Abstract
Purpose
While methods for imaging tumor hypoxia with positron emission tomography (PET) have been developed, optimal methods for interpreting and utilizing these datasets in the clinic remain unclear. In this study we analyzed hypoxia PET images of head and neck cancer patients and compared imaging metrics with human papilloma virus (HPV) status and clinical outcome.
Methods
41 patients treated as part of a phase III trial of the hypoxic cytotoxin tirapazamine (TROG 02.02) were imaged with PET using fluorodeoxyglucose (FDG) and fluoroazomycin arabinoside (FAZA). FDG and FAZA PET images were interpreted qualitatively and quantitatively, and compared with tumor T stage, HPV status, and treatment outcome using multivariate statistics.
Results
PET signals in the tumor and lymph nodes exhibited significant intra- and inter-patient variability. The FAZA hypoxic volume demonstrated a significant correlation with tumor T stage. PET-hypoxic tumors treated with cisplatin exhibited significantly worse treatment outcomes relative to PET-oxic tumors or PET-hypoxic tumors treated with tirapazamine.
Conclusion
Quantitative analysis of FAZA PET yielded metrics that correlated with clinical T stage and were capable of stratifying patient outcome. These results encourage further development of this technology, with particular emphasis on establishment of robust quantitative methods.
Keywords: Hypoxia, PET, Head and neck cancer, Radiation therapy
Introduction
Over 60 years ago it was shown that hypoxia inhibits the ability of radiation to kill mammalian cells [1]. The existence of hypoxic regions in solid tumors was hypothesized [2] and subsequently confirmed [3], establishing as a key limiting factor in cancer radiotherapy. Given the biological and clinical significance of tumor hypoxia, a number of technologies for quantifying tumor oxygenation have been developed and applied, including electrode-based measurements as well as hypoxia-sensing probes suitable for use in immunohistochemistry and non-invasive molecular imaging. Molecules that accumulate in hypoxic cells have been conjugated to positron-emitter isotopes to produce hypoxia radiotracers for positron emission tomography (PET), including [18F]-Fluoromisonidazole (FMISO), [18F]-Fluoroazomycin arabinoside (FAZA), [18F]-2-(2-Nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide (EF5), 3-[18F]-2-(4-((2-nitro-1H-imidazol-1-yl)methyl)-1H-1,2,3,-triazol-1-yl)-propan-1-ol (HX4), [18F]-fluoroerythronitroimidazole (FETNIM), and a number of others.
Tumors of the head and neck are prime candidates for use of these methods because of their location away from regions of physiologic uptake of these tracers as well as the incidence and clinical significance of hypoxia in these lesions [4–6]. However, despite positive initial reports of the utility of several hypoxia PET radiotracers to identify clinically important features of head and neck cancers [7], debate continues over how to optimally utilize them in the clinical management of these patients. A variety of qualitative and quantitative analysis methods have been proposed, but their relative association with patient outcome is not known. Tumor human papillomavirus (HPV) status has been shown to be strongly associated with both local control and overall patient survival following treatment [8, 9], however previous studies have not elucidated the relationship between HPV and PET-visible hypoxia as predictors of treatment outcome [10]. We therefore investigated the relationship between FDG and FAZA PET signals, tumor HPV status, and patient outcome in a sample of 41 head and neck cancer patients treated prospectively on the randomized Tasmanian Radiation Oncology Group (TROG) trial 02.02 comparing chemoradiotherapy to chemoradiotherapy plus the hypoxic cell cytotoxin tirapazamine. We extracted a panel of qualitative and quantitative features from FDG and FAZA PET datasets of primary tumors and involved lymph nodes, and evaluated their association with patient outcome and to tumor HPV status.
