Abstract
Background
Tenofovir disoproxil fumarate (TDF), a prodrug of tenofovir (TFV), may be ideal for topical HIV preexposure prophylaxis because it has higher tissue and cell permeability than TFV, is not adversely impacted by seminal proteins, and its active metabolite, TFV-diphosphate (TFV-DP), has a long intracellular half-life. We engineered a TDF eluting polyurethane reservoir intravaginal ring (IVR) to provide near constant mucosal antiretroviral concentrations.
Methods
A first-in-human randomized placebo-controlled trial was conducted to assess the safety and pharmacokinetics of the TDF IVR in healthy, sexually abstinent women (15 TDF and 15 placebo). Drug concentrations were measured in cervicovaginal fluid (CVF) obtained by swab, cervical tissue, plasma, and dried blood spots (DBS) over 14 days of continuous ring use.
Results
There were 43 total, 23 reproductive tract, and 8 product-related Grade 1 adverse events. Steady state CVF TFV concentrations were achieved proximal (vagina, ectocervix) and distal (introitus) to the TDF IVR one day after ring insertion. Median tissue TFV-DP concentrations 14 days after TDF IVR placement were 120 fmol/mg (interquartile range 90, 550). CVF collected from the cervix one week and two weeks after TDF IVR insertion provided significant protection against ex vivo HIV challenge. Eleven of 14 (78%) participants had detectable TFV-DP DBS concentrations 14 days after TDF IVR placement, suggesting that DBS may provide a surrogate marker of adherence in future clinical trials.
Conclusions
A TDF IVR is safe, well tolerated, and results in mucosal TFV concentrations that exceed those associated with HIV protection. The findings support further clinical evaluation of this TDF IVR.
Keywords: HIV prevention, intravaginal ring, tenofovir disoproxil fumarate, topical preexposure prophylaxis, vaginal microbicide, pharmacokinetics
Introduction
Worldwide 16 million women ≥15 years are living with HIV [1]. Two trials demonstrated that the formulation of oral tenofovir disoproxil fumarate (TDF) and emtricitabine (Truvada) can be effective as preexposure prophylaxis (PrEP) in women to prevent heterosexual HIV transmission [2, 3]. Another study of daily oral Truvada in women was discontinued early due to futility and subsequently linked to low adherence [4]. Attempts at preventing HIV transmission in women with tenofovir (TFV) vaginal gel dosed daily or pericoitally yielded disappointing results, which were also linked to low adherence [4, 5]. Pericoital dosing of TFV 1% gel failed to reduce HIV acquisition in 2059 HIV-uninfected women; 70% of whom were younger than 25 [5]. Difficulties with adherence to oral and gel formulations suggest that sustained drug delivery with long-acting systemic therapy or intravaginal rings (IVRs) may prove more effective.
TDF IVR delivery for HIV prevention in women may have distinct advantages over other formulations, including the need for less frequent dosing and fewer systemic side effects. Importantly, TDF is 100-fold more potent than TFV against HIV in vitro and in animal models, reflecting greater tissue permeability and cellular uptake [6–8]. TDF retains antiviral activity when virus is introduced in seminal plasma or when cells are washed with seminal plasma as might occur following sex. In contrast, a decrease in anti-HIV activity of dapivirine was observed with seminal plasma wash, reflecting protein binding and rapid transit of drug out of cells [9]. A polyurethane reservoir IVR engineered to deliver TDF completely protected non-human primates (NHP) against 16 weekly intravaginal challenges with simian HIV (SHIV) and was highly protective in a model combining depot medroxyprogesterone acetate with 12 weekly vaginal viral challenges. Similar vaginal fluid TFV concentrations were observed proximal and distal to the ring and protection was associated with mean vaginal fluid TFV concentrations of 1.8×105 ng/mL (range 1.1×104–6.6×105 ng/mL). Notably, mean TFV concentrations >103 ng/mL persisted for days after ring removal, suggesting that protection may be provided if the ring were removed pericoitally [10, 11].
Building on these studies, a randomized, placebo-controlled trial was conducted to assess the safety of a TDF IVR and pharmacokinetics (PK) of TDF and its metabolites in cervicovaginal fluid (CVF), tissue and blood during use and after ring removal. Exploratory objectives included adherence, acceptability, and pharmacodynamic (PD) assessment by measuring HIV inhibitory activity of CVF using an ex vivo challenge model.
Materials and Methods
Study Design
The study was approved by the Albert Einstein College of Medicine Institutional Review Board and registered in clinicaltrials.gov (NCT02006264). All participants provided written informed consent. The primary outcome was to assess the safety of TDF and placebo IVRs by comparing the proportion of women in each arm who experienced Grade 1 or higher genitourinary (GU) events as defined by the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events (AE) [12] judged to be related to study product. Secondary outcomes were systemic and genital PK of TDF and its metabolites and included descriptive concentration–time curves for analytes, peak concentration (Cmax), time to peak concentration (Tmax) in plasma and CVF, and drug half-life after ring removal in CVF.
Thirty women (18–45 years) were recruited from New York City between November 2013-September 2014. Inclusion criteria were use of low-dose combined oral contraceptive pills, regular menstrual cycles, and willingness to abstain from vaginal product use and sex for the study duration. Exclusion criteria included pregnancy, breastfeeding, HIV, GU infection, vaginitis, intermenstrual bleeding, abnormal Pap test, hepatitis B infection, and abnormal renal or liver function.
