Abstract
Objective
Listeners with sensorineural hearing loss (SNHL) typically experience reduced speech perception, which is not completely restored with amplification. This likely occurs because cochlear damage, in addition to elevating audiometric thresholds, alters the neural representation of speech transmitted to higher centers along the auditory neuroaxis. While the deleterious effects of SNHL on speech perception in humans have been well-documented using behavioral paradigms, our understanding of the neural correlates underlying these perceptual deficits remains limited. Using the scalp-recorded Frequency Following Response (FFR), the authors examine the effects of SNHL and aging on subcortical neural representation of acoustic features important for pitch and speech perception, namely the periodicity envelope (F0) and temporal fine structure (TFS) (formant structure), as reflected in the phase-locked neural activity generating the FFR.
Design
FFRs were obtained from 10 listeners with normal hearing (NH) and 9 listeners with mild-moderate SNHL in response to a steady-state English back vowel /u/ presented at multiple intensity levels. Use of multiple presentation levels facilitated comparisons at equal sound pressure level (SPL) and equal sensation level (SL). In a second follow-up experiment to address the effect of age on envelope and TFS representation, FFRs were obtained from 25 NH and 19 listeners with mild to moderately-severe SNHL to the same vowel stimulus presented at 80 dB SPL. Temporal waveforms, Fast Fourier Transform (FFT) and spectrograms were used to evaluate the magnitude of the phase-locked activity at F0 (periodicity envelope) and F1 (TFS).
Results
Neural representation of both envelope (F0) and TFS (F1) at equal SPLs was stronger in NH listeners compared to listeners with SNHL. Also, comparison of neural representation of F0 and F1 across stimulus levels expressed in SPL and SL (accounting for audibility) revealed that level-related changes in F0 and F1 magnitude were different for listeners with SNHL compared to listeners with normal hearing. Further, the degradation in subcortical neural representation was observed to persist in listeners with SNHL even when the effects of age were controlled for.
Conclusions
Overall, our results suggest a relatively greater degradation in the neural representation of TFS compared to periodicity envelope in individuals with SNHL. This degraded neural representation of TFS in SNHL, as reflected in the brainstem FFR, may reflect a disruption in the temporal pattern of phase-locked neural activity arising from altered tonotopic maps and/or wider filters causing poor frequency selectivity in these listeners. Lastly, while preliminary results indicate that the deleterious effects of SNHL may be greater than age-related degradation in subcortical neural representation, the lack of a balanced age-matched control group in this study does not permit us to completely rule out the effects of age on subcortical neural representation.
Keywords: Neural phase-locking, envelope, temporal fine structure, sensorineural hearing loss
INTRODUCTION
Complex periodic stimuli, including speech, contain a temporal structure that is characterized by a slowly varying temporal feature (periodicity envelope) that is superimposed on a more rapidly varying temporal feature (temporal fine structure (TFS)). Behavioral studies have consistently demonstrated intact periodicity envelope processing in sensorineural hearing loss (SNHL), as reflected by equivalent gap detection thresholds and temporal modulation transfer functions (TMTFs) (Florentine & Buus 1984; Bacon & Viemeister 1985; Bacon & Gleitman 1992), in listeners with mild-moderate SNHL and individuals with normal hearing (NH). Physiologic studies at the auditory nerve level in cats and chinchillas with induced SNHL have demonstrated intact, or even enhanced, periodicity envelope encoding (Kale & Heinz 2010) accompanied by degraded TFS encoding (Miller et al. 1997; Wong et al. 1998; Henry & Heinz, 2012, 2013), as compared to the NH group. TFS encoding deficits in SNHL have been attributed to a degradation in neural phase-locking due to wider auditory filters consequent to hearing loss, as well as shifts in frequency-place mapping, which is evident in the downward shift of characteristic frequencies (CFs) in chinchillas with noise-induced hearing loss (NIHL). Thus, there appears to be an emerging consensus that the representation of TFS is appreciably degraded with little or no change in the representation of envelope cues in individuals with SNHL.
To date, knowledge about the nature of neural encoding of periodicity envelope and TFS in normal and impaired ears is largely derived from auditory nerve recordings in animals. The scalp recorded human Frequency Following Response (FFR) elicited by a complex sound reflects sustained phase-locked activity to both the periodicity envelope (referred to as simply “envelope” for the remainder of the paper) and TFS (Krishnan, 2002; Smalt et al. 2012; Anderson et al. 2013) among a population of neural elements in the rostral brainstem (Smith et al.1975; Glaser et al. 1976). Specifically, the phase-locked neural activity underlying FFR has been shown to preserve both the envelope and TFS information of a variety of steady-state and time-variant complex sounds, including two-tone approximations of vowels (Krishnan 1999); steady-state vowels (Krishnan 2002; Aiken & Picton 2008); complex tones with missing fundamentals (Hall, 1979; Greenberg et al. 1987; Krishnan & Plack 2011; Smalt et al. 2012), time-variant tonal sweeps (Krishnan & Parkinson, 2000); time-variant consonant-vowel (CV) syllables (Plyler & Ananthanarayan, 2001) and Mandarin tones (Krishnan et al. 2004, 2005). Thus the phase-locked neural activity underlying the FFR provides an effective physiologic window to examine the neural representation of these temporal cues in the human brainstem, and how they may be altered consequent to SNHL.
Of particular relevance to the current study are the two published FFR reports on neural representation of speech in NH and SNHL listeners (Plyler & Ananthanarayan, 2001; Anderson et al. 2013). Plyler and Ananthanarayan (2001) compared neural representation of time-varying TFS cues represented by formant transitions in CV syllables in listeners with NH and mild-moderate SNHL using the FFR. Their results showed that the phase-locked neural activity in the SNHL group, unlike the NH group, was not able to follow the formant transition presented in the CV syllables, suggesting degraded TFS representation due to disrupted neural phase-locking. Also, the authors noted no improvement in neural representation of the formant transition with increasing presentation level, suggesting that the observed degradation was not accounted for by audibility. More recently, Anderson and colleagues (2013) used the FFR elicited by a CV syllable, in quiet and in noise, to compare brainstem neural representation of envelope and TFS cues in listeners with NH and mild-moderate SNHL. Consistent with previous findings at the auditory nerve level, they observed an enhancement of the representation of the envelope of the CV syllable for the SNHL listeners relative to NH listeners. However, in contrast to both behavioral and neurophysiologic studies, Anderson and colleagues (2013) noted little or no degradation in the TFS representation in listeners with SNHL. The authors suggest that the absence of TFS degradation in their data may be due to their use of a single high presentation level, and the possibility of age-related effects, common to both groups, obscuring TFS degradation specific to SNHL.
Given the conflicting results from the two previous FFR reports, we examine here the level-dependent neural representation of envelope and TFS of steady-state speech stimuli in NH and SNHL listeners, as reflected in the scalp-recorded FFR. The use of multiple stimulus presentation levels will enable us to not only compare neural representations of envelope and TFS in the normal and impaired auditory systems at equal sound pressure levels (SPLs) and equal sensation levels (SLs) to address the issue of audibility, but will also allow us to evaluate if the changes in neural representation of the two cues with level are different for the two groups. Preliminary investigations will also be conducted on the effects of aging on neural representation of envelope and TFS in both normal hearing and SNHL. The primary motivation for the present study is to gain a better understanding of the nature of alterations in the neural representation of envelope and TFS cues consequent to SNHL. This knowledge may facilitate development of optimal signal processing strategies for hearing prosthetic devices in order to optimally restore neural representation of these cues.
MATERIALS AND METHODS
Participants
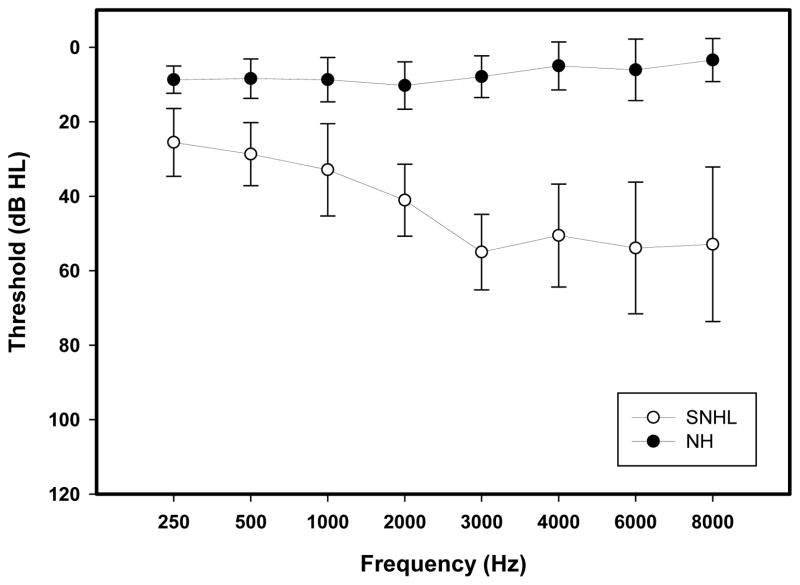
Ten adult listeners with normal hearing (male = 4, female = 6; age range = 21–32 years (mean = 24.55 years; SD = 3.35 years)) and nine listeners with mild to moderately-severe SNHL (male = 6, female= 3; age range = 21–71 years (mean = 50.66 years; SD = 17.80 years)) participated in the experiment examining the effects of stimulus intensity on neural representation of envelope and TFS. Also, FFR data obtained in response to a steady-state English back vowel /u/ at 80 dB SPL from twenty-five normal hearing listeners (male = 8, female = 17; age range: 21–55 years (mean = 27.72 years, S.D.= 9.33 years)) and nineteen listeners with mild to moderately-severe SNHL (male = 11, female = 8 age range: 21–89 years (mean = 54.26 years, S.D. = 19.40 years)) were evaluated in the follow-up experiment to address the role of age on envelope and TFS representation. All the subjects who took part in the primary experiment were also part of the follow-up experiment investigating the effects of aging on neural representation of envelope and TFS. Air and bone conduction thresholds at octave frequencies from 250–8000 Hz were at or better than 25 dB HL in NH listeners (average PTA (0.5, 1, 2 kHz): 9.1 dB HL) and ranged between 26–70 dB HL (average PTA (0.5, 1, 2 kHz): 32.59 dB HL, average PTA (2, 3, 4 kHz): 43.05 dB HL) in SNHL participants (Figure 1). All participants were paid and gave informed consent in compliance with a protocol approved by the Institutional Review Board at Purdue University.