Materials and Methods
Patients
Patients were collected from a previous open-label, randomized phase III trial of radiation and cisplatin combined with the hypoxic cytotoxin tirapazamine that was performed in 82 centers across 16 countries in Australia, New Zealand, North America, Europe, and South America between April 2002 and September 2005 (TROG 02.02). The study protocol including this subanalysis was approved by the institutional ethics committees. Eligibility criteria for this phase III trial have been described previously [11]. Additional eligibility criteria imposed for this analysis included pre-treatment FAZA PET-CT, an applied radiotherapy dose >60 Gy, and the absence of any major deviations in radiotherapy quality that would be predicted to have an adverse effect on tumor control [12].
Positron Emission Tomography Imaging and Analysis
All patients underwent both an 18F-2-fluoro-2-deoxy-glucose (FDG) PET scan as well as a FAZA PET scan at the Peter McCallum Cancer Centre on separate days prior to the initiation of treatment. FDG was obtained through the licensed manufacturer Cyclotek Pty Ltd. (Melbourne, Australia, license no. MI-12092005-LI-000904-2). 18F-FAZA was produced at the Peter MacCallum Cancer Centre using a previously reported synthesis methodology [13]. Static PET images were acquired one hour after intravenous injection of FDG or 2 hours after intravenous injection of FAZA at a dose of 5.2 MBq/kg using a dedicated PENN-PET300H PET scanner (UGM Medical System Inc., Philadelphia, PA) or a Discovery LS PET/CT scanner (GE Medical Systems, Milwaukee, WI). PET scans were acquired over a range of at least 24 cm from the base of the skull to the top of the lungs, including all tumor and involved lymph nodes in the neck. The PENN-PET300H imaged a single field-of-view over a 15 minute period, while the Discovery LS was operated in whole body mode, scanning a series of bed positions for 5 minutes each. FDG and FAZA PET images were acquired within one month of each other. Following acquisition of both PET datasets, the FAZA images were coregistered with FDG PET images using a semiautomated mutual information-based PET-to-PET rigid registration method implemented within the RT_Image software package [14]. This method has been applied previously to register metabolic and hypoxia PET scans, resulting in average errors on the order of 2 mm (approximately half the width of a PET voxel) [15]. The FDG PET was then used to identify tumor and involved nodal areas as regions of interest for FAZA analysis. The FAZA images were initially analyzed qualitatively by two blinded readers, with lesions in each dataset scored from 0 (uptake less than background) to 4 (focal uptake markedly greater than background). The FAZA scan was interpreted to be positive if there was greater activity (score of 2 or 3) within the sites of tumoral uptake of FDG than the activity present in adjacent or mirrored soft tissue sites. The PET images were also analyzed quantitatively using RT_Image. The maximum SUVs over the primary tumor, lymph nodes, and total tumor volume were calculated for FAZA and FDG. The volume of FDG-avid tissue, defined as the metabolic tumor volume (MTV), was measured for the tumor and involved nodes as described previously [16]. The histogram analysis of Mortensen et al. was applied to this patient cohort to characterize the distribution of FAZA signals in order to calculate a threshold value separating normoxic and hypoxic voxels [10]. Hypoxic tumors were identified based on the quantitative analysis as those with at least one voxel with a FAZA signal greater than the calculated threshold. The hypoxic tumor volume (HTV) was defined as the volume in the FAZA PET dataset with an intensity greater than the determined threshold. The HTV analysis was restricted to the MTV. Finally, the fractional hypoxic tumor volume (fHTV) was calculated by dividing the HTV by the MTV for the primary tumor, lymph nodes, and total tumor volume.
p16 Status
p16 immunohistochemistry was performed as previously described to ascertain the HPV status of each patient [9]. Slides were semiquantitatively scored for staining intensity in the cell nucleus and cytoplasm. Intensity was scored as 0 (none), 1 (weak), 2 (moderate) or 3 (strong), with 0 or 1 defined as p16 negative and 2 or 3 defined as p16 positive.