At screening, participants had a pregnancy test, gynecological examination, Pap and nucleic acid amplification testing for Neisseria gonorrheae, Chlamydia trachomatis and Trichomonas vaginalis (Gen-Probe, Inc., San Diego, CA). Swabs of the lateral vaginal wall were collected for pH (Whatman pH paper, pH 3.8–5.5), and Gram stain for Nugent scoring. Blood was collected for Treponema pallidum enzyme immunoassay (Trep-Sure™ EIA, Trinity Biotech, Inc., Jamestown, NY), serotype specific antibodies for HSV-1 and HSV-2 (BioPlex 2200, Bio-Rad Laboratories, Inc., Hercules, CA), hepatitis B serologies, complete blood count, and kidney and liver function tests. An oral swab was collected for the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test (OraSure Technologies, Inc, Bethlehem, PA).
Enrollment (visit 2) occurred within 30 days of screening. Swabs were collected for pH, Nugent score, microbiome studies, and detection of semenogelin using the Rapid Stain Identification (RSID) test (Independent Forensics, Lombard, IL). Randomization (visit 3) was conducted after cessation of menses to allow for 2 weeks of continuous ring use without bleeding. Speculum exam and colposcopy were performed, and swabs for pH, Nugent score, and semen test were collected. Dry Dacron swabs were individually held at the introitus, vaginal wall and ectocervix for 2 minutes for assessment of drug levels and antiviral activity. Eligible participants were randomized 1:1 to TDF or placebo ring. The randomization was computer generated by the study statistician. This study was not double-blinded because the TDF and placebo rings are not identical in appearance, though all participants and laboratory staff were blinded to study treatment. Each ring was inserted and removed by a clinician. Participants were instructed to abstain from vaginal product use and sexual activity from 48 hours prior to enrollment until 7 days after ring removal.
Study visits 4–9 were conducted 1, 3 (range 3–5), 7, and 14 days after ring insertion and 2 (range 2–4) and 7 days after ring removal. The ring was removed 14 days after insertion. Speculum exam, swabs for pH, Nugent score, semen, drug levels and antiviral activity, and blood collection were performed at each visit. Swabs for microflora analysis were collected at Day 14 and after ring removal. Two ectocervical biopsies were collected at Day 14 for PK analysis. Rectal swabs for drug levels were optional and collected 7 and 14 days after IVR placement. Dried blood spots (DBS) for TFV-DP were obtained 7 and 14 days after TDF IVR insertion. AEs were collected at each visit. A computer-assisted quantitative survey was administered at randomization, 3, and 14 days after IVR insertion.
Manufacture and Characterization of TDF and Placebo Rings
Hydrophilic elastomer HydroThane AL 25-93A (AdvanSource Biomaterials, Inc., Wilmington, MA) tubing (wall thickness=0.7 mm) was extruded, tubing was cut to 17.1±0.1 cm in length, and one end was sealed as described previously [10, 13]. The open tube was filled with a mixture of TDF (Gilead Sciences, Inc., Foster City, CA) and NaCl (US Pharmacopeia [USP] grade, Spectrum Chemicals, New Brunswick, NJ). The formulation of TDF and NaCl (86:14) was filled to achieve a final concentration of 365±15 mg of TDF and 55±5 mg of NaCl per TDF IVR. For the placebo formulation, one-end sealed tubes were filled with 55±5 mg of NaCl per IVR. The open end of the filled tubes was sealed to form a rod and the ends of each rod were butt-welded together to form a ring. To set the shape, the rings were placed in a heated circular-shaped mold and then cooled. The rings were packaged in heat-sealed pouches (LPS Industries, Moonachie, NJ) and placed at 65°C for 5 days to load the wall of the IVR with TDF. The ring dimensions were similar to those of the commercially available NuvaRing® with outer and cross-sectional diameters of 55 and 5.5 mm respectively. IVRs were analyzed for residual drug content after removal by chemical extraction as described previously [6].
Dacron swabs of the surface and fluid extracted from the core of used IVRs were collected for quantitative culture analysis, as previously described [14]. Each swab was placed in a Port-A-Cul transport tube (Becton Dickson, Sparks, MD) and transported on ice to the Magee-Women’s Research Institute, Pittsburgh, PA within 24 hours of collection. Aerobic and anaerobic organisms were identified to the genus, species, or groups of bacteria using phenotypic tests. Lactobacillus species were tested for hydrogen peroxide production [15]. Broad-range 16S rRNA gene PCR with pyrosequencing using the 454 Life Sciences Titanium technology (Roche, Branford, CT) was used to document the vaginal microbiota with ring use [16, 17]. Samples from enrollment (visit 2), Day 14 (visit 7) and 1 week after removal (visit 9) were multiplexed using 6 bp barcodes (see Table, Supplementary Digital Content [SDC] 1), and sequences were classified (see Table, SDC 2) using the phylogenetic placement tool pplacer [18] and a curated reference set of vaginal bacteria [16]. An average of 4078 reads per sample was generated. Sequence reads have been deposited to the NCBI Short Read Archive.