Figure 1.
Average pure tone thresholds at audiometric test frequencies between 250 Hz–8000 Hz for the ear tested in NH (filled circles) and SNHL (open circles) listeners. Error bars represent standard errors.
Stimuli
FFRs were elicited by a synthetic steady state, English back vowel /u/ (as in WHO’D) with F0 = 120 Hz, F1= 360 Hz, F2 = 970 Hz, F3 = 2667 Hz, and F4 = 3007 Hz). This particular vowel was chosen because the first two formants occur well below 1500 Hz, the upper limit for neural phase-locking in the rostral brainstem. Also, this vowel has been previously used successfully to elicit robust FFRs in normal hearing listeners (Krishnan 2002). Stimulus duration was 265 milliseconds, including a 10 millisecond cosine-squared ramp used to minimize spectral splatter and onset responses. FFRs were recorded from each participant in response to monaural stimulation at multiple sound pressure levels (60–85 dB SPL in NH listeners and 70–95 dB SPL in HI listeners, in 5 dB steps) at a repetition rate of 2.76/s using an alternating onset polarity. The stimulus files were routed through a digital to analog module and presented through a magnetically shielded insert earphone (Etymotic, ER-3A). Stimuli were presented to the right ear in normal hearing participants and to the ear with mild-moderate SNHL in the participants with hearing loss. The presentation order of the stimuli was randomized both within and across participants. The 10 dB higher presentation levels for the SNHL group was necessary to elicit discernible FFRs in this group. Also, the range of levels used here allows us to compare responses at both equal SPLs and at equal SLs. Care was taken to ensure that the maximum presentation level used in either group was below the uncomfortable loudness level (UCL). All stimuli were generated and played out using an auditory evoked potential system (SmartEP, Intelligent Hearing Systems (IHS); Miami, FL, USA). Apart from the stimulus intensity level, which was fixed at 80 dB SPL, all stimulus and recording parameters were identical in the follow-up experiment addressing age-related effects.
FFR data acquisition
Participants reclined comfortably in an acoustically and electrically shielded booth. They were instructed to relax and refrain from extraneous body movements to minimize movement artifacts. Subjects were allowed to sleep through the two-hour data acquisition session. Almost all participants slept through the recording session and were awakened at the end of the session.
FFRs were recorded differentially using two electrode configurations: one between a non-inverting (positive) electrode placed on the midline of the forehead at the hairline (Fz) and linked inverting (reference) electrodes placed on the right (A2) and left mastoids (A1); and the other between the same non-inverting electrode on the forehead and an inverting electrode on the 7th cervical vertebra (C7). Another electrode placed on the mid-forehead (Fpz) served as the common ground. FFRs were recorded simultaneously from the two different electrode configurations, and subsequently averaged for each stimulus condition to yield a response with a higher signal-to-noise ratio (SNR) (Krishnan et al. 2009). All inter-electrode impedances were maintained below 1 kΩ. The EEG inputs were amplified by 200,000 and bandpass filtered from 50 to 3000 Hz (6 dB/octave roll-off, RC response characteristics). Each response waveform represents the average of 4000 stimulus presentations over a 300 millisecond analysis window. The experimental protocol took approximately 120 minutes to complete.
FFR data analysis
Extraction of the Envelope and TFS FFR
As noted under the stimulus section, FFRs were recorded using alternating onset polarity. Split buffer averaging employed in the IHS’ SmartEP data acquisition software stores averaged FFRs to condensation and rarefaction onset polarities in two different buffers. For each recording, the averaged FFRs in the split buffer were added to extract the envelope FFR, or subtracted to extract the FFR to the TFS. The neural response to the polarity-inverted stimulus has an envelope structure identical to that evoked by the original stimulus. However, the neural response to the fine structure is inverted when stimulus polarity is inverted as compared to that evoked in response to the original stimulus. Thus, addition of the FFRs to these two polarities yield FFRs primarily phase-locked to the envelope of the stimulus (FFRENV), and subtraction of these responses yield FFRs phase-locked to the fine structure of the stimulus (FFRSPEC) (Krishnan 2002; Aiken & Picton 2008). This method to extract FFRENV and FFRSPEC is valid and well-established and has been used in previous FFR studies (Krishnan 2002; Aiken & Picton 2008; Elsisy & Krishnan 2008; Smalt et al. 2012; Anderson et al. 2013) as well as in auditory nerve single unit studies (Joris 2003; Heinz & Swaminathan 2009; Kale & Heinz 2010).
Spectral Analysis
Fast Fourier Transform (FFT) analysis was used to decompose the complex FFR into its component frequencies. The magnitudes of the spectral peaks in the response corresponding to stimulus F0 (FFRENV) and the formant-related harmonics (FFRSPEC) were measured for each individual and across experimental conditions. Unless otherwise indicated, the magnitude of FFT peak at 120 Hz (stimulus F0) will reflect the strength of FFRENV representation while the magnitude of the first formant-related harmonic (360 Hz) will reflect the strength of fine structure (FFRSPEC) representation. To supplement the FFT analysis, narrow-band spectrograms obtained from individual FFRs were grand averaged to provide a qualitative visual image of the joint time-frequency representation of the spectral content in the subcortical response.
Comparisons at equal sound pressure level (SPL) and equal sensation level (SL)
The FFRs obtained at four presentation levels (70, 75, 80 and 85 dB SPL) common to both groups were used for the equal SPL comparisons. For equal SL comparisons we utilized a derived SL measure where the audiometric pure tone average (PTA) at 250, 500 and 1000 Hz for each individual was subtracted from the stimulus presentation level(s) used for FFR data acquisition. For example, the derived SL in the case of Subject 1 in the NH group when the stimulus presentation level was 65 dB SPL was calculated as follows: Presentation level (65 dB SPL) – Subject PTA (21.5 dB SPL) = 43.5 dB SL. This procedure was repeated for all subjects within the NH and SNHL group for each stimulus presentation level. The resultant SLs obtained for all individuals in a group for a particular stimulus presentation level were averaged to yield a single SL per group (NH and SNHL) per stimulus level. For example, the average SL value for the NH group for a stimulus presented at 65 dB SPL was computed to be 48.97 dB SL (SL range: 43.5–55.16 dB SL). Similarly, at a stimulus intensity of 85 dB SPL in the HI group, the average SL was calculated to be 49.57 dB SL (range: 43.83–58.83 dB SL). Hence, 65 dB SPL in the NH group was considered to be approximately equal in SL to 85 dB SPL in the SNHL group. Thus, the FFR data obtained at 65, 70 and 75 dB SPL in the NH group was compared to the data obtained at 85, 90 and 95 dB SPL in the HI group, respectively, to realize comparisons at approximately 50, 55 and 60 dB SL. It should be noted here that although the derived SLs for a given presentation level varied across subjects, we did not observe a systematic trend where response magnitude was always larger for listeners with higher derived SLs (SNHL group: n=4, mean F0 magnitude=0.0842) compared to listeners with lower derived SLs (SNHL group: n=4, mean F0 magnitude=0.088).
The FFR to vowel /u/ predominantly contains energy only at frequencies below about 1000 Hz (magnitudes of formants F3 and F4 in the FFR are very low). Hence, the PTA at 250, 500, and 1000 Hz was chosen as the base for deriving SL, because this frequency range best represents the dominant spectral components of the vowel /u/ captured by the FFR. Further, even though there were very minor differences between the equated SLs (for example, 48.97 dB SL in NH was compared with 49.57 dB SL in SNHL), since three different SLs were tested with similar results in the current study and were all highly supra-threshold, we would not predict a major difference in the results based on those minor differences.
Statistical Analyses
A multiple regression model with presentation level (in dB SPL) as the continuous variable and group (2 levels; NH and SNHL) as the categorical variable, was conducted on natural log transformed F0 magnitudes to see if presentation level and hearing status predicted brainstem representation of F0 (or envelope). In addition, a two-way repeated measures analysis of variance (RMANOVA) with group (2 levels; NH and SNHL), the between-subjects factor, and presentation level (4 levels; 70, 75, 80 and 85 dB SPL), the within-subject factor, was conducted on natural log transformed F0 magnitudes in order to evaluate the effects of hearing loss on brainstem neural representation of F0 (envelope) when stimuli were presented at equal SPL. A second two-way RMANOVA with group (2 levels; NH and SNHL), the between-subjects factor, and sensation level (3 levels; 50, 55 and 60 dB SL), the within-subject factor, was conducted on natural log transformed F0 magnitudes in order to evaluate the effects of hearing loss on brainstem neural representation of F0 (envelope) when stimuli were presented at equal SL. Similar multiple regression and two-way RMANOVA models were used to analyze natural log transformed F1 magnitudes to study the effects of hearing loss and presentation level (SPL and SL) on brainstem neural representation of F1 (TFS). It should be noted here that F0 and F1 magnitudes were natural log transformed to satisfy model assumptions for statistical analysis. The sphericity assumption was confirmed for all mixed-design ANOVAs by specifying appropriate covariance structures.