Endpoints
Locoregional failure was defined as a disease progression at the primary tumor and/or involved lymph nodes. Time to locoregional failure was measured from the date of the start of the treatment to the date of first locoregional failure. Distant failure and death were treated as censoring events for time to locoregional failure. Overall treatment failure was defined as disease progression at any site, locoregional and/or distant, or death. Failure-free survival was measured from the date of the start of the treatment to the date of first failure or death.
Statistical Analysis
Patients were grouped based on their p16 status in HPV positive and negative groups. For the purposes of this analysis, all patients with non-oropharyngeal primary tumors were considered HPV negative. Kaplan-Meier curves were computed for the endpoints described above, and the treatment arms were compared using the exact logrank test. The impact of hypoxia metrics on the time-to-event outcomes was assessed using the logrank test for trend. The hypoxic tumor volume in the primary tumor, lymph nodes, and tumor plus nodes was compared between HPV positive and negative patients using the Wilcoxon rank sum test.
Results
Patient Population
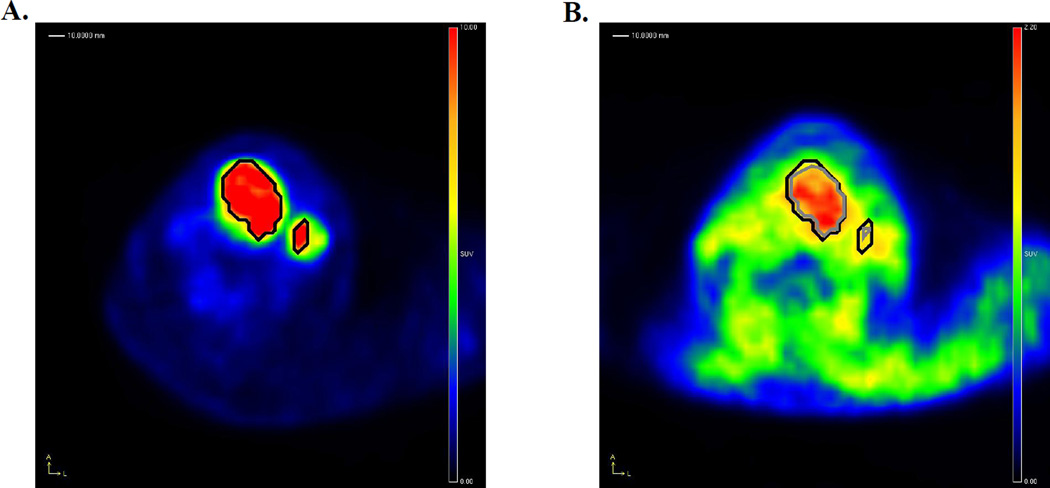
A total of 41 patients were included in this study. The characteristics of this patient sample are described in Table 1 according to treatment arm, either chemoradiotherapy (CRT) or chemoradiotherapy plus tirapazamine (CRT + TPZ). Fourteen (34%) of the oropharyngeal tumors were determined to be HPV positive, 24 (59%) were HPV negative, and 3 (7%) tumors were of unknown HPV status. Only one of the non-oropharyngeal tumours was p16 positive, but was included in the HPV negative group as per a priori decision. On subsequent analysis using an in situ hybridization assay for HPV DNA [9], this case was found to be HPV negative. FDG and FAZA PET datasets were acquired from all patients within a median of 7 days of each other (range: 1–29 days). 24 patients were imaged using the PENN-PET300H scanner, while 17 were imaged on the Discovery LS PET/CT. Representative FDG and FAZA PET images from this patient cohort are shown in Figure 1.
Table 1.
