Abstract
Summary
Physical exercise benefits bone structure and mineralization, especially in children. Immediately following high-impact exercise, PTH increased and returned to resting values within 24 h in both groups, while sclerostin increased in men but not in boys. The underlying mechanisms and implication of this age-related differential response are unclear.
Introduction
Circulating sclerostin, a negative regulator of bone, decreases during puberty and increases in adulthood. Parathyroid hormone (PTH) is inversely related to sclerostin. In mice, sclerostin decreases following 24 h of mechanical stimulation. Its response to exercise in humans and, especially in children, in whom high-impact physical exercise benefits bone structure and mineralization is unclear. The aim of this study was to investigate the acute response of sclerostin to a single exercise session of high mechanical loading and the corresponding changes in PTH in boys and men.
Methods
Twelve boys (10.2±0.4 years old) and 17 young men (22.7±0.8 years old) underwent a protocol of plyometric exercises (total 144 jumps). Blood samples were collected pre-, 5 min, 1 h, and 24 h post-exercise.
Results
Boys had significantly higher resting values of sclerostin compared with men (150±37 vs. 111±34 pg/ml, respectively, p=0.006). Following exercise, sclerostin markedly increased in men but this response was attenuated in boys (at 5 min: 51±38 vs. 14±21 %, respectively, p=0.005). PTH levels were similar in boys and men at rest and throughout the 24-h study period, increasing significantly (p<0.001) 5 min after exercise, decreasing after 60 min post-exercise and returning to resting values within 24 h.
Conclusion
Although the PTH response was similar in boys and men, the sclerostin response was greater in men. The combined increases in PTH and sclerostin immediately post-exercise appear contrary to the accepted osteogenic effect of exercise. The underlying mechanisms and full implication of the differential response between children and adults need to be further examined.
Keywords: Bone, Children, Exercise, Mechanical loading, Osteocyte, Sost
Introduction
Osteocytes are believed to be the mechanosensors in bone tissue [1]. One possible way in which osteocytes coordinate the osteogenic response to mechanical loading is through sclerostin, an osteocyte-specific glycoprotein, which negatively regulates bone formation through inhibition of the Wnt/β-catenin pathway [2]. In mice, deletion of the Sost gene (and sclerostin expression) results in high bone mineral density [3], while overexpression of Sost results in low bone mass [4]. In humans, resting levels of sclerostin appear to increase early in life until puberty, subsequently declining during late puberty and reaching a nadir in young adulthood [5]. Sclerosteosis and van Buchem disease are both characterized by low sclerostin levels, which allow for greater bone formation, resulting in high bone mineral density [6, 7]. Sclerostin is also inversely related to parathyroid hormone (PTH) in cases of hyper- or hypothyroidism, as well as in healthy elderly men and women [8]. The relationship between resting PTH and sclerostin levels in healthy children or young adults is not clear.
In response to mechanical loading, it has been suggested that sclerostin production by osteocytes is suppressed, thereby increasing the osteogenic effect of the Wnt/β-catenin pathway and resulting in increased bone formation [9]. Several animal studies have demonstrated that chronic mechanical stimulation of bone (2 days) results in reduced osteocyte expression of sclerostin 24 h after loading [1, 9, 10]. Conversely, chronic unloading increases sclerostin expression [1, 9]. No such data exist in humans, but high levels of physical activity (walking, running) have been shown to result in reduced resting sclerostin levels in pre-menopausal women [11]. The relationship between PTH and sclerostin response to chronic exercise or physical activity in humans has not been examined.
The acute sclerostin response to exercise is unclear. Information about such response could be valuable in understanding the mechanism(s) responsible for the effect of mechanical loading on bone cells. In mice, reduced sclerostin levels and mRNA were reported 24 h following 2 days of bone loading [1, 10], but the immediate effect was not reported. We have previously demonstrated that, at rest, bone turnover markers are higher in boys compared with those in men and that one session of jumping exercise leads to increases in bone formation markers in both boys and men [12]. Moreover, boys appeared to be more responsive to the mechanical (jumping) stimuli.
The purpose of this study was to investigate the acute response of sclerostin and PTH to a single exercise session of high mechanical loading in boys and men. Based on the previous findings of reduced sclerostin levels 24 h following 2 days of bone loading in mice [1, 10], we hypothesized a reduction in sclerostin 24 h following exercise in both groups. Based on current views that late childhood and early adolescence serve as a critical period in terms of bone accrual and its response to weight-bearing exercise [13], and our previous findings of increased bone turnover following exercise in children [12], we hypothesized that the post-exercise sclerostin decrease would be greater in boys compared with that in men. We also postulated that the PTH response would be inversely related to sclerostin.