RESULTS
Effects of stimulus level on the neural representation of envelope
Neural representation of envelope: presentation level in dB SPL
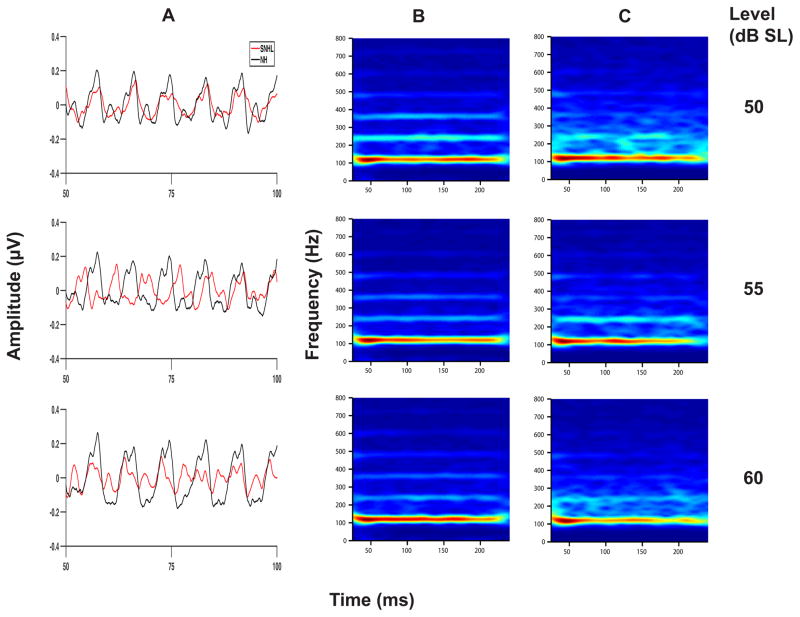
Grand averaged FFR waveforms, (overlaid for the two groups in Panel A), and spectrograms (Panels B and C) representing neural phase-locking to envelope of the vowel /u/ for NH and SNHL listeners at stimulus levels representing low, mid and high intensity (70, 75, 80 and 85 dB SPL) are shown in Figure 2. FFR temporal waveform amplitude (Panel A, Figure 2) appears to increase with increase in stimulus intensity level for both groups, with generally smaller amplitudes across levels for the SNHL group. Consistent with the waveform data, spectral bands at F0 in the SNHL group (Panel C, Figure 2) are weaker, less distinct, and exhibit spectral smearing compared to the more robust and spectrally distinct bands in NH subjects (Panel B, Figure 2) at all SPLs. Mean spectral magnitude of F0, plotted as a function of stimulus level in dB SPL for both groups in Figure 3, clearly reinforce the observations on the waveform and spectral data. Specifically, while F0 magnitude increases with intensity for both groups, F0 magnitude for the SNHL group was smaller compared to the NH group, at all levels expressed in dB SPL. Results of multiple regression analysis showed a significant stimulus level effect for both groups (NH: t1 = 4.03, p = 0.0001; SNHL: t1 = 4.53, p = 0.0001) but the slope of F0 magnitude change with level was not different for the two groups (t1 = 0.68, p = 0.4987). In addition, a two-way RMANOVA (hearing status (NH, SNHL) and stimulus level (presentation levels in dB SPL)) was performed on only the FFR data obtained at equal presentation levels (70, 75, 80 and 85 dB SPL) for the two groups. Results of this analysis revealed a significant main effect for both hearing status (F1,18 = 27.75, p < 0.0001) and stimulus level (F3,49 = 11.16, p < 0.0001) with no significant interaction effect (F 3,49 = 0.49, p = 0.6926). Tukey-adjusted post-hoc multiple comparisons indicated that neural representation of F0 was stronger in the NH group compared to the SNHL group across all four sound pressure levels. Further, F0 representation for both groups was stronger when the stimulus level was 85 or 80 dB SPL as compared to 70 or 75 dB SPL. Taken together, the weaker neural representation of F0 in the SNHL group across all levels may suggest degradation in neural phase-locking to the stimulus envelope; and the essentially parallel F0 magnitude growth slopes for both groups suggests a similar pattern of growth in F0 magnitude with level.
Figure 2.
Grand average waveforms for NH and SNHL (overlaid in panel A), and spectrograms (panel B: normal hearing and panel C: SNHL) plotted as a function of stimulus level in dB SPL for the FFRENV condition.
Figure 3.
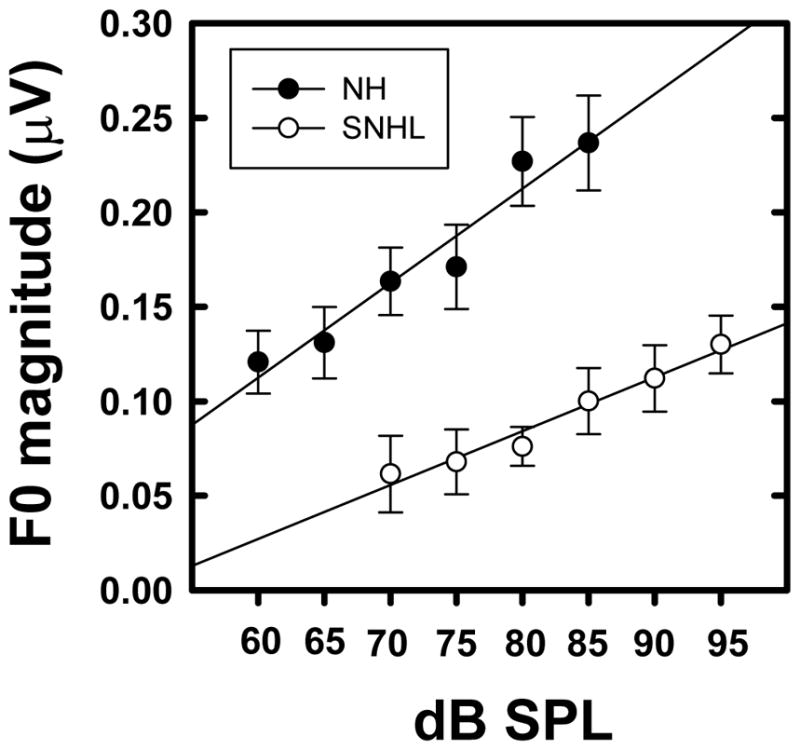
Mean F0 magnitudes (natural log transformed) plotted as a function of stimulus level (in dB SPL) for normal hearing (filled circles) and SNHL (open circles). Error bars represent standard errors.
Neural representation of envelope: comparison at equal sensation level (SL)
Neural representation of F0 was compared for the two groups at equal SLs (50, 55, and 60 dB SL) to determine if differences between the groups were related to signal audibility. Grand averaged FFR waveforms, (overlaid for the two groups in Panel A), and spectrograms (Panels B and C) representing neural phase-locking to envelope of the vowel /u/ for NH and SNHL listeners at equal sensation levels (50, 55, and 60 dB SL) are shown in Figure 4. FFR waveform amplitude (Panel A, Figure 4) does not appear to increase appreciably with increase in stimulus intensity level for either group, although generally smaller amplitudes are noted across levels for the SNHL group. Spectral bands at F0 (Panels B and C, Figure 4) appear to be equally robust with little or no change across SLs for both groups. However, the NH group shows more robust spectral bands at F0 harmonics. Mean F0 amplitude (Figure 5) increases gradually with SL for both groups with slightly greater overall amplitudes for the NH group. While two-way RMANOVA results yielded a significant main effect for level in SL (F2,31 = 4.17, p = 0.0249), it failed to produce a significant main effect for hearing status (F1,17 = 1.90, p = 0.1858). The interaction effect (F2,31 = 0.31, p = 0.4068) was also not significant. Tukey-adjusted post-hoc multiple comparisons indicated that overall, F0 representation was stronger when the stimulus level was 60 dB SL as compared to 50 or 55 dB SL. This finding is consistent with the results of the multiple regression analysis, which showed improvements in F0 representation as a function of sound pressure level in both groups. However, the lack of a statistically significant difference between the NH and SNHL groups at equal SL must be interpreted with caution. It is possible that the neural representation of stimulus envelope, as reflected by F0 magnitude, is comparable for both groups when compared at equal SLs, i.e. when compared at equal audibility. This, in turn, would indicate that audibility, at least in part, might account for degraded F0 representation in the SNHL group. However, caution should be exercised with this interpretation because the lack of a statistically significant difference between the groups may be due to the variability in group means associated with the heterogeneity of the groups (differences in thresholds, PTAs, and the derived SLs) and the small sample size.
Figure 4.
Grand average waveforms for NH and SNHL (overlaid in panel A), and spectrograms (panel B: normal hearing and panel C: SNHL) plotted as a function of stimulus level in dB SL for the FFRENV condition.
Figure 5.