Properties of the study patient population based on randomization arm.
| Characteristic | CRT arm (n=19) |
CRT + TPZ arm (n=22) |
|---|---|---|
| Age | ||
| Median | 59 (42–72) | 59 (46–77) |
| Gender | ||
| Male (%) | 84 (n=16) | 82 (n=18) |
| Site | ||
| Hypopharynx (%) | 0 (n=0) | 9 (n=2) |
| Larynx (%) | 11 (n=2) | 14 (n=3) |
| Oral cavity (%) | 11 (n=2) | 9 (n=2) |
| Oropharynx (%) | 79 (n=15) | 68 (n=15) |
| T stage | ||
| T3–4 (%) | 79 (n=15) | 82 (n=18) |
| N stage | ||
| N2–3 (%) | 89 (n=17) | 86 (n=19) |
| HPV | ||
| Negative (%) | 53 (n=10) | 64 (n=14) |
| PET | ||
| FAZA positive (%) | 58 (n=11) | 32 (n=7) |
| Median MTV (cc) | 23.8 (3.8–80.4) | 16.7 (3.8–48.0) |
| Median HTV (cc) | 5.7 (0.0–52.3) | 1.8 (0.0–23.8) |
| Median fHTV | 0.3 (0.0–0.9) | 0.1 (0.0–0.6) |
| Median FDG SUVmax | 10.7 (4.6–21.1) | 10.4 (3.2–21.1) |
| Median FAZA SUVmax | 1.7 (0.9–6.1) | 1.6 (0.6–2.4) |
Figure 1.
Analysis of FDG and FAZA PET images. A shows an FDG PET scan of a patient with a tumor of the larynx, showing the computed MTV for the primary tumor (central lesion) and involved lymph node (peripheral lesion) as a black contour. This contour was then overlaid on the coregistered FAZA PET image shown in B, and used to determine the FAZA positive volumes for the primary tumor and lymph node. In this patient it is apparent that the majority of the primary tumor is hypoxic according to FAZA, while the lymph node is less FAZA avid and correspondingly less hypoxic. Note the lower contrast between FAZA uptake in tumor compared to background soft tissues than observed with FDG.
PET Imaging and HPV Status
All patients underwent FDG and FAZA PET imaging prior to treatment. Of the 24 HPV negative patients, 15 (62%) were classified as having no PET-visible hypoxia according to the qualitative grading scheme. 4 (17%) exhibited FAZA accumulation in the primary tumor but not in lymph nodes, 2 (8%) showed FAZA accumulation in the lymph nodes but not the primary tumor, and 3 (12%) were FAZA positive in both the primary tumor and lymph nodes. For the 14 HPV positive patients, 6 (43%) showed no PET evidence of tumoral hypoxia, 1 (7%) exhibited FAZA uptake in the primary tumor, 4 (29%) exhibited FAZA uptake in the lymph nodes, and 3 (21%) showed uptake in both the primary tumor and lymph nodes. Analysis of the distribution of FAZA signals across all patients according to the method of Mortensen et al. [10] resulted in a threshold hypoxic tumor:muscle FAZA ratio of 1.44.