Methods
Participants
Twelve boys (10.2±0.4 years) and 17 men (22.7±0.8 years) participated in the study. Exclusion criteria included the following: (1) obesity (body fat percentage ≥30 %), (2) previous or current fracture, (3) premature growth or growth delay, and (4) use of drugs known to affect bone. Note that some of the participants took part in another study and have been previously described [12] (secondary analysis).
Procedures
The study and all related procedures were cleared by the University Research Ethics Board. Prior to testing, an informed consent and an assent form were signed by the participant and the parent/guardian. Participants were instructed to refrain from alcohol and caffeine use, as well as from vigorous or impact exercise for a minimum of 24 h prior to testing. In order to control for potential circadian rhythm variation, all testing took place in the morning (9 a.m.), about 60–90 min after a light breakfast. Participants completed a questionnaire package which included medical information, maturity, physical activity, and dietary intake. A resting blood sample was drawn pre-exercise (see details below), 5 min and 1 h post-exercise, as well as 24 h post-exercise. Anthropometric measures included height, body mass, and body composition.
Exercise protocol
The exercise protocol was modified from the one used by MacKelvie et al. [14] in long-term school-based interventions designed to increase bone accrual and was previously described [12]. Briefly, participants performed an exercise circuit, which consisted of six jump stations for a total of 144 jumps (three sets of eight repetitions, with 3 min recovery between sets). In an attempt to provide a similar impact loading in the two groups, drop jumps and hurdle heights were adjusted to participants’ height (drop jumps: 75 cm for men and 40 cm for boys; hurdle jumps: 40 cm for men and 15 cm for boys). Landing impact was assumed to be related to body mass. Participants were asked to jump to their highest potential for each jump and were familiarized with each of the exercises before performing the full circuit. Heart rate during the exercise was >80 % of the age-predicted maximum heart rates in both groups (men, 172±1 bpm; boys, 178±1 bpm). Mean vertical acceleration values were measured using accelerometry and were found to be within the vigorous exercise intensity zone for both groups.
Baseline measurements
Height (Ellard Instruments, Monroe, WA, USA) and mass (InBody 520 system Biospace 2008) were measured while participants wore light clothing and no shoes. Body fat percentage was assessed using skinfold thickness measurements, using a Harpenden caliper, and the Slaughter et al. [15] equations. Sexual maturity was self-assessed by boys using drawings of secondary sexual characteristics (pubic hair), as defined by Tanner [16]. Somatic maturity was determined by calculating the years from the estimated age of peak height velocity (PHV), using the equation suggested by Mirwald et al. [17]. Current and past-year physical activity were assessed using the Godin-Shephard leisure time exercise questionnaire [18] and the past year physical activity questionnaire (PYPAQ) [19]. Dietary intake was evaluated over 24 h, using the 24-h nutrition recall questionnaire and analyzed using Nutritionist Pro™ (Axxya Systems, USA).
Biochemical analysis
Resting venous blood samples were drawn using serum tubes (BD Vacutainer®) pre-exercise (9:00–9:30 a.m.), 5 min and 1 h post-exercise, as well as 24 h post-exercise from a vein in the antecubital fossa. The serum was separated and aliquoted within 1 h post-blood draw into 0.5-ml polyethylene tubes which were then stored at −80 °C until analysis.
Serum PTH was determined using a commercial ELISA assay (ALPCO, cat# 21-IPTHU-E01), with a detection range from 7 to 700 pg/ml and a sensitivity of 1.57 pg/ml. This assay determines intact PTH 1–84, which is the biologically active form of the hormone. PTH intra-assay coefficient of variation (CV) ranged from 3.1 to 5.4 with an average of 3.5; whereas inter-assay CV ranged from 1 to 7.6 % (3.3 average). Serum sclerostin (SOST) was determined using a commercial ELISA Assay (R&D cat# DSST00) with a detection range from 31.3 to 2000 pg/ml and a sensitivity of 3.8 pg/ml. SOST intra-assay CV ranged from 0.5 to 4 with an average of 2.5; whereas inter-assay CV ranged from 2.3 to 12 (4.5 average).
Statistical analysis
Group differences in baseline measures were examined using an independent t test. Group differences over time in sclerostin and PTH concentrations were examined using a two-way analysis of variance for repeated measures (between-subject effect: group, within-subject effect: time). Bivariate correlations were examined using Pearson product-moment correlation coefficients. Data were analyzed using SPSS version 21.0 and are reported as means and standard error.
Results
The mean age and physical characteristics for both groups are presented in Table 1. Seven of the boys were prepubertal (stage 1), two boys were early pubertal (stage 2), and three boys were mid-pubertal (stage 3) [16]. Results of the physical activity questionnaires and nutritional intake were reported elsewhere [12]. Briefly, no differences were observed between groups in terms of current or past-year physical activity. There were no differences between groups in calcium and vitamin D intake. Daily energy intake, expressed relative to body mass, was significantly higher in boys (p<0.006). No differences were observed between groups in fat and protein intake, when expressed relative to body mass.