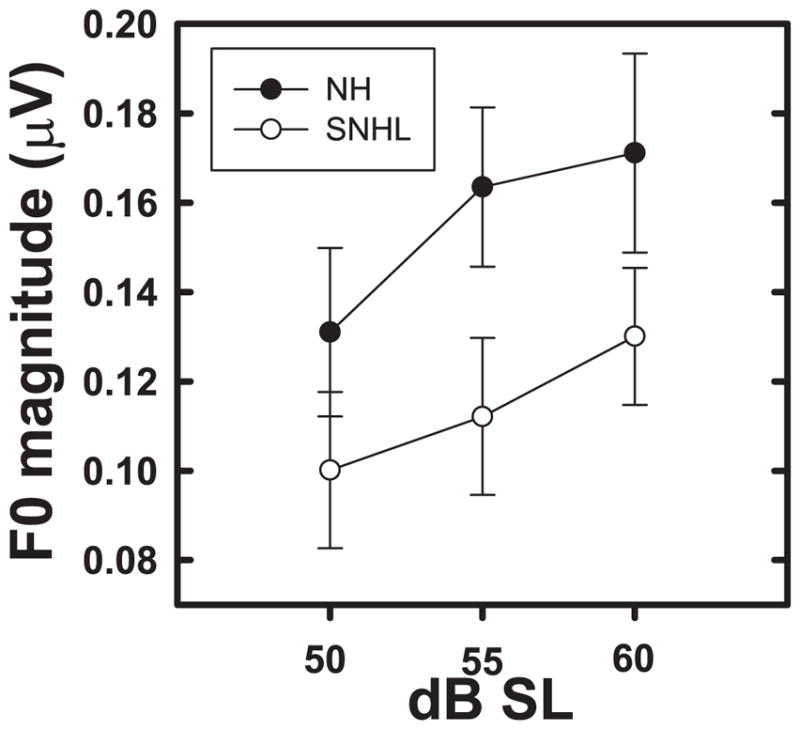
Mean F0 magnitudes (natural log transformed) plotted as a function of stimulus level (in dB SL) for normal hearing (filled circles) and SNHL (open circles). Error bars represent standard errors.
Effects of stimulus level on the neural representation of TFS
Neural representation of TFS: presentation level in dB SPL
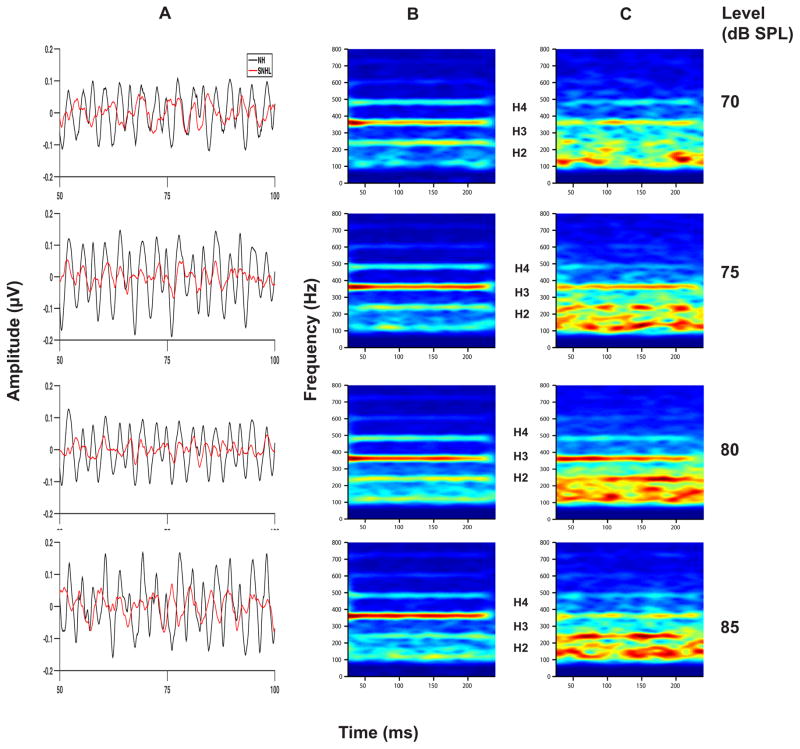
Grand averaged FFR waveforms, (overlaid for the two groups in Panel A), and spectrograms (Panels B and C) representing neural phase-locking to the temporal fine structure of the vowel stimulus for NH and SNHL listeners at stimulus levels representing low, mid and high intensity (70, 75, 80 and 85 dB SPL) are shown in Figure 6. FFR temporal waveform amplitude (Panel A, Figure 6) appears to increase with increase in stimulus intensity level for both groups, with generally smaller amplitudes across levels for the SNHL group. Spectrograms for the NH group (Panel B, Figure 6) show distinct and robust spectral bands at formant-related harmonics (H2, H3 and H4) at all stimulus levels. In contrast, the spectrograms for the SNHL group (Panel C, Figure 6) show weaker, less distinct, spectrally smeared bands (H1, H2) of activity with a clear spectral band present only at H3. The spectral smearing appears to worsen with increase in intensity. Mean spectral magnitude of F1 (H3=360 Hz), plotted as a function of stimulus level for both groups in Figure 7, shows greater magnitude for NH groups compared to SNHL group at all levels. Also, the slope of F1 magnitude versus intensity function appears to be steeper for the NH group compared to the shallower slope for the SNHL group, particularly at levels below 80 dB SPL. Results of multiple regression analysis showed that a significant stimulus level effect was present for both groups (NH: t1 =5.72, p < 0.0001; SNHL: t1 = 2, p=0.04) and that the slope of F1 magnitude change with level was significantly different for two groups (t= 2.33, p=0.02). In addition, a two-way RMANOVA (hearing status: NH, SNHL; and equal presentation levels in dB SPL) was performed on the FFR data obtained at equal presentation levels (70, 75, 80 and 85 dB SPL) for the two groups. Results of this analysis revealed significant main effects for hearing status (F1,18 = 27.43, p < 0.0001) as well as stimulus level (F3,49 = 3.52, p = 0.0216) while the interaction effect was non-significant (F3,49 = 1.02, p = 0.3921). Tukey-adjusted post-hoc multiple comparisons indicated that F1 representation was stronger in the NH group as compared to the SNHL group across all four sound pressure levels. Further, F1 representation across groups was stronger when the stimulus level was 85 or 80 dB SPL as compared to 70 dB SPL. These results suggest that the weaker neural representation of F1 in the SNHL group across all levels may suggest degradation in neural phase-locking to the stimulus TFS. Further, the significant difference in F1 magnitude growth slopes for both groups indicates, at least, that TFS representation does not improve with presentation level in the SNHL group in the manner observed for envelope representation. This in turn indicates that audibility alone might not entirely account for the degradation of the neural representation of TFS in the SNHL group.
Figure 6.
Grand average waveforms for NH and SNHL (overlaid in panel A), and spectrograms (panel B: normal hearing and panel C: SNHL) plotted as a function of stimulus level (in dB SPL) for the FFRTFS condition
Figure 7.
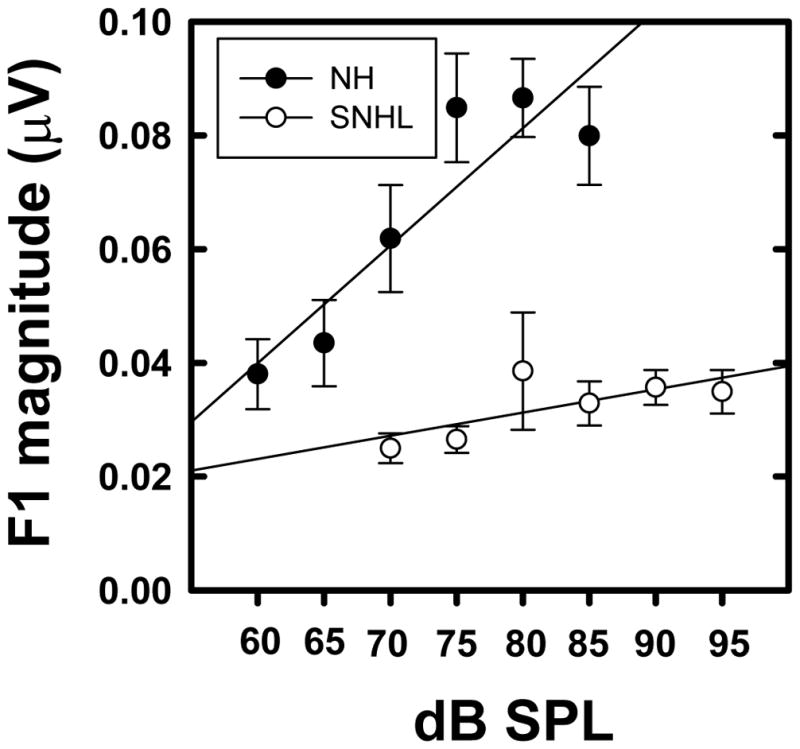
Mean F1 magnitudes (natural log transformed) plotted as a function of stimulus level (in dB SPL) for normal hearing (filled circles) and SNHL (open circles). Error bars represent standard errors.