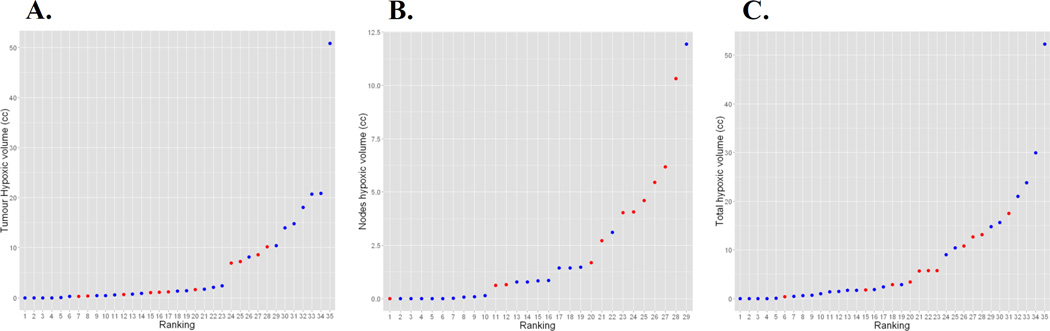
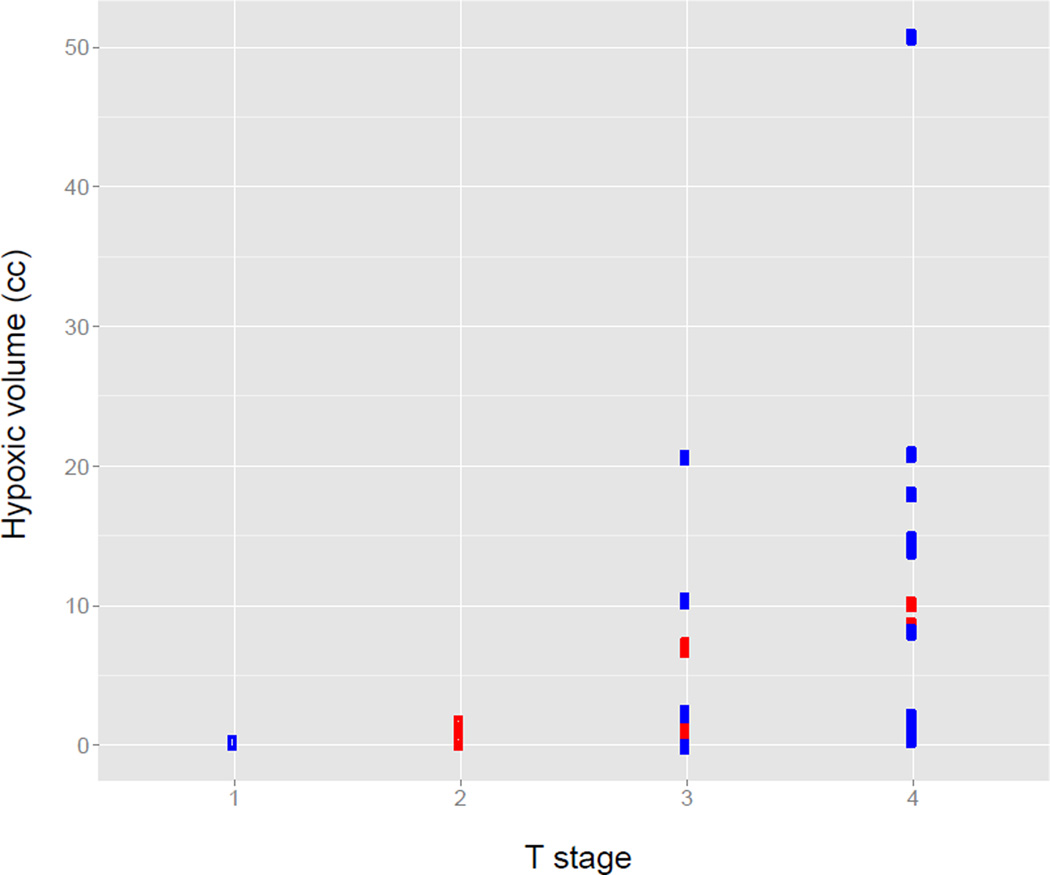
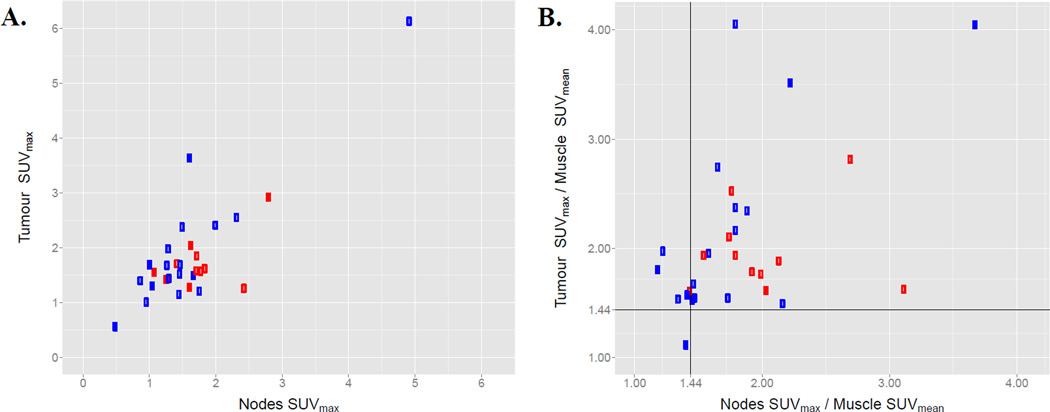
Table 2 summarizes the quantitative imaging metrics derived from the FDG PET images for HPV positive and negative tumors. The cumulative MTVs for HPV positive and negative tumors are similar, however the MTVs for HPV negative primary tumors are significantly larger (p=0.01) while the MTVs for HPV negative involved lymph nodes are significantly smaller than those for HPV positive tumors (p=0.001). There are no significant differences in the SUV values between the two HPV groups. A similar analysis of the FAZA PET images is shown in Table 3 and in Figure 2. As with the MTV values, the HTVs for the HPV negative involved lymph nodes are significantly smaller than those for the HPV positive tumors (p=0.004). There was a statistically significant correlation between T stage and hypoxic tumor volume assessed using the Kendall Tau rank correlation (p=0.002, tau=0.42), with higher T stage associated with a higher FAZA tumor HTV (Figure 3). A correlation was observed between the SUVmax values measured for the tumor and the nodal volumes (r=0.81), as seen in Figure 4. This correlation is weaker when normalizing by the muscle SUVmean (r=0.45).
Table 2.
FDG PET metrics stratified based on HPV status