Pharmacokinetics
Biopsies and swab-collected fluids were weighed, stored in separate cryovials, flash frozen in liquid nitrogen and stored at −80°C prior to analysis. TFV concentrations were quantified using liquid chromatographic-tandem mass spectrometric (LC-MS/MS) methods [19]. For TDF, sample preparation involved extraction of the analyte from the swab using a 1:1 (v/v) methanol:water solution. Post-extraction, evaporation to dryness and reconstitution, 0.05 mL sample was combined with 0.05 mL of the structural analog desmethyl-TDF (Toronto Research Chemicals, Toronto, ON) and 0.5 mL 1:1 (v/v) methanol:water. A 7.5 µL injection was introduced to the LC-MS/MS system for analysis. The lower limits of quantification (LLOQ) for TFV in plasma, tissue, CVF and rectal fluid (RF) are 0.31 ng/mL, 0.25 ng/sample and 0.625 ng/swab, respectively. For TDF, the LLOQ of the assay was 0.0625 ng/swab. For swabs and tissue, concentrations were converted to ng/mg based on the net weight of the specimen swab or biopsy. Values below the assay-specific LLOQ were reported as below the limit of quantification (BLQ). TFV-DP concentrations were also measured in tissue homogenates using validated LC-MS/MS assays [19]. LLOQ of the assay was 50 fmol/sample, and final concentrations were converted to ng/mg based on the net weight of tissue provided.
Dried Blood Spots
To measure intracellular TFV-DP 25 µL of blood from EDTA tubes were placed onto 903 Protein Saver Cards (Whatman/GE Healthcare, Piscataway, NJ), which were allowed to dry for ≥3 hours at ambient temperature. Each card was placed in a low gas-permeability plastic bag with humidity indicator card and desiccant pack. The bags were stored at −80°C. A micropuncher was used to remove a disc 3 mm in diameter from the DBS. The disc was then extracted with 500 µL of 70:30 methanol-H2O and sonication. The supernatant was analyzed using a validated LC-MS/MS assay [20]. The LLOQ of the assay was 25 fmol/sample.
Anti-HIV activity of CVF
To measure CVF HIV inhibitory activity, 3×105 Jurkat-Tat-CCR5 cells were incubated with cervical swab eluants diluted 1:1 with culture medium (RPMI-1640 containing L-glutamine, 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin) for 2 hours prior to challenge with HIV-1BaL (equivalent to 103 TCID50). Input virus was removed after 2 hours by washing the cells thrice with culture medium. HIV infection was assessed by measuring p24 levels in supernatants collected 5 days post-infection by AlphaLISA (PerkinElmer, Inc., Waltham, MA).
Statistical analysis
Demographic data and AEs were compared using chi-squared or Fisher’s exact tests for categorical variables and t-tests or Wilcoxon rank-sum test for continuous variables. Friedman tests were used to examine changes in Nugent scores and drug concentrations accounting for repeated measures taken from the same women over time. Bonferroni adjustments were applied for post-hoc comparisons between time-points. The half-life of TFV in CVF from different locations was compared using the Friedman test. CVF HIV inhibition after ring insertion compared to enrollment was assessed with the Wilcoxon signed-rank test. A sample size of 30 (15 per arm) was selected a priori with the goal of having 12 per group complete the study. The sample size is based upon the size of similar Phase 1 studies of microbicide products. All statistical analyses were performed using SAS version 9.3 (SAS Inc., Cary, NC) and GraphPad Prism Version 6 (GraphPad Software, La Jolla, CA).
Results
Study Participants
Thirty females were enrolled; 29 completed the study and 1 requested early termination (see Figure, SDC 3). There was 1 missed visit among all who completed the study. There were no significant differences in demographics or other characteristics (Table 1).
Table 1.
Demographic and clinical characteristics of participants
| TDF IVR N=15 |
Placebo IVR N=15 |
p value | |
|---|---|---|---|
| Age (years; mean ± SD) | 30.6 ± 6.9 | 28.7 ± 5.9 | 0.41 |
| Race | 0.81 | ||
| Black | 4 (27) | 5 (33) | |
| White | 6 (40) | 4 (27) | |
| Other | 5 (33) | 6 (40) | |
| Ethnicity | 0.71 | ||
| Hispanic | 5 (33) | 6 (40) | |
| Non-Hispanic | 10 (67) | 9 (60) | |
| Education (years completed) | 15 (14–16) | 15 (14–17) | 0.33 |
| Body Mass Index | 25 (23–28) | 24 (23–28) | 0.44 |
| Current cigarette smoker | 2 (13) | 0 | 0.48 |
| Ever been pregnant | 7 (47) | 7 (47) | >0.99 |
| History of receptive anal sex | 9 (60) | 10 (67) | 0.71 |
| History of receptive oral sex | 14 (93) | 15 (100) | >0.99 |
| Prior NuvaRing® use | 1 (6) | 2 (13) | >0.99 |
| History of sexually transmitted infection | 2 (13) | 2 (13) | >0.99 |
| History of bacterial vaginosis | 2 (13) | 2 (13) | >0.99 |
| HSV seropositivity | |||
| HSV-1 seropositive | 8 (53) | 12 (80) | 0.12 |
| HSV-2 seropositive | 3 (20) | 5 (23) | 0.68 |
| Nugent score at ring insertion visit | 3.3 (1.0–6.8) | 2.5 (0.5–4.5) | 0.26 |
TDF = tenofovir disoproxil fumarate; IVR = intravaginal ring; N=number; SD = standard deviation Categorical variables reported as number (%); Continuous variables reported as median (interquartile range [25%- 75%]), unless otherwise specified
Safety of IVRs
Twenty-nine AEs occurred in 12 women in the TDF arm and 14 were reported in 7 placebo recipients. Twenty-three AEs involved the reproductive tract, 8 of which were judged to be product-related in 6 participants (40%) who received TDF versus 1 (7%) who received placebo (p=0.08). All product-related AEs were due to vaginal or cervical discharge and were mild (Grade 1). An additional 8 reproductive tract AEs were related to study procedures and 7 were due to intermenstrual bleeding (see Table, SDC 4). There were 2 non-product-related Grade 2 AEs, no Grade 3–4 AEs, and no serious AEs. There were 10 colposcopic findings; 6 were present at enrollment and 4 were due to speculum trauma.