Neural representation of TFS: comparison at equal sensation level (SL)
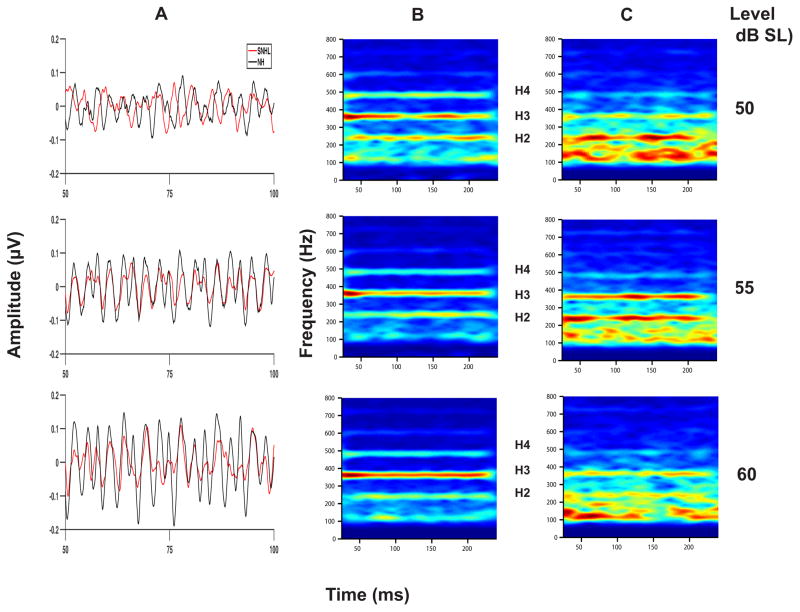
Shown in Figure 8 are the grand averaged FFR waveforms (overlaid for the two groups in Panel A) and spectrograms (Panels B and C) representing neural phase-locking to the TFS of the vowel stimulus presented at equal sensation levels (50, 55 and 60 dB SL). Once again, FFR temporal waveform amplitude (Panel A, Figure 8) appears to increase with increase in stimulus intensity level for both groups, with generally smaller amplitudes across levels for the SNHL group. While both groups show spectral bands at the formant-related harmonics (H2, H3, and H4) at all sensation levels, the SNHL group (Panel C) exhibit less distinct, broader, and smeared bands of neural activity spanning these three harmonics. Mean F1 amplitude (Figure 9) exhibits a small increase as level is changed from 50 to 55 dB SL, with appreciably greater amplitude at 60 dB SL for the NH group. In contrast, the F1 magnitude for the SNHL group shows little or no increase with increase in SL. These observations are confirmed by the two-way RMANOVA results, which yielded significant main effects for hearing loss (F1,17 = 7.22, p = 0.0156), as well as for stimulus level (F2,17 = 9.67, p = 0.0016) and a significant interaction effect (F2,17=5.27, p = 0.0165). Post-hoc simple main effects testing using Tukey-adjusted multiple comparisons indicate no group differences at 50 or 55 dB SL; however, F1 representation in NH listeners is significantly better than that in the SNHL group at 60 dB SL. Further, a significant improvement in F1 representation is noted as SL increases from 50 to 55 to 60 dB SL in the NH group. However, no changes in F1 representation as a function of SL were observed in the SNHL group. The improvement in F1 representation as a function of SL in the NH group coupled with the invariance of the reduced F1 magnitude across SLs in the SNHL suggests that the effect of stimulus level on TFS representation is dependent on hearing status. This finding is consistent with the results of the multiple regression analysis performed on F1 representation, and adds to the evidence suggesting that SNHL alters the TFS and envelope representation differently.
Figure 8.
Grand average waveforms for NH and SNHL (overlaid in panel A), and spectrograms (panel B: normal hearing and panel C: SNHL) plotted as a function of stimulus level (in dB SL) for the FFRTFS condition.
Figure 9.
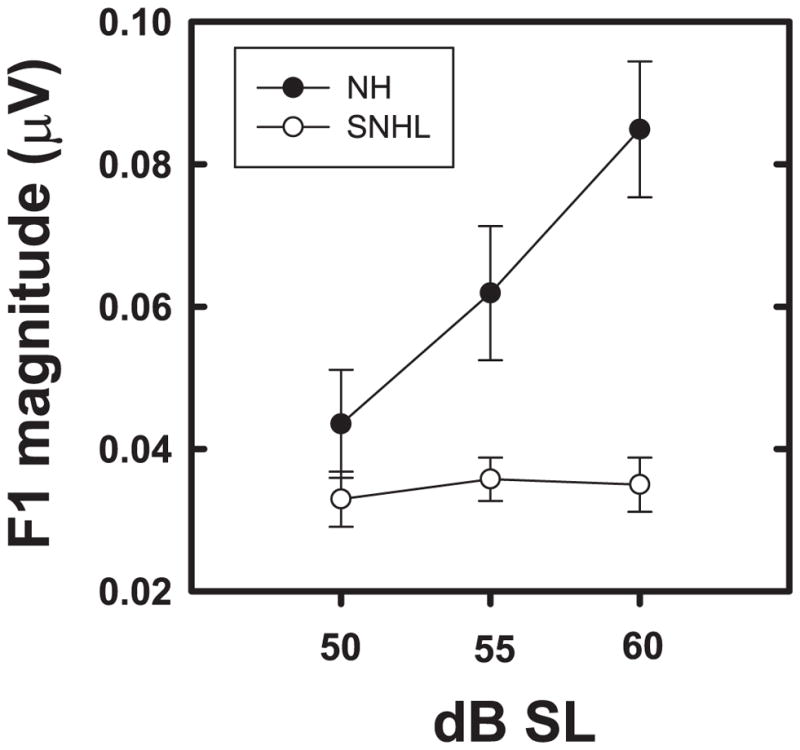
Mean F1 magnitudes (natural log transformed) plotted as a function of stimulus level (in dB SL) for normal hearing (filled circles) and SNHL (open circles). Error bars represent standard errors.
Influence of aging on neural representation
The mean age of SNHL participants (54.26 years) was greater than the mean age of NH participants (27.72 years). In order to determine if age-related effects, in addition to hearing loss, also contributed to the degraded neural representation in the older SNHL group, we compared the neural representation of both envelope and TFS data at 80 dB SPL. This level was chosen because it was the highest level at which FFRs could be comfortably recorded in all participants in the extended subject pool, including three older (>50 years) NH listeners and four younger (<30 years) listeners with SNHL. An analysis of covariance (ANCOVA) model was used to examine the effect of age (covariate) on F0 magnitude (dependent variable) in NH and SNHL groups (categorical variable) at 80 dB SPL. A significant main effect was observed for hearing loss (F1, 39 = 4.51, p = 0.0402) but not for age (F1,3 = 0.12, p = 0.73). The interaction effect between age and hearing loss was not significant (F1, 39 = 0.00, p = 0.94) either. The model was re-run after dropping the non-significant interaction term as a two-way ANOVA model, yielding a significant effect for hearing loss (F1, 40 = 24.86, p < 0.0001). The effect for age was not significant (F1, 40 = 0.14, p = 0.71) in the reduced model either, indicating that age does not appear to account for the observed group differences in F0 magnitude in this particular data-set.
A similar ANCOVA model applied to analyze the effects of SNHL and age on F1 magnitude indicated that none of the main effects (hearing loss: F1,39 = 0.86, p = 0.36; age: F1,39 = 0.78, p = 0.38) were significant in the full model. A reduced two-way ANOVA model rerun after dropping the non-significant interaction term yielded a significant main effect for hearing loss (F1, 40 = 17.41, p = 0.0002), but not for age (F1, 40 = 0.12, p = 0.72), again suggesting that age-related changes does not appear to contribute to the degraded TFS representation in SNHL group in this particular data-set.
DISCUSSION
The major findings of this study demonstrate that (i) neural phase-locking to both envelope (F0) and TFS (F1) is degraded in individuals with mild to moderately-severe SNHL; (ii) F0 (envelope) magnitude increases similarly with intensity for both groups, suggesting that differences in envelope representation may, at least in part, be related to signal audibility; (iii) F1 (TFS) magnitude for the hearing impaired individuals exhibits appreciably smaller changes in magnitude with intensity, suggesting a relatively greater deterioration in the neural representation of TFS (compared to envelope) that cannot be accounted for by audibility alone; and (iv) the observed changes in the neural representation of envelope and TFS in individuals with SNHL appears to be more consistent with consequences of a peripheral hearing loss rather than age-related changes.
Degradation in neural representation of envelope (F0)
Our observation of degraded F0 representation in subjects with SNHL as compared to NH subjects at equal SPLs, with an improvement in F0 magnitude with increasing stimulus levels for both groups leading to no group differences at equal SLs, is consistent with behavioral results that show significantly weaker envelope detection in SNHL listeners compared to NH listeners for stimuli presented at equal SPL, but not at equal SL. Equivalent gap detection thresholds and temporal modulation transfer functions (Florentine & Buus 1984; Bacon & Viemeister 1985; Bacon & Gleitman 1992) have been observed in listeners with normal hearing and SNHL at equal sensation levels. However, the equal SL comparisons in the current study must be interpreted with caution. In this study, the SL associated with any given presentation level for either group (NH or SNHL) is a derived measure based on the PTA for each subject within the group. Given the inevitable variations in PTA, this computation may yield a wide range of SL values within the SNHL group for a given presentation level. In addition, the small sample size may lead to reduced statistical power. Together, these factors could introduce enough variability to render group comparisons statistically insignificant. In the future, in order to minimize such variability, the behavioral threshold for the stimulus (in this case, vowel /u/) itself could be used as the base for calculating SL, rather than the PTA. Despite the challenges associated with the statistical analysis of equal SL comparisons, the equivalent slopes observed for the F0-SPL growth functions strongly suggest that the degraded envelope representation observed in SNHL as compared to NH at equal SPLs is likely a consequence of reduced audibility.
It is likely that the degradation in F0 representation and its improvement with increased stimulus levels for the listeners with SNHL reflects a change from reduced to improved signal audibility in the impaired high frequency regions. Reduced signal audibility in the higher frequency regions and/or broader filter bandwidths have been implicated in the deterioration of neural representation of envelope consequent to cochlear hearing impairment (Bacon & Veimeister 1985; Bacon & Gleitmann 1992; Leek & Summers 1996). Several physiologic studies have demonstrated that envelope information in complex sounds is encoded by interactions between higher unresolved harmonics (Cariani & Delgutte 1996a, b; Meddis & O’Mard 1997; Sayles & Winter 2008). Consistent with this, behavioral studies have shown that F0 discrimination in listeners with SNHL is better for harmonic complex sounds with only the higher unresolved components (Arehart 1994). Summers and Leek (1998) found that fundamental frequency difference limens (F0DLs) had the best correlation with threshold at 2 kHz in listeners with SNHL, further supporting the role of high frequency audibility in F0 perception.