Table 1.
Physical characteristics and body composition for men and boys
| Men (n=17) | Boys (n=12) | p value | |
|---|---|---|---|
| Age (years) | 22.7±0.8 | 10.2±0.4 | <0.001 |
| Height (cm) | 180.4±1.6 | 142.6±2.0 | <0.001 |
| Body mass (kg) | 79.0±2.7 | 34.4±1.5 | <0.001 |
| Body fat (%) | 15.9±1.5 | 13.4±1.1 | NS |
| Years to PHV | – | −2.9±0.2 | – |
All values are expressed as means±SE
PHV peak height velocity
Resting sclerostin values were significantly higher in boys compared with those in men (Table 2). No significant differences were observed in resting PTH concentration between groups. As expected, resting PTH levels in both groups, combined, were negatively correlated with resting sclerostin levels (r=−0.42, p=0.025).
Table 2.
Resting PTH and sclerostin levels in men and boys
| Men (n=17) | Boys (n=12) | p value | |
|---|---|---|---|
| PTH (pg·ml−1) | 44.54±4.30 | 37.05±3.26 | 0.27 |
| Sclerostin (pg·ml−1) | 111.5±8.3 | 150.4±10.7 | 0.006 |
All values are expressed as means±SE
The change in sclerostin levels over time are demonstrated in Fig. 1. There was a significant group-by-time interaction (p<0.001), reflecting an increase in sclerostin 5 min post-exercise in men but not in boys. While sclerostin levels were consistently higher in boys before and after exercise, group effect did not reach statistical significance (p=0.065).
Fig. 1.
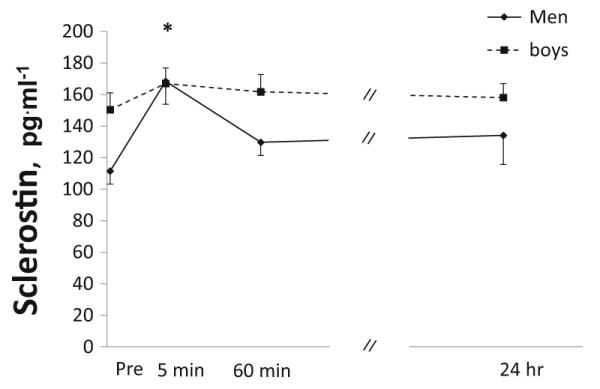
Sclerostin concentrations at baseline (immediately prior to exercise) and post-exercise (5 min, 1 h, and 24 h) in boys and men. Values are expressed as mean±SE. *Significant increase in men but not in boys (group-by-time interaction)
The change in PTH over time is demonstrated in Fig. 2. PTH increased 5 min post-exercise and then decreased at 60 min post-exercise (time effect, p<0.001). Values at 24 h post-exercise were similar to pre-exercise, resting values. No differences were observed between groups (no group effect, p=0.90; no group-by-time interaction, p=0.21). The post-exercise change in PTH was related to the post-exercise change in sclerostin in men (r=0.51, p=0.03) but not in boys.
Fig. 2.
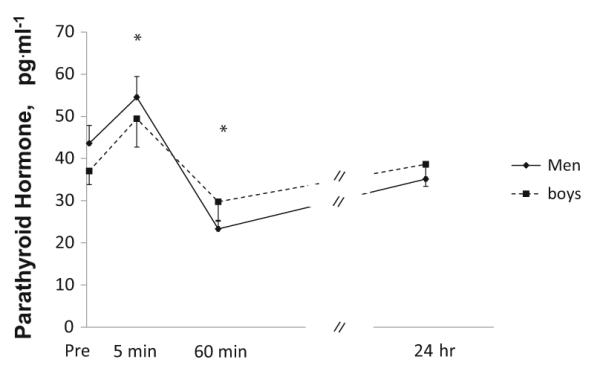
Parathyroid concentrations at baseline (immediately prior to exercise) and post-exercise (5 min, 1 h, and 24 h) in boys and men. Values are expressed as mean±SE. *Significantly different from baseline in both groups (time effect)
Discussion
To our knowledge, this is the first study to examine the acute responses of sclerostin and PTH to a single exercise session in humans. Specifically, we examined the responses to high mechanical loading in boys and in men. Contrary to our hypotheses, the results demonstrate that sclerostin levels increased immediately post-exercise in young men, with no change in boys, following one session of high-impact exercise. The same exercise resulted in a similar PTH response in boys and men with PTH increasing immediately after exercise, then decreasing within 1 h in both boys and men.