| HPV status |
Tumor location |
Metabolic tumor volume | SUVmax | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Mean | Maximum | Minimum | Median | Mean | Maximum | ||
| Negative | Primary | 1.75 | 14.17 | 18.23 | 65.22 | 3.18 | 10.94 | 11.52 | 21.11 |
| Nodes | 0.00 | 4.72 | 4.95 | 13.03 | 1.85 | 6.48 | 8.62 | 18.44 | |
| Total | 3.76 | 21.27 | 23.18 | 70.13 | 3.18 | 10.94 | 11.57 | 21.11 | |
| Positive | Primary | 2.27 | 6.80 | 9.70 | 16.41 | 3.20 | 9.48 | 10.67 | 21.10 |
| Nodes | 2.77 | 13.42 | 17.03 | 63.94 | 2.76 | 10.26 | 10.53 | 17.84 | |
| Total | 8.04 | 22.00 | 24.58 | 80.35 | 5.11 | 11.00 | 12.27 | 21.10 | |
Table 3.
FAZA PET metrics stratified based on HPV status
| HPV status |
Tumor location |
Hypoxic tumor volume | SUVmax | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Mean | Maximum | Minimum | Median | Mean | Maximum | ||
| Negative | Primary | 0.00 | 1.37 | 7.08 | 50.80 | 0.57 | 1.43 | 1.75 | 6.14 |
| Nodes | 0.00 | 0.45 | 1.27 | 11.93 | 0.50 | 1.46 | 1.59 | 4.93 | |
| Total | 0.00 | 1.68 | 8.03 | 52.27 | 0.57 | 1.50 | 1.80 | 6.14 | |
| Positive | Primary | 0.08 | 1.07 | 2.87 | 10.16 | 0.81 | 1.57 | 1.62 | 2.93 |
| Nodes | 0.00 | 4.33 | 5.22 | 15.23 | 1.03 | 1.72 | 1.76 | 2.81 | |
| Total | 0.37 | 6.54 | 8.09 | 17.51 | 1.03 | 1.82 | 1.87 | 2.93 | |
Figure 2.