Unlike our findings, two recent published reports show enhanced envelope representation in chinchillas with noise-induced SNHL (Kale & Heinz, 2010), and in FFRs obtained in humans with SNHL (Anderson et al. 2013). Kale and Heinz (2010) suggest that very steep rate-level functions, often observed in moderate-severe hearing losses due to increased inner hair cell loss, likely account for improved envelope representation at the single unit level in SNHL. These authors have suggested that this envelope enhancement is present to a lesser degree in mild-moderate hearing losses where there is lesser inner hair cell involvement and the rate-level functions are more gradual. The relatively milder degree of hearing loss in the listeners used in our study may explain the absence of enhanced F0 representation. Alternatively, the FFR represents ensemble neural activity and therefore represents the summed neural activity of a population of neurons with different thresholds and rate level functions which could obscure the enhancement observed by Kale and Heinz (2010) for selective single units with steep rate-level functions.
Anderson and colleagues (2013) attribute enhanced envelope representation observed in the SNHL FFR to reduced inhibitory mechanisms in hearing impairment. Reduced inhibitory and enhanced excitatory mechanisms subsequent to hearing loss have been documented in animal studies (Vale & Sanes 2002). Anderson and colleagues further hypothesize that wider auditory filter bandwidths in subjects with SNHL may allow a greater amount of energy through, which in turn may be represented as enhanced envelope measures. However, such envelope enhancement was observed only when the stimulus was adjusted for audibility and/or in the presence of background noise. Differences in the way “equal audibility” was defined in Anderson et al. (2013) as compared to the current study might account for the absence of enhanced F0 representation in the present study. Anderson and colleagues used a National Acoustics Laboratory (NAL) algorithm to adjust the stimulus for audibility only in the SNHL group; on the other hand, in the present study, equal SL was determined by comparing overall stimulus presentation level to pure tone average at 0.25, 0.5 and 1 kHz. Alternatively, it is possible that the envelope FFR to stimulus /da/ used by Anderson et al. (2013), containing more high frequency energy than the vowel /u/ used in this study, would be more robust due to additional contributions from envelope modulation resulting from interaction between higher harmonic components within the broader cochlear filters in individuals with SNHL.
Degradation in neural representation of temporal fine structure (F1)
Like the degradation in F0, neural representation of F1 was weaker in listeners with SNHL as compared to normal hearing listeners at any given stimulus presentation level. However, in contrast to the steady growth in the envelope (F0) representation with intensity, the representation of the fine structure component (harmonic representing the first formant (F1)) showed reduced or no change with intensity in the SNHL listeners, suggesting that SNHL has a differential effect on envelope and TFS representation with increasing stimulus presentation level. That is, in order for the SNHL group to yield a similar value of both subcortical envelope and TFS representation as compared to the NH group, the stimulus presentation level must be higher for the SNHL group as compared to the NH group. However, equivalent slopes of the F0-SPL growth functions in NH and SNHL indicate an approximately constant difference in intensity levels between NH and SNHL listeners required to reach similar values of envelope representation. On the other hand, in the case of TFS representation the non-parallel F1-SPL functions in NH and SNHL suggest a variable difference in intensity levels between NH and SNHL listeners required to reach similar values of TFS representation. Therefore, there is a relatively greater decline in TFS representation as compared to envelope representation in SNHL across intensity levels. These results are consistent with findings from Plyler and Ananthanarayan (2001), who showed weak neural representation of formant transition in SNHL listeners with comparable hearing loss, which did not improve with increase in intensity level. Diminished subcortical TFS representation in SNHL is consistent with neurophysiologic findings (Henry & Heinz 2013). Degraded neural representation of TFS may be attributed to diffuse or impaired phase-locking synchrony secondary to reduced frequency selectivity in SNHL (Palmer & Moorjani, 1993; Miller et al. 1997). The reduced frequency selectivity might occur due to tonotopic re-mapping, which could disrupt place-dependent TFS encoding (Henry & Heinz 2013), and possible age effects.
Broader cochlear filters in hearing loss reduce frequency resolution (Glasberg & Moore 1986) which can cause deficits in TFS cues available and render the hearing impaired listener dependent on envelope cues arising as a result of modulation of unresolved harmonics (Moore & Carlyon 2005). Behavioral findings from Leek and Summers (1996) indicate that wider than normal auditory filter bandwidths in SNHL can reduce the difference between the peaks and valleys of formant frequencies (spectral contrast), which plays an important role in vowel perception. Broadly resolved frequency components passing through wider than normal auditory filters may cause diffuse patterns of phase-locking to a wide frequency range, as opposed to “tight” phase-locking confined to a narrow band of frequencies in normal hearing listeners. Degraded F1 representation in listeners with SNHL in the present study may be attributable, at least in part, to degraded phase locking consequent to reduced frequency selectivity.
The majority of the listeners with SNHL participating in the present study had high frequency hearing loss with normal/near-normal audiometric thresholds at frequencies below 1 kHz, suggesting auditory filter bandwidths within, or close to, normal limits at low frequencies. Neural representation of the low frequency F1 (F1=360 Hz) was degraded despite near-normal audiometric thresholds in the lower frequencies in these subjects, implying that reduced frequency selectivity cannot wholly account for the observed TFS deficit. Several psychophysical (Moore & Peters 1992; Simon & Yund 1993) and speech perception studies (Horwitz et al. 2002; Lorenzi et al. 2009) lend support to this view, demonstrating that impaired frequency selectivity is not always predictive of degraded pitch perception in individuals with SNHL. Simon and Yund (1993) observed differences in Frequency Difference Limen (FDL) measures in ears with the same pure tone threshold, and similar FDLs in ears with different pure tone thresholds. Poor correlations were observed in listeners with SNHL between FDLs and psychophysical tuning curves (Tyler et al. 1983) and FDLs and auditory filter bandwidths (Moore & Peters 1992). Horwitz and colleagues (2002) as well as Lorenzi and colleagues (2009) have shown that identification of speech signals processed to reflect TFS cues alone and low-pass filtered at 1.5 kHz is significantly affected in hearing impaired individuals who have normal or near normal audiometric thresholds at frequencies below 1.5 kHz. Hopkins and Moore (2011) found no significant correlations between TFS sensitivity and equivalent rectangular bandwidths (ERBs) for any center frequency, thus indicating that TFS representation is not affected by frequency selectivity. Further strengthening this argument, Hopkins and Moore found that TFS sensitivity was affected even for subjects with normal low frequency hearing with normal ERB values at low center frequencies but impaired high frequency hearing. Kale and Heinz (2010) also found similar results in single unit data, where envelope and TFS encoding in chinchillas with noise-induced hearing loss was unrelated to their frequency selectivity. Collectively, these findings suggest that reduced frequency selectivity may only partially account for the diminished F1 representation observed in SNHL.
It is our view that the degradation in the fine structure representation in individuals with SNHL reflects a disruption in the temporal pattern of neural activity consequent to a peripheral hearing loss that has adverse effects on neural timing (and therefore synchronization of neural activity) that is cumulative along the auditory neuroaxis. The relatively greater deterioration of TFS representation compared to the envelope representation observed here supports this view. Specifically, timing disruption via temporal jitter may have more pronounced effects on faster changes (TFS) than slower changes (envelope). Auditory nerve single unit population studies have established that synchronous firing of neural elements is required for a robust and accurate representation of speech signals. Further, formant frequencies, which are critical in vowel perception, are encoded through “synchrony capture”. Synchrony capture is a neural encoding phenomenon wherein auditory nerve fibers demonstrate enhanced neural phase-locking synchrony to harmonics near formant frequencies, while phase-locking to other harmonic components located further away from the formants is diminished. When comparing neural phase-locking ability in auditory nerve fibers in cats with normal and impaired hearing, Miller and colleagues (1997) observed the cats with noise-induced hearing loss had significantly reduced or absent synchrony capture. Similarly, Palmer & Moorjani (1993) found reduced synchrony capture for F1 and F2 in guinea pigs with kanamycin-induced hearing loss, while robust encoding of the lower frequency F0 was preserved. The phenomenon of synchrony capture is not restricted to the single unit population response, but has also been documented in the ensemble response (the brainstem FFR) to steady state speech in normal hearing listeners (Krishnan 2002). Consistent with the above findings, strong formant-related harmonic encoding, or “synchrony capture” was present in normal hearing individuals, but reduced or absent in the SNHL FFR in the present study, again suggesting reduced precision in brainstem neural phase-locking consequent to SNHL.
A consistent finding in the auditory nerve single unit studies reviewed above, as well as the FFR data in the present study, is the preservation of robust representation of lower fundamental frequencies with a degradation in neural phase-locking to higher formant frequencies. Such a selective disruption in synchrony of neural firing in response to higher formant frequencies in SNHL may be a consequence of tonotopic remapping, which can affect place-dependent encoding of TFS information. Henry and Heinz (2013) found that phase-locking in neural fibers with CFs > 2.5 kHz encoded not only high frequency envelope information but also TFS information at much lower frequencies between 0.5–1.5 kHz in mild-moderate hearing loss. While low frequency TFS is encoded by all fibers irrespective of CF, high frequency TFS information between 2.5–5 kHz is not encoded at all. Normal encoding of envelope cues in the presence of diminished high frequency TFS encoding at the single unit level may explain the differential effect of SNHL on envelope and TFS representation observed in the ensemble brainstem response.
