoma-unfavorable clinical stage I and II. J Am Coll Radiol. 2011;8:302–308. doi: 10.1016/j.jacr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Terezakis SA, Metzger ML, Hodgson DC, Schwartz CL, Advani R, Flowers CR, et al. ACR Appropriateness Criteria Pediatric Hodgkin Lymphoma. Pediatr Blood Cancer. 2014;61:1305–1312. doi: 10.1002/pbc.24983. [DOI] [PubMed] [Google Scholar]
- 4.Keegan TH, Clarke CA, Chang ET, Shema SJ, Glaser SL. Disparities in survival after Hodgkin lymphoma: a population-based study. Cancer Causes Control. 2009;20:1881–1892. doi: 10.1007/s10552-009-9382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keegan TH, Glaser SL, Clarke CA, Gulley ML, Craig FE, Digiuseppe JA, et al. Epstein-Barr virus as a marker of survival after Hodgkin's lymphoma: a population-based study. J Clin Oncol. 2005;23:7604–7613. doi: 10.1200/JCO.2005.02.6310. Epub 2005 Sep 26. [DOI] [PubMed] [Google Scholar]
- 6.Jachimowicz RD, Engert A. The challenging aspects of managing adolescents and young adults with Hodgkin's lymphoma. Acta Haematol. 2014;132:274–278. doi: 10.1159/000360205. [DOI] [PubMed] [Google Scholar]
- 7.Xavier AC, Costa LJ. Changes in the use of radiation therapy for early classical Hodgkin lymphoma in adolescents and young adults: implications for survival and second malignancies. Leuk Lymphoma. 2015;56:2339–2343. doi: 10.3109/10428194.2014.983097. [DOI] [PubMed] [Google Scholar]
- 8.Aizer AA, Falit B, Mendu ML, Chen MH, Choueiri TK, Hoffman KE, et al. Cancer-specific outcomes among young adults without health insurance. J Clin Oncol. 2014;32:2025–2030. doi: 10.1200/JCO.2013.54.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg AR, Kroon L, Chen L, Li CI, Jones B. Insurance status and risk of cancer mortality among adolescents and young adults. Cancer. 2015;121:1279–1286. doi: 10.1002/cncr.29187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins AS, Lerro CC, Barr RD. Insurance status and distant-stage disease at diagnosis among adolescent and young adult patients with cancer aged 15 to 39 years: National Cancer Data Base, 2004 through 2010. Cancer. 2014;120:1212–1219. doi: 10.1002/cncr.28568. [DOI] [PubMed] [Google Scholar]
- 11.Smith EC, Ziogas A, Anton-Culver H. Association between insurance and socioeconomic status and risk of advanced stage Hodgkin lymphoma in adolescents and young adults. Cancer. 2012;118:6179–6187. doi: 10.1002/cncr.27684. [DOI] [PubMed] [Google Scholar]
- 12.Olszewski AJ, Shrestha R, Castillo JJ. Treatment selection and outcomes in early-stage classical hodgkin lymphoma: analysis of the national cancer data base. J Clin Oncol. 2015;33:625–633. doi: 10.1200/JCO.2014.58.7543. [DOI] [PubMed] [Google Scholar]
- 13.Fritz F, Percy C, Jack A, Shanmugaratnan K, Sobin L, Parkin DM, et al., editors. International Classification onf Diseases for Oncology. Third. Geneva: World Health Organization; 2000. [Google Scholar]
- 14.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds P, Hurley SE, Quach AT, Rosen H, Von Behren J, Hertz A, et al. Regional variations in breast cancer incidence among California women, 1988–1997. Cancer Causes Control. 2005;16:139–150. doi: 10.1007/s10552-004-2616-5. [DOI] [PubMed] [Google Scholar]
- 16.Urayama KY, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA. Factors associated with residential mobility in children with leukemia: implications for assigning exposures. Ann Epidemiol. 2009;19:834–840. doi: 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke CA, Glaser SL. Population-based surveillance of HIV-associated cancers: utility of cancer registry data. J Acquir Immune Defic Syndr. 2004;36:1083–1091. doi: 10.1097/00126334-200408150-00012. [DOI] [PubMed] [Google Scholar]