There were no changes in Nugent scores over the dosing period. At randomization, 3 participants in the TDF and 1 in the placebo group had bacterial vaginosis (BV). Three of 4 (2 TDF, 1 placebo) who had BV at randomization also had BV at the removal visit. The composition of vaginal microbiota was monitored using broad-range PCR with pyrosequencing of amplified 16S rRNA genes (see Figure, SDC 5). The bacterial community was mostly stable in placebo recipients. Transitions from L. crispatus- or L. jensenii-dominant to L. iners-dominant communities were noted in 5 TDF compared to 1 placebo recipient. One TDF recipient had Streptococcus agalactiae-dominant community after ring removal.
Product Acceptability and Protocol Adherence
Participants completed a computer-assisted survey. None reported ring removal or expulsions, 2 reported physical discomfort, and 4 were aware of the ring during normal daily activities. Twelve women (9 TDF and 3 placebo) reported that the vagina was wetter after ring use. All strongly liked or somewhat liked the ring, all reported that it was very easy or somewhat easy to wear, and all definitely or probably would recommend it to others. Although all participants reported sexual abstinence for the study duration, 8 (4 in each arm) tested positive at 1 or more visits for the presence of semen. Three participants (2 TDF, 1 placebo) were positive during ring use and 5 were positive prior to insertion or after removal.
Pharmacokinetics
CVF and tissue
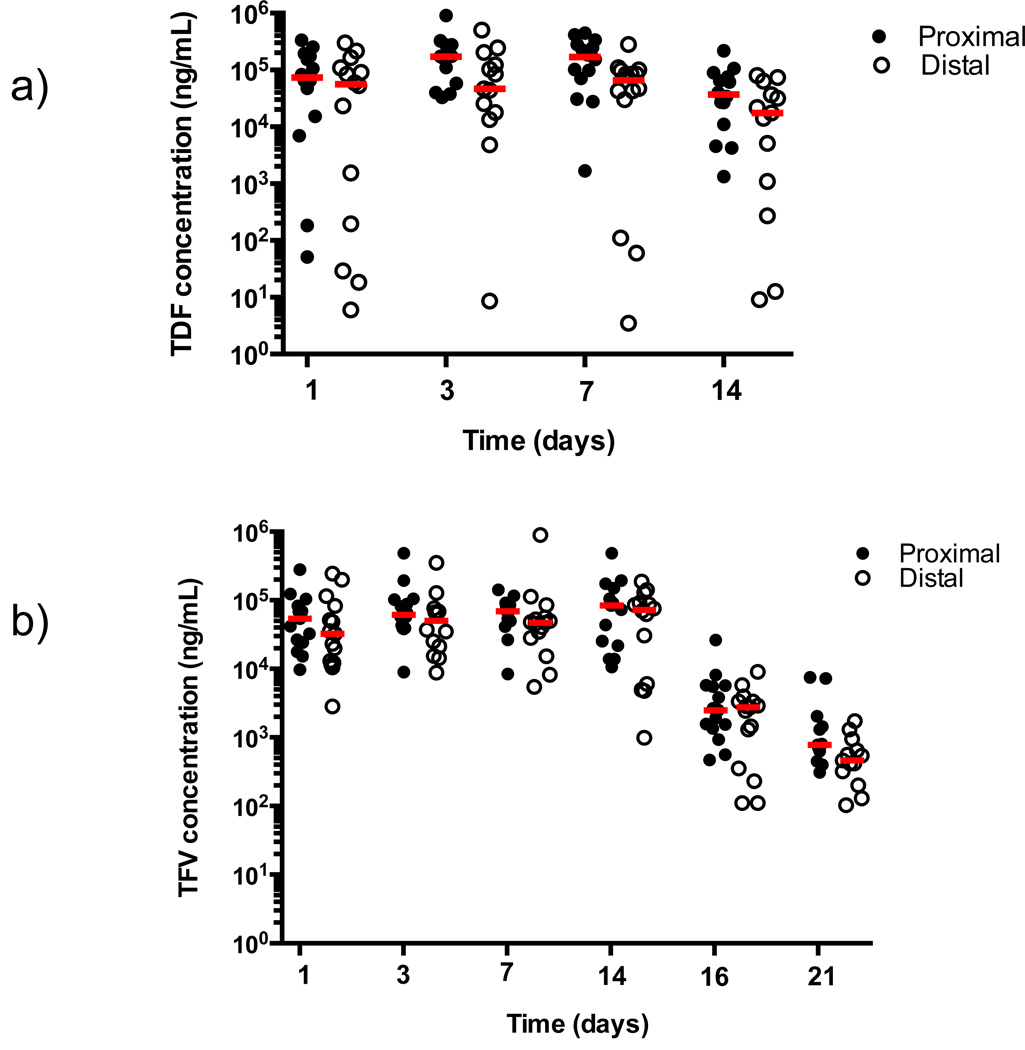
The TDF IVR provided high tenofovir disoproxil (TD) (the fumarate disassociates from TDF in solution) and TFV CVF concentrations (Figure 1 and Table 2). TD concentrations were more variable than TFV (Figure 1a). The median average vaginal TD and TFV concentrations during ring use were 1.1×105 and 7×104 ng/mL, respectively (Table 2) and concentrations did not differ significantly between sites or change significantly from Day 1-Day 14 (p=0.42, vagina; p=0.70, ectocervix; p=0.63, introitus) (Figure 2), indicating that the drug reached steady state conditions in the vagina one day after ring insertion. Moreover, although drug concentrations declined, the median TFV concentration remained above 103 ng/mL 2–4 days after ring removal. There was no quantifiable TD in CVF after ring removal, which is consistent with its hydrolysis to TFV [21]. Following removal, the median [interquartile range, IQR] initial (2-day) half-life of TFV in CVF from the vagina, ectocervix, and introitus is estimated to be 14 [11–24], 12 [10–21] and 11 [9–17] hours, respectively (p=0.78).