Influence of age related changes on the neural representation of envelope and TFS
One of the perennial challenges when studying neural encoding in human subjects with SNHL is the relationship between age and audiometric thresholds, and the potential influence of age-related effects on neural encoding. High frequency SNHL often occurs with advancing age. If age and hearing loss are not appropriately controlled during statistical analysis, it is possible that the neural degradation observed in the SNHL group could reflect a combination of the effects of aging and SNHL. While there was a lack of a balanced age-matched control group in the current study, there were three older normal hearing adults and four younger adults with SNHL included in the study. Based on an analysis of covariance with age as a covariate, our results showed that hearing loss to be the main contributor to the observed degradation in neural representation of both envelope and TFS, with age not contributing significantly. The majority of recent studies examining the effects of age on the brainstem FFR (Clinard et al. 2010; Marmel et al. 2013; Bones & Plack 2015) suggest that brainstem neural representation of temporal cues undergoes some degradation with advancing age. At first glance, the results from the current study appear to be inconsistent with these findings. However, a careful inspection of the existing literature on the effects of aging on neural representations of simple and complex stimuli provides some arguments that might explain these inconsistent findings. Bones and Plack (2015) found a decline in brainstem temporal representation of complex stimuli (musical dyads) with increasing age. However, while the audiometric thresholds in the young and aged participants in this study were within normal limits between 250–2000 Hz, the hearing threshold at audiometric frequencies higher than 2000 Hz in either group were not taken into consideration. As age is strongly correlated with high frequency hearing loss, Bones and Plack acknowledge that high frequency hearing loss could be a confounding variable in their results. In other words, the effect of hearing loss at frequencies beyond 2 kHz on subcortical neural representation of speech in the aged population cannot be ruled out in their study. In attempting to explain their findings, Bones and Plack reason that while the older adult participants may have high frequency hearing loss at frequencies beyond 2000 Hz, they have normal hearing in the lower frequencies, which overlaps with the stimulus frequency range. As a consequence, there should be no effects of high frequency SNHL on representation of low frequencies in the brainstem. However, several psychophysical (Moore & Peters 1992; Simon & Yund 1993) and speech perception studies (Horwitz et al. 2002; Lorenzi et al. 2009), described in detail earlier in the discussion, indicate that perception of low frequency stimuli is affected in individuals with normal/near-normal low frequency thresholds and high frequency SNHL. These behavioral observations are consistent with the results of the current study, and may explain the discrepancy between our findings and results from Bones and Plack.
Marmel et al. (2013) found a negative correlation between age and a “synchrony” metric obtained from brainstem FFR when using a pure tone stimulus of 660 Hz. Age effects on brainstem representation were also noted by Clinard et al. (2010) when young and old listeners were presented with pure tone stimuli. However, Clinard et al. (2010) remark that these age effects are frequency specific. Specifically, their results indicate that age-related effects are seen in the brainstem FFR when it is elicited using relatively higher frequencies such as 1000 Hz, but not in response to low frequency signals at or below 500 Hz. Clinard et al. (2010) hypothesize that such frequency specific effects may occur as higher frequencies that have shorter periods may be more susceptible to the temporal jitter effects that occur consequent to aging. This line of reasoning may also explain findings from Marmel et al. (2013); the degradation in neural phase-locking with increasing age observed in this study may have occurred due to the use of a pure tone stimulus of frequency higher than 500 Hz. Based on these findings and given that both the F0 (120 Hz) and F1 (360 Hz) in the stimulus in the current experiment were below 500 Hz, it is reasonable to assume that the differences in subcortical neural representation observed in NH and SNHL participants are not influenced significantly by the differences in their ages.
Further, an overall trend observed in speech perception studies (Gordon-Salant 2005; Pichora-Fuller & Singh 2006) is that age effects are not usually present in quiet environments with adequate audibility, or for simple auditory tasks, but become evident in challenging listening conditions such as listening in background noise. Based on these findings, one might extrapolate that the effects of age on neural representation of temporal cues may not be as significant in the quiet listening conditions employed in the current experiment.
Finally, however, given that there was not a balanced age-matched control group of older normal hearing adults or younger adults with SNHL in this study, the effects of age on subcortical neural representation of temporal cues cannot be comprehensively ruled out. Further systematic examination of the interactions between SNHL, age and brainstem encoding is required in order to tease apart the effects of hearing loss and aging on neural representation of complex stimuli.
Conclusions
To date, our knowledge about how SNHL alters representation of envelope and TFS is largely derived from behavioral studies in humans and physiologic studies in animals. Results of our evaluation of the neural representation of envelope and TFS in normal and impaired ears as reflected in the scalp-recorded human frequency following responses to a large extent complement results of previous behavioral and physiologic studies. Specifically, our results showed degraded TFS representation in the presence of essentially normal envelope representation as a function of stimulus level in subjects with SNHL. Deficits in envelope representation in SNHL observed at lower sensation levels likely occur due to reduced audibility in higher frequency regions that are necessary for neural representation of F0 derived from modulations of unresolved harmonics. In contrast, the persistence of degraded neural phase locking of TFS even when audibility is restored may be due to a combination of impaired temporal synchrony due to altered tonotopicity and loss of frequency selectivity due to wider auditory filter bandwidths. Our results suggesting that age-related factors do not appear to contribute to the observed degradation in F0 and F1 representation should be considered preliminary and requires confirmation by a more complete and systematic evaluation of age-related changes. The FFR provides an effective physiologic window to evaluate how SNHL alters representation of envelope and fine structure information relevant to speech perception. Given its ability to reflect experience dependent plasticity, the FFR may also serve as a tool to track “secondary plasticity” following amplification and auditory training through pre- and post-training/amplification recordings in listeners with SNHL.
Acknowledgments
S.A. designed and performed experiments, analyzed data and wrote the paper; A.K. and E.B. provided critical revisions. The authors wish to thank Dr. Gavin M. Bidelman (University of Memphis), Dr. Christopher J. Smalt (MIT Lincoln Laboratory) and Venkatakrishnan Vijayaraghavan for assistance with MATLAB coding, Dr. Bruce Craig and Zachary Hass (Department of Statistics, Purdue University) for guidance on statistical analysis. Research supported by NIH R01 DC008549 (A.K.) and the Department of Speech, Language and Hearing Sciences (Purdue University).
Footnotes
Portions of this article were presented at the 36th Meeting of the Association for Research in Otolaryngology (ARO), Baltimore, M.D., February 16–20, 2013, 35th Meeting of the Association for Research in Otolaryngology (ARO), San Diego, California, February 25–29, 2012 and 41st Annual Scientific & Technology Conference, American Auditory Society, Scottsdale, AZ, March 6–8, 2014. The major part of this work has been presented in the first author’s dissertation (Ananthakrishnan, S. (2013). Neural encoding of complex signals in the healthy and impaired auditory systems).