- 18.Vaccher E, Spina M, Tirelli U. Clinical aspects and management of Hodgkin's disease and other tumours in HIV-infected individuals. Eur J Cancer. 2001;37:1306–1315. doi: 10.1016/s0959-8049(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 19.Glaser SL, Clarke CA, Gulley ML, Craig FD, DiGiuseppe JA, Dorfman RF, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 20.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 21.LeMieux MH, Solanki AA, Mahmood U, Chmura SJ, Koshy M. Risk of second malignancies in patients with early-stage classical Hodgkin's lymphoma treated in a modern era. Cancer Med. 2015;4:513–518. doi: 10.1002/cam4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keegan TH, Tao L, DeRouen MC, Wu XC, Prasad P, Lynch CF, et al. Medical care in adolescents and young adult cancer survivors: what are the biggest access-related barriers? J Cancer Surviv. 2014;8:282–292. doi: 10.1007/s11764-013-0332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons HM, Schmidt S, Harlan LC, Kent EE, Lynch CF, Smith AW, et al. Young and uninsured: Insurance patterns of recently diagnosed adolescent and young adult cancer survivors in the AYA HOPE study. Cancer. 2014;120:2352–2360. doi: 10.1002/cncr.28685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchhoff AC, Lyles CR, Fluchel M, Wright J, Leisenring W. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012;118:5964–5972. doi: 10.1002/cncr.27537. [DOI] [PubMed] [Google Scholar]
- 25.Moy B, Polite BN, Halpern MT, Stranne SK, Winer EP, Wollins DS, et al. American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011;29:3816–3824. doi: 10.1200/JCO.2011.35.8903. [DOI] [PubMed] [Google Scholar]
- 26.Wolfson J, Ruccione K, Reaman GH. Health care reform 2010: expected favorable impact on childhood cancer patients and survivors. Cancer J. 2010;16:554–562. doi: 10.1097/PPO.0b013e3181feee83. [DOI] [PubMed] [Google Scholar]
- 27.Soares A, Biasoli I, Scheliga A, Luiz RR, Costa MA, Land M, et al. Socioeconomic inequality and short-term outcome in Hodgkin's lymphoma. Int J Cancer. 2007;120:875–879. doi: 10.1002/ijc.22417. [DOI] [PubMed] [Google Scholar]
- 28.Green DM. Late effects of treatment for cancer during childhood and adolescence. Curr Probl Cancer. 2003;27:127–142. doi: 10.1016/s0147-0272(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 29.Zeltzer LK, Lu Q, Leisenring W, Tsao JC, Recklitis C, Armstrong G, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2008;17:435–446. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- 30.Casillas J, Kahn KL, Doose M, Landier W, Bhatia S, Hernandez J, et al. Transitioning childhood cancer survivors to adult-centered healthcare: insights from parents, adolescent, and young adult survivors. Psychooncology. 2010;19:982–990. doi: 10.1002/pon.1650. [DOI] [PubMed] [Google Scholar]
- 31.Brown ML, Yabroff KR. Economic impact of cancer in the United States. In: Schottenfeld DFJJ, editor. Cancer Epidemiology and Prevention. 3rd. New York, NY: Oxford Univ; 2006. pp. 202–214. [Google Scholar]
- 32.Gruber J, Perry I. Will the Affordable Care Act make health insurance affordable? Issue Brief (Commonw Fund) 2011;2:1–15. [PubMed] [Google Scholar]
- 33.Nicholson JL, Collins SR. Young, uninsured, and seeking change: health coverage of young adults and their views on health reform. Findings from the Commonwealth fund Survey of Young Adults (2009) Issue Brief (Commonw Fund) 2009;73:1–22. [PubMed] [Google Scholar]
- 34.Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32:250–256. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 35.Ahluwalia IB, Mack KA, Murphy W, Mokdad AH, Bales VS. State-specific prevalence of selected chronic disease-related characteristics--Behavioral Risk Factor Surveillance System, 2001. MMWR Surveill Summ. 2003;52:1–80. [PubMed] [Google Scholar]
- 36.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. Jama. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 37.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. Epub 2005 Sep 2. [DOI] [PubMed] [Google Scholar]
- 38.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29:4045–4053. doi: 10.1200/JCO.2011.36.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118:333–341. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care. 2014 doi: 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J. Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 43.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–187. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 45.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 46.Adams SH, Newacheck PW, Park MJ, Brindis CD, Irwin CE., Jr Health insurance across vulnerable ages: patterns and disparities from adolescence to the early 30s. Pediatrics. 2007;119:e1033–e1039. doi: 10.1542/peds.2006-1730. [DOI] [PubMed] [Google Scholar]