Figure 1. Drug Levels from Swabs Collected Proximal and Distal to TDF IVR.
Samples were collected proximal (vaginal wall, closed symbols) and distal (introitus, open symbols) to IVR placement for TDF (a) and TFV (b) concentrations at the indicated time-points (1, 3–5, 7, and 14 days after ring insertion and 2–4 and 7 days after ring removal). Each data point represents a single sample and the red bar corresponds to the median for each time-point. TDF levels are missing from the vaginal and introitus at Day 3 from 1 participant and the majority of samples at Days 16 and 21 are below the limit of quantification (BLQ) and are not shown. TFV levels are missing from the vagina and introitus at Day 3 from 1 participant, 1 sample from the introitus at Day 14 could not be reported, and 2 samples from the vagina and introitus at Day 21 are BLQ.
Table 2.
Summary of drug concentrations in CVF, plasma, and tissue
| Matrix & Analyte |
N | Cave (ng/mL) median (IQR) |
N | Cmax (ng/mL) median (IQR) |
N | Tmax median (IQR) |
N | C14 (ng/mL) median (IQR) |
N | AUC0–14 (ng/mL) median (IQR) |
|---|---|---|---|---|---|---|---|---|---|---|
| CVF VAG TDF | 14 | 1.1×105 (5.6×104–1.5×105) | 14 | 2.4×105 (1.4×105–3.6×105) | 14 | 6 (3–7) | 14 | 3.7×104 (9.4×103–7.9×104) | 14 | 2.0×106 (6.9×105–3.2×106) |
| CVF ECX TDF | 14 | 6.6×104 (2.6×104–1.3×105) | 14 | 2.1×105 (8.1×104–3.2×105) | 14 | 5 (4–7) | 12 | 7.0×103 (1.0×103–4.4×104) | 13 | 1.3×106 (3.4×105–2.1×106) |
| CVF INT TDF | 14 | 4.1×104 (1.6×104–7.5×104) | 14 | 9.6×104 (3.7×104–1.7×105) | 14 | 4 (1–6) | 13 | 1.7×104 (6.8×102–5.0×104) | 13 | 6.1×105 (2.4×105–1.2×106) |
| CVF VAG TFV | 15 | 7.0×104 (4.4×104–1.3×105) | 15 | 9.1×104 (7.3×104–1.9×105) | 15 | 14 (1–14) | 15 | 8.4×104 (2.2×104–1.5×105) | 15 | 1.1×106 (6.7×105–2.1×106) |
| CVF ECX TFV | 15 | 5.8×104 (3.5×104–8.4×104) | 15 | 8.5×104 (5.2×104–1.7×105) | 15 | 7 (3–14) | 13 | 4.6×104 (2.3×104–1.3×105) | 13 | 9.7×104 (5.7×105–1.3×106) |
| CVF INT TFV | 15 | 6.3×104 (1.6×104–1.1×105) | 15 | 9.2×104 (3.6×104–2.0×105) | 15 | 4 (1–14) | 14 | 7.3×104 (5.8×103–1.0×105) | 14 | 9.4×105 (2.5×105–1.3×106) |
| Tissue TFV | 15 | 5.4 (2.8–8.8) ng/mg | ||||||||
| Tissue TFV-DP | 15 | 120 (90–550) fmol/mg | ||||||||
| Plasma TFV | 15 | 1.9 (1.2–2.4) | 12 | 14 (14–14) | 15 | 1.5 (BLQ-2.1) | 15 | 9.4 (6.9–14.4) |