Distributions of hypoxic volumes, sorted in increasing order, within the tumor (A), nodes (B), and total tumor volume including the primary and nodes (C). Blue markers denote HPV negative tumors and red HPV positive.
Figure 3.
The relationship between hypoxic tumor volumes and T stage. HPV negative tumors are indicated by blue markers and HPV positive tumors by red markers.
Figure 4.
The relationship between FAZA PET signals in the primary tumor and nodal volumes. A shows a scatter plot of tumor SUVmax against nodal SUVmax. B shows a scatter plot of the same metrics scaled by the SUVmean of the reference muscle tissue for that patient. Black lines identify the empirically determined SUVmax/muscle SUVmean hypoxia threshold of 1.44. Blue markers denote HPV negative tumors and red HPV positive.
An alternate method of analyzing the degree of hypoxia using the fractional hypoxic tumor volume (fHTV) is considered in Table 4. The fHTV values range from 0.00 to 0.95 for the primary tumor, from 0.00 to 0.92 for the lymph nodes, and from 0.00 to 0.90 for the total tumor volume. The distribution of fHTV values for the primary tumor and the total tumor volume are not significantly different between the HPV positive and negative tumors, however the fHTV values for the lymph nodes are smaller (p=0.05) for the HPV negative tumors (median 0.13) than for the HPV positive tumors (median 0.30). This is in accord with the lower HTV observed for the lymph nodes of HPV negative tumors, as seen in Table 3.
Table 4.
Fractional hypoxic tumor volumes stratified based on HPV status.
| HPV status | Tumor location | Fractional hypoxic tumor volume | |||
|---|---|---|---|---|---|
| Minimum | Median | Mean | Maximum | ||
| Negative | Primary | 0.00 | 0.12 | 0.28 | 0.95 |
| Nodes | 0.00 | 0.13 | 0.17 | 0.92 | |
| Total | 0.00 | 0.12 | 0.25 | 0.90 | |
| Positive | Primary | 0.02 | 0.17 | 0.30 | 0.74 |
| Nodes | 0.00 | 0.30 | 0.33 | 0.73 | |
| Total | 0.03 | 0.33 | 0.35 | 0.62 | |
Treatment Outcome
The treatment failures observed in this patient population are summarized in Table 5. A total of 6 failures (35%) were seen in the patients with tumor or nodal volumes qualitatively classified as oxic, while 6 failures (29%) were observed in the patients qualitatively classified as hypoxic. The failure rates for the patients treated with chemoradiotherapy and with chemoradiotherapy plus tirapazamine were 40% and 17% for the oxic tumors, and 56% and 8% for the hypoxic tumors, respectively. Stratified based on HPV, the failure rates for patients in the chemoradiotherapy and chemoradiotherapy plus tirapazamine arms were 80% and 15% for the HPV negative tumors, and 11% and 0% for the HPV positive tumors, respectively.
Table 5.
Patterns of failure stratified based on qualitative hypoxia score, HPV status, and treatment arm.
| Qualitative FAZA PET analysis |
HPV status |
Treatment arm |
Failure type | |||||
|---|---|---|---|---|---|---|---|---|
| Death | Local | Loco- regional |
Combined | Distant | None | |||
| Negative | Negative | CIS | 0 | 3 | 1 | 0 | 0 | 1 |
| TPZ | 0 | 1 | 0 | 0 | 1 | 3 | ||
| Positive | CIS | 0 | 0 | 0 | 0 | 0 | 5 | |
| TPZ | 0 | 0 | 0 | 0 | 0 | 2 | ||
| Positive | Negative | CIS | 2 | 1 | 0 | 1 | 0 | 1 |
| TPZ | 0 | 0 | 0 | 0 | 1 | 8 | ||
| Positive | CIS | 1 | 0 | 0 | 0 | 0 | 3 | |
| TPZ | 0 | 0 | 0 | 0 | 0 | 3 | ||