The combined post-exercise transient increases in PTH and sclerostin, specifically in men, are contrary to the accepted osteogenic chronic effect of exercise. There are several possible explanations for this apparent discrepancy between the acute and chronic effects of exercise. It is possible that the post-exercise sclerostin serum levels in the current study reflect pre-existing sclerostin from the canalicular-lacunar system, which was “flushed” out through exercise-induced fluid movement in bone. However, this does not explain the differential response between boys and men. It is also plausible that regulators in the Wnt pathway, other than sclerostin (e.g., DKK1), independently influence bone’s response to acute exercise. Of note, one may also speculate that the exercise session resulted in an initial catabolic response, especially in men, to be followed later by an anabolic response. Indeed, immediately following intense exercise, Mezil et al. [20] demonstrated elevated bone resorption markers in young men. Twenty-four hours following exercise, we observed an increase in bone-specific alkaline phosphatase, reflecting an increase in bone formation, in both boys and men [12]. This type of catabolic-anabolic coupling is in line with previous findings of reduced IGF-1 levels shortly following intense aerobic training in adolescents [21]. It is also parallel to the acute inflammatory response immediately post-exercise [20], followed by a general reduced inflammatory status following training [22]. Thus, the immediate increase in PTH and sclerostin may reflect an initial catabolic response, which is later followed by an anabolic response to exercise.
The differential sclerostin response in boys and men may be related to the boys’ higher resting values. Assuming that an increase in sclerostin would increase osteoclastogenesis and bone resorption [23], the fact that sclerostin did not increase in boys may serve as a protective effect, moderating the post-exercise catabolic response. This is in line with the accepted view that late childhood and early adolescence, the developmental stages of our participants, serve as a critical period for bone accretion [13]. Alternatively, although the exercise was designed to provide a similar loading stimulus for boys and men (same number of jumps, height adjusted), it may not have provided the same stress on the tissues. That is, boys’ habitual physical activity typically involves more jumping-type of activities (e.g., ball games). Therefore, they may have been more habituated to this type of stimulus.
Examining the immediate bone cell response to exercise can provide insight into the mechanism(s) responsible for the effect of mechanical loading on bone cells and bone tissue. The differential sclerostin response between boys and men observed in this study suggests that the internal structure or cellular properties of bone in children result in different transduction of mechanical stimuli.
Acknowledgments
The authors would like to thank all the participants and their parents who volunteered to participate in this study. The study was funded by a Brock University grant and by NIH Grant P01HD-04872.
Footnotes
Statement of human rights All procedures performed in this study were in accordance with the ethical standards of Brock University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of interest None
References
- 1.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 2.Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, Danzig J, Robling AG. Mechanobiology of the skeleton. Sci Signal. 2009;2(pt3) doi: 10.1126/scisignal.268pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 4.Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirmani S, Amin S, McCready LK, Atkinson EJ, Melton LJ, 3rd, Muller R, Khosla S. Sclerostin levels during growth in children. Osteoporos Int. 2012;23:1123–1130. doi: 10.1007/s00198-011-1669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 7.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R. Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab. 2013;98:3873–3883. doi: 10.1210/jc.2013-2113. [DOI] [PubMed] [Google Scholar]
- 9.Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–1234. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardawi MS, Rouzi AA, Qari MH. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab. 2012;97:3691–3699. doi: 10.1210/jc.2011-3361. [DOI] [PubMed] [Google Scholar]
- 12.Kish K, Mezil Y, Ward WE, Klentrou P, Falk B. Effects of plyometric exercise session on markers of bone turnover in boys and young men. Eur J Appl Physiol. 2015 doi: 10.1007/s00421-015-3191-z. [DOI] [PubMed] [Google Scholar]
- 13.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? A systematic review. Br J Sports Med. 2002;36:250–257. doi: 10.1136/bjsm.36.4.250. discussion 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112:e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 15.Slaughter MH, Lohman TG, Boileau BA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 16.Tanner JM. Growth at adolescence. Blackwell Scientific Publications; Oxford: 1962. [Google Scholar]
- 17.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 19.Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 20.Mezil YA, Allison D, Kish K, Ditor D, Ward WE, Tsiani E, Klentrou P. Response of bone turnover markers and cytokines to high-intensity low-impact exercise. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 21.Eliakim A, Brasel JA, Mohan S, Barstow TJ, Berman N, Cooper DM. Physical fitness, endurance training, and the growth hormone-insulin-like growth factor I system in adolescent females. J Clin Endocrinol Metab. 1996;81:3986–3992. doi: 10.1210/jcem.81.11.8923848. [DOI] [PubMed] [Google Scholar]
- 22.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, McFarlin BK, Coen PM, Talbert E. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 23.Morse A, McDonald MM, Kelly NH, Melville KM, Schindeler A, Kramer I, Kneissel M, van der Meulen MC, Little DG. Mechanical load increases in bone formation via a sclerostin-independent pathway. J Bone Miner Res. 2014;29:2456–2467. doi: 10.1002/jbmr.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]