N = number; IQR = interquartile range (25th% –75th%); Cave = average concentration during ring use (visits 4–7); Cmax = maximum concentration; Tmax = time to maximum concentration in days; C14 = concentration at 14 days; AUC0–14 = area under the curve from day 0 to 14 in concentration-days; CVF = cervicovaginal fluid; VAG = vagina; TDF = tenofovir disoproxil fumarate; ECX = ectocervix; INT = introitus; TFV = tenofovir; TFV-DP = tenofovir diphosphate
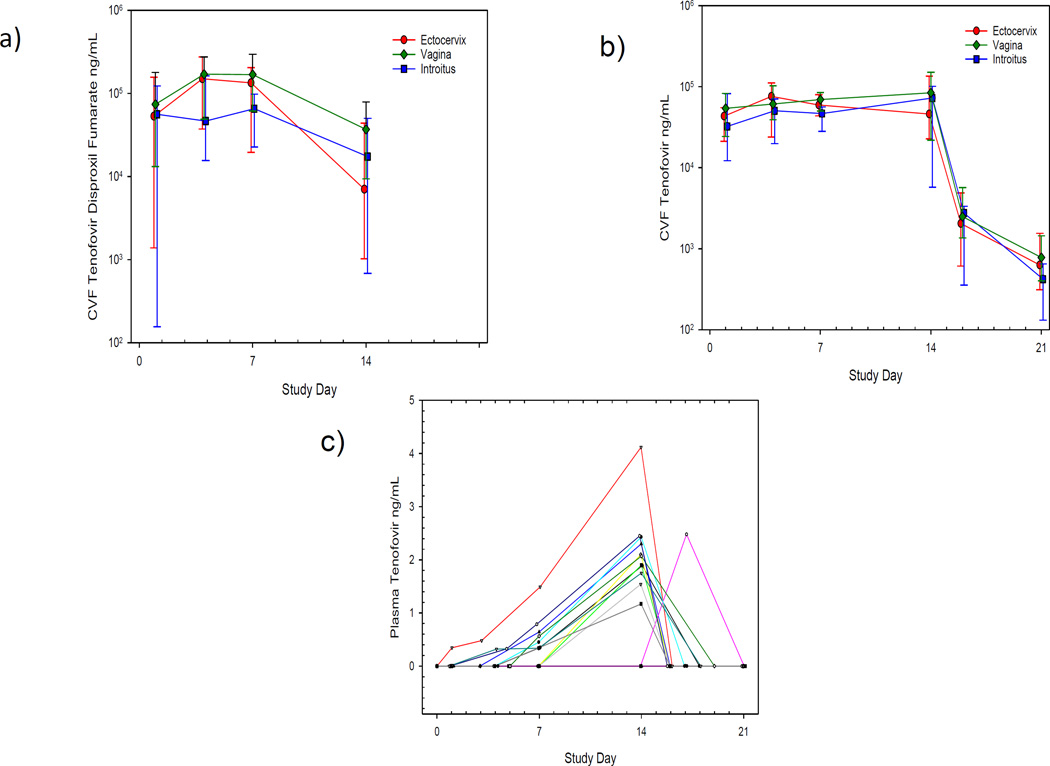
Figure 2. CVF and Plasma Concentrations Versus Time.
CVF TDF (a) and CVF TFV (b) concentration versus time plots are shown for the 14-day dosing period and the week following IVR removal. Median with asymmetric upper and lower quartiles is shown. Plots are shown of plasma TFV concentrations in relation to time for the 15 women who received a TDF IVR (c).
Ectocervical biopsies were collected on Day 14 for quantification of tissue TFV and TFV-DP concentrations. Median [IQR] tissue TFV and TFV-DP levels were 5.4 [2.8–8.8] ng/mg and 120 [90–550] fmol/mg, respectively (Table 2).
Rectal fluid
TFV was quantifiable in all 5 participants who consented to anoscopy at Day 7 (443 ng/mL [216–1939]) and in 4 of 5 (80%) at Day 14 (767 ng/mL [279–3222]). The median CVF vaginal to RF ratio was 104 [59–505] on Day 7 and 240 [36–420] on Day 14.
Plasma and DBS
Plasma TFV concentrations were quantifiable in 53% (8/15) women at Day 7 (0.34 ng/mL [BLQ, 0.56]) and 73% (11/15) women at Day 14 (1.5 ng/mL [BLQ, 2.1]). TFV-DP concentrations in DBS were measured to explore the possibility that DBS may provide a marker of recent and cumulative adherence in future studies. TFV-DP was detected in 78% (11/14) women at Day 14 (117 fmol/punch [63–260]). Only one woman had detectable TFV-DP in DBS on Day 7, which is consistent with the median red blood cell (RBC) TFV-DP half-life of ~17 days [15.7–20.2] following oral therapy [20].
Pharmacodynamics
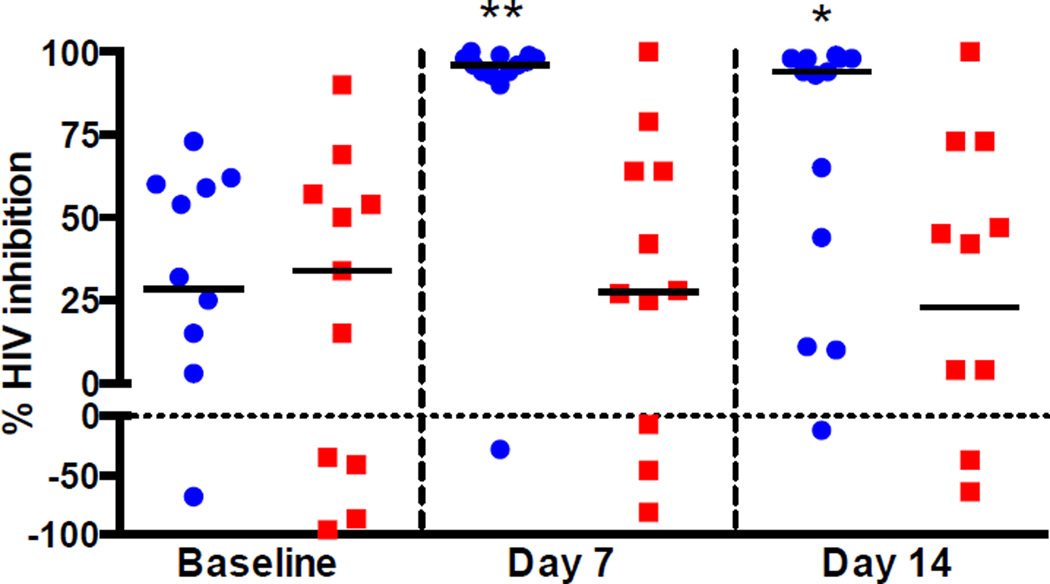
To assess PD of luminal drug, Jurkat-Tat-CCR5 T cells were challenged with HIV-1BaL in the presence of CVF collected at enrollment, one and two weeks after ring insertion. At enrollment, there was variable anti-HIV activity with CVF from 5 women enhancing HIV infection. The inhibitory activity increased significantly in the TDF group from a median [IQR] of 29% [−50–60] to 96% [94–99] at Day 7 (p=0.005) and to 94% [28–98] on Day 14 (p=0.025), whereas there was no change in CVF antiviral activity in the placebo group (Figure 3).