Financial Disclosures/Conflicts of Interest: This research was funded by the Department of Speech, Language and Hearing Sciences (Purdue University)
References
- 1.Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hearing research. 2008b;245(1–2):35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S, Parbery-Clark A, White-Schwoch T, et al. Effects of hearing loss on the subcortical representation of speech cues. The Journal of the Acoustical Society of America. 2013;133(5):3030–8. doi: 10.1121/1.4799804. http://doi.org/10.1121/1.4799804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arehart KH. Effects of harmonic content on complex-tone fundamental- frequency discrimination in hearing-impaired listeners. The Journal of the Acoustical Society of America. 1994;95:3574. doi: 10.1121/1.409975. [DOI] [PubMed] [Google Scholar]
- 4.Bacon SP, Gleitman RM. Modulation detection in subjects with relatively flat hearing losses. Journal of Speech, Language and Hearing Research. 1992;35(3):642. doi: 10.1044/jshr.3503.642. [DOI] [PubMed] [Google Scholar]
- 5.Bacon SP, Viemeister NF. Temporal modulation transfer function in normal hearing and hearing impaired subjects. Audiology. 1985;24:117–134. doi: 10.3109/00206098509081545. [DOI] [PubMed] [Google Scholar]
- 6.Bones O, Plack CJ. Losing the music: Aging affects the perception and subcortical neural representation of musical harmony. The Journal of Neuroscience. 2015;35(9):4071–4080. doi: 10.1523/JNEUROSCI.3214-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cariani Pa, Delgutte B. Neural correlates of the pitch of complex tones. II. Pitch shift, pitch ambiguity, phase invariance, pitch circularity, rate pitch, and the dominance region for pitch. Journal of neurophysiology. 1996a;76(3):1717–34. doi: 10.1152/jn.1996.76.3.1717. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8890287. [DOI] [PubMed] [Google Scholar]
- 8.Cariani P, Delgutte B. Neural correlates of the pitch of complex tones. I. Pitch and pitch salience. Journal of Neurophysiology. 1996b doi: 10.1152/jn.1996.76.3.1698. Retrieved from http://jn.physiology.org/content/76/3/1698.short. [DOI] [PubMed]
- 9.Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hearing research. 2010;264(1–2):48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsisy H, Krishnan A. Comparison of response characteristics of acoustical and neural distortion product at 2f1–f2 in normal hearing adults. International Journal of Audiology. 2008;47:431–438. doi: 10.1080/14992020801987396. [DOI] [PubMed] [Google Scholar]
- 11.Florentine M, Buus S. Temporal gap detection in sensorineural and simulated hearing impairments. Journal of Speech, Language and Hearing Research. 1984;27(3):449. doi: 10.1044/jshr.2703.449. [DOI] [PubMed] [Google Scholar]
- 12.Glasberg BR, Moore BC. Auditory filter shapes in subjects with unilateral and bilateral cochlear impairments. The Journal of the Acoustical Society of America. 1986;79:1020. doi: 10.1121/1.393374. [DOI] [PubMed] [Google Scholar]
- 13.Gordon-Salant S. Hearing loss and aging: New research findings and clinical implications. The Journal of Rehabilitation Research and Development. 2005;42(4s):9. doi: 10.1682/JRRD.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg S, Marsh JT, Brown WS, et al. Neural temporal coding of low pitch. I. Human frequency-following responses to complex tones. Hearing Research. 1987;25(2–3):91–114. doi: 10.1016/0378-5955(87)90083-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3558136. [DOI] [PubMed] [Google Scholar]
- 15.Glaser EM, Suter CM, Dasheiff R, et al. The human frequency-following response: its behavior during continuous tone and tone burst stimulation. Electroencephalography and Clinical Neurophysiology. 1976;40(1):25–32. doi: 10.1016/0013-4694(76)90176-0. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/55345. [DOI] [PubMed] [Google Scholar]
- 16.Hall J. Auditory brainstem frequency following responses to waveform envelope periodicity. Science. 1979;205(4412):1297–1299. doi: 10.1126/science.472748. Retrieved from http://www.sciencemag.org/content/205/4412/1297.short. [DOI] [PubMed] [Google Scholar]
- 17.Heinz MG, Swaminathan J. Quantifying envelope and fine-structure coding in auditory nerve responses to chimaeric speech. Journal of the Association for Research in Otolaryngology: JARO. 2009;10(3):407–23. doi: 10.1007/s10162-009-0169-8. http://doi.org/10.1007/s10162-009-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry KS, Heinz MG. Diminished temporal coding with sensorineural hearing loss emerges in background noise. Nature Neuroscience. 2012;15(10):1362–4. doi: 10.1038/nn.3216. http://doi.org/10.1038/nn.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry KS, Heinz MG. Effects of sensorineural hearing loss on temporal coding of narrowband and broadband signals in the auditory periphery. Hearing Research. 2013:1–9. doi: 10.1016/j.heares.2013.01.014. http://doi.org/10.1016/j.heares.2013.01.014. [DOI] [PMC free article] [PubMed]
- 20.Hopkins K, Moore BCJ. The effects of age and cochlear hearing loss on temporal fine structure sensitivity, frequency selectivity, and speech reception in noise. The Journal of the Acoustical Society of America. 2011;130(1):334–49. doi: 10.1121/1.3585848. http://doi.org/10.1121/1.3585848. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz AR, Dubno JR, Ahlstrom JB. Recognition of low-pass-filtered consonants in noise with normal and impaired high-frequency hearing. The Journal of the Acoustical Society of America. 2002;111:409. doi: 10.1121/1.1427357. [DOI] [PubMed] [Google Scholar]
- 22.Joris PX. Interaural time sensitivity dominated by cochlea-induced envelope patterns. The Journal of neuroscience. 2003;23(15):6345–6350. doi: 10.1523/JNEUROSCI.23-15-06345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kale S, Heinz MG. Envelope coding in auditory nerve fibers following noise-induced hearing loss. Journal of the Association for Research in Otolaryngology. 2010;11(4):657–673. doi: 10.1007/s10162-010-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan A. Human frequency-following responses to two-tone approximations of steady-state vowels. Audiology and Neurotology. 1999;4(2):95–103. doi: 10.1159/000013826. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan A, Parkinson J. Human frequency-following response: representation of tonal sweeps. Audiology and Neurotology. 2000;5(6):312–321. doi: 10.1159/000013897. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan A. Human frequency-following responses: representation of steady-state synthetic vowels. Hearing Research. 2002;166(1–2):192–201. doi: 10.1016/s0378-5955(02)00327-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12062771. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan Ananthanarayan, Xu Y, Gandour J, et al. Encoding of pitch in the human brainstem is sensitive to language experience. Brain research. Cognitive brain research. 2005;25(1):161–8. doi: 10.1016/j.cogbrainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan Ananthanarayan, Xu Y, Gandour JT, et al. Human frequency-following response: representation of pitch contours in Chinese tones. Hearing research. 2004;189(1–2):1–12. doi: 10.1016/S0378-5955(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan A, Gandour JT, Bidelman GM, Swaminathan J. Experience dependent neural representation of dynamic pitch in the brainstem. Neuroreport. 2009;20(4):408. doi: 10.1097/WNR.0b013e3283263000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan A, Plack CJ. Neural encoding in the human brainstem relevant to the pitch of complex tones. Hearing research. 2011;275(1):110–119. doi: 10.1016/j.heares.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Leek M, Summers V. Reduced frequency selectivity and the preservation of spectral contrast in noise. The Journal of the Acoustical Society of America. 1996;(April):1796–1806. doi: 10.1121/1.415999. Retrieved from http://link.aip.org/link/?JASMAN/100/1796/1. [DOI] [PubMed]
- 32.Lorenzi C, Debruille L, Garnier S, et al. Abnormal processing of temporal fine structure in speech for frequencies where absolute thresholds are normal. The Journal of the Acoustical Society of America. 2009;125(1):27–30. doi: 10.1121/1.2939125. http://doi.org/10.1121/1.2939125. [DOI] [PubMed] [Google Scholar]
- 33.Marmel F, Linley D, Carlyon RP, Gockel HE, Hopkins K, Plack CJ. Subcortical neural synchrony and absolute thresholds predict frequency discrimination independently. Journal of the Association for Research in Otolaryngology. 2013;14(5):757–766. doi: 10.1007/s10162-013-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meddis R, O’Mard L. A unitary model of pitch perception. The Journal of the Acoustical Society of America. 1997;102(3):1811–20. doi: 10.1121/1.420088. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9714929. [DOI] [PubMed] [Google Scholar]
- 35.Miller RL, Schilling JR, Franck KR, et al. Effects of acoustic trauma on the representation of the vowel “eh” in cat auditory nerve fibers. The Journal of the Acoustical Society of America. 1997;101(6):3602–16. doi: 10.1121/1.418321. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9193048. [DOI] [PubMed] [Google Scholar]
- 36.Moore BC, Peters RW. Pitch discrimination and phase sensitivity in young and elderly subjects and its relationship to frequency selectivity. The Journal of the Acoustical Society of America. 1992;91(5):2881–2893. doi: 10.1121/1.402925. [DOI] [PubMed] [Google Scholar]
- 37.Moore BC, Carlyon RP. Pitch. Springer; New York: 2005. Perception of pitch by people with cochlear hearing loss and by cochlear implant users; pp. 234–277. [Google Scholar]
- 38.Palmer AR, Moorjani PA. Responses to speech signals in the normal and pathological peripheral auditory system. Progress in brain research. 1992;97:107–115. doi: 10.1016/s0079-6123(08)62268-2. [DOI] [PubMed] [Google Scholar]
- 39.Pichora-Fuller MK, Singh G. Effects of Age on Auditory and Cognitive Processing: Implications for Hearing Aid Fitting and Audiologic Rehabilitation. Trends in Amplification. 2006;10(1):29–59. doi: 10.1177/108471380601000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plyler PN, Ananthanarayan aK. Human frequency-following responses: representation of second formant transitions in normal-hearing and hearing-impaired listeners. Journal of the American Academy of Audiology. 2001;12(10):523–33. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11791939. [PubMed] [Google Scholar]
- 41.Sayles M, Winter IM. Reverberation challenges the temporal representation of the pitch of complex sounds. Neuron. 2008;58(5):789–801. doi: 10.1016/j.neuron.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 42.Simon H, Yund E. Frequency discrimination in listeners with sensorineural hearing loss. Ear and hearing. 1993 doi: 10.1097/00003446-199306000-00006. Retrieved from http://journals.lww.com/ear-hearing/Abstract/1993/06000/Frequency_Discrimination_in_Listeners_with.6.aspx. [DOI] [PubMed]
- 43.Smalt CJ, Krishnan A, Bidelman GM, et al. Distortion products and their influence on representation of pitch-relevant information in the human brainstem for unresolved harmonic complex tones. Hearing Research. 2012;292(1–2):26–34. doi: 10.1016/j.heares.2012.08.001. http://doi.org/10.1016/j.heares.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith J, Marsh J, Brown W. Far-field recorded frequency-following responses: evidence for the locus of brainstem sources. Electroencephalography and Clinical Neurophysiology. 1975;39(5):465–472. doi: 10.1016/0013-4694(75)90047-4. Retrieved from http://www.sciencedirect.com/science/article/pii/0013469475900474. [DOI] [PubMed] [Google Scholar]
- 45.Summers V, Leek M. F0 processing and the seperation of competing speech signals by listeners with normal hearing and with hearing loss. Journal of Speech, Language and Hearing Research. 1998 doi: 10.1044/jslhr.4106.1294. Retrieved from http://jslhr.asha.org/cgi/content/abstract/41/6/1294. [DOI] [PubMed]
- 46.Tyler RS, Wood EJ, Fernandes M. Frequency resolution and discrimination of constant and dynamic tones in normal and hearing-impaired listeners. The Journal of the Acoustical Society of America. 1983;74(4):1190–9. doi: 10.1121/1.390043. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6643841. [DOI] [PubMed] [Google Scholar]
- 47.Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. European Journal of Neuroscience. 2002;16(12):2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- 48.Wong J, Miller R, Calhoun B. Effects of high sound levels on responses to the vowel/ε/in cat auditory nerve. Hearing Research. 1998;123:61–77. doi: 10.1016/s0378-5955(98)00098-7. Retrieved from http://www.sciencedirect.com/science/article/pii/S0378595598000987. [DOI] [PubMed] [Google Scholar]