Figure 3. Ex Vivo Challenge of CVF.
Jurkat-Tat-CCR5 cells were challenged with HIV-1BaL in the absence or presence of CVF collected at enrollment and after ring use. Blue circles represent the anti-HIV activity of CVF from participants who received TDF, red squares indicate the activity of those who received placebo, and the black bars correspond to the median percent HIV inhibition for each time-point relative to control cells challenged in the presence of control fluid (water diluted 1:1 with complete medium and a final concentration of 200 µg/mL BSA). Each CVF sample was tested in triplicate. Compared to enrollment, there was a significant increase in percent HIV inhibition at one week and two weeks in the TDF group (* p<0.05, ** p<0.01).
Characterization of IVRs after use
TDF recovered from 15 TDF IVRs after use was 260±20 mg, which is consistent with an average in vivo release rate of 6.2±1.4 mg/day based on product content of 365±15 mg TDF (mean±standard deviation). This in vivo release rate is similar to the 14-day average in vitro release rate of 5.0±0.16 mg/day in acetate buffer at pH 4.2 and 37°C. The mean concentration of mono-POC TFV recovered from rings was 13±5 mg, which represents ~8.0±2.7 mol% of total drug and is comparable to the 6–10% measured in rings after 30 days of in vitro release testing. Semiquantitative cultures of the ring surface showed colonization with common vaginal flora (see Table, SDC 6) and no bacteria were isolated from the core of 26 rings.
Discussion
This first clinical study of a polyurethane reservoir TDF ring demonstrates that it is safe and well tolerated. Vaginal discharge, which is frequently reported in IVR studies [22, 23], was more common in the TDF compared to the placebo group, but there were no other product-related AEs.
Steady state concentrations of TFV in CVF were achieved 1 day after ring insertion, which is consistent with rapid and sustained drug release in the vaginal compartment. CVF TFV concentrations were similar at the vagina, cervix, and introitus, and were in excess of the concentration associated with protection in the CAPRISA study (103 ng/mL) [24], though the timing in which CVF concentrations were measured relative to coitally-dependent gel application in CAPRISA is unknown. CVF TFV concentrations following ring use were also above the median TFV concentration (5×104 ng/mL) measured after 6 weeks of daily oral TDF in Microbicide Trials Network 001 [19], a regimen proven to be 100% effective in the Partners PrEP study if taken with high adherence [25].
CVF TFV concentrations were also similar to those achieved with macaque-sized rings in which the animals were completely protected from repeated SHIV vaginal challenges [10]. Consistent with NHP studies, we found that concentrations remained >103 ng/mL after ring removal, which may be important as women may remove a ring before sex [26].
As expected, low plasma TFV concentrations were detected early after IVR insertion and continued to increase throughout the dosing period. The plasma concentrations were similar to serum concentrations found after 6 weeks of daily vaginal TFV gel dosing (Cmax=3.9 ng/mL), but were significantly lower than those observed with oral daily TDF dosing (332 ng/mL) [19]. Further studies are needed to determine steady state plasma concentrations with the TDF IVR, but we anticipate that it will be similar to concentrations achieved with TFV gel, and thus unlikely to be associated with systemic toxicity. The higher plasma concentration at Day 14 suggests that plasma TFV may serve as a biomarker of cumulative ring use over 2 weeks. Additionally, we detected TFV-DP in RBCs using DBS. This is the first IVR study to incorporate DBS and the findings suggest that this assay, which is similar to hair levels as a measure of drug exposure over 1–2 months, could be used in the future as a marker of longer-term adherence in efficacy trials. Steady state tissue levels may not be achieved for 3–4 weeks presuming that the half-life of TFV-DP is ~130 hours [27].
Future studies of longer duration in sexually active women are needed. Based on in vitro and in vivo data, we anticipate that the ring will continue to deliver ~5–6 mg/day of TDF for 30–45 days and will result in very rapid steady state tissue TFV-DP concentrations that exceed those achieved following oral TDF PrEP in adherent women, but with significantly lower systemic concentrations, thus avoiding potential toxicities. A limitation of the study is that participants were asked to abstain from sex. However, the rapid intracellular accumulation of TFV-DP after topical dosing, prolonged intracellular half-life, and sustained ring delivery suggest that there would be minimal effects of sex on PK/PD. This will be tested in a planned three-month Phase 1 study.
Additional studies with greater power are needed to better characterize the impact of TDF rings on the vaginal microbiota, preferably using molecular methods to determine if TDF rings promote colonization with Lactobacillus iners or other bacterial species.
In conclusion, the favorable safety and PK data support further clinical investigation of this TDF IVR. A three-month trial is planned in sexually active women in the US and Kenya. The high prevalence of BV in Kenya will allow assessment of PK in the setting of an altered vaginal microbiome. Women will undergo sampling early after IVR insertion to determine how rapidly protective levels are achieved and days after removal to determine the rate of decay.
Supplementary Material
Acknowledgements
Funding statement: The work was supported by grants from the National Institutes of Health [AI076980, AI03461, TR001073, HHSN272200800014C]. We acknowledge Gilead Sciences, Inc. for providing tenofovir disoproxil fumarate and regulatory assistance. We thank Hans Spiegel and Cherlynn Mathias for assisting with protocol development and study operations, Jason McConnell for helping with the manufacture of rings, Ashley Huber for conducting antiviral assays, Tina Fiedler for helping with classification of sequence reads, Andrew Wiser for assisting with the microbiome studies, and CONRAD for providing advice. We also acknowledge the site staff and study participants.
Footnotes
Author contributions:
MJK, CWH, PFK, BCH, and DNF contributed to study design, MJK, LE and JMA were involved in participant recruitment and clinical follow-up, PFK, RT, BF designed and manufactured the rings, PMM, LE, MAM, RT, LR, SS performed the experiments, MJK, PMM, YL, SS, PLA, CWH, BCH analyzed the data, MJK and BCH wrote the manuscript. All authors reviewed the manuscript for intellectual content and approved the final version.
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.UNAIDS. The Gap Report. 2014
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rees H, Delany-Moretiwe S, Baron D, Lombard C, Gray G, Myer L, et al. Conference on Retroviruse and Opportunistic Infections. Seattle, WA: 2015. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. [Google Scholar]
- 6.Mesquita PM, Rastogi R, Segarra TJ, Teller RS, Torres NM, Huber AM, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012;67:1730–1738. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nixon B, Jandl T, Teller RS, Taneva E, Wang Y, Nagaraja U, et al. Vaginally delivered tenofovir disoproxil fumarate provides greater protection than tenofovir against genital herpes in a murine model of efficacy and safety. Antimicrob Agents Chemother. 2014;58:1153–1160. doi: 10.1128/AAC.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seay K, Khajoueinejad N, Zheng JH, Kiser P, Ochsenbauer C, Kappes JC, et al. The Vaginal Acquisition and Dissemination of HIV-1 Infection in a Novel Transgenic Mouse Model is Facilitated by Coinfection with HSV-2 and is Inhibited by Microbicide Treatment. J Virol. 2015;89:9559–9570. doi: 10.1128/JVI.01326-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesquita PM, Srinivasan P, Johnson TJ, Rastogi R, Evans-Strickfaden T, Kay MS, et al. Novel preclinical models of topical PrEP pharmacodynamics provide rationale for combination of drugs with complementary properties. Retrovirology. 2013;10:113. doi: 10.1186/1742-4690-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A. 2013;110:16145–16150. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JM, Srinivasan P, Teller RS, Lo Y, Dinh CT, Kiser PF, et al. Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate-treated macaques from multiple SHIV exposures. J Acquir Immune Defic Syndr. 2015;68:1–5. doi: 10.1097/QAI.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0. 2004 Dec; Clarification dated August 2009. In.
- 13.Johnson TJ, Clark MR, Albright TH, Nebeker JS, Tuitupou AL, Clark JT, et al. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob Agents Chemother. 2012;56:6272–6283. doi: 10.1128/AAC.01431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurman AR, Kimble T, Herold B, Mesquita PM, Fichorova RN, Dawood HY, et al. Bacterial Vaginosis and Subclinical Markers of Genital Tract Inflammation and Mucosal Immunity. AIDS Res Hum Retroviruses. 2015;31:1139–1152. doi: 10.1089/aid.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabe LK, Hillier SL. Optimization of media for detection of hydrogen peroxide production by Lactobacillus species. J Clin Microbiol. 2003;41:3260–3264. doi: 10.1128/JCM.41.7.3260-3264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7:e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan S, Morgan MT, Liu C, Matsen FA, Hoffman NG, Fiedler TL, et al. More than meets the eye: associations of vaginal bacteria with gram stain morphotypes using molecular phylogenetic analysis. PLoS One. 2013;8:e78633. doi: 10.1371/journal.pone.0078633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsen FA, Kodner RB, Armbrust EV. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics. 2010;11:538. doi: 10.1186/1471-2105-11-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, et al. Tenofovir and tenofovir disoproxil fumarate pharmacokinetics from intravaginal rings. AIDS. 2012;26:707–710. doi: 10.1097/QAD.0b013e3283509abb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roumen F. Contraceptive efficacy and tolerability with a novel combined contraceptive vaginal ring, NuvaRing. Eur J Contracept Reprod Health Care. 2002;7(Suppl 2):19–24. discussion 37-19. [PubMed] [Google Scholar]
- 23.Oddsson K, Leifels-Fischer B, de Melo NR, Wiel-Masson D, Benedetto C, Verhoeven CH, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176–182. doi: 10.1016/j.contraception.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Kashuba AD, Gengiah TN, Werner L, Yang KH, White NR, Karim QA, et al. Genital Tenofovir Concentrations Correlate With Protection Against HIV Infection in the CAPRISA 004 Trial: Importance of Adherence for Microbicide Effectiveness. J Acquir Immune Defic Syndr. 2015;69:264–269. doi: 10.1097/QAI.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montgomery ET, van der Straten A, Cheng H, Wegner L, Masenga G, von Mollendorf C, et al. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav. 2012;16:1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- 27.Louissaint NA, Cao YJ, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–1450. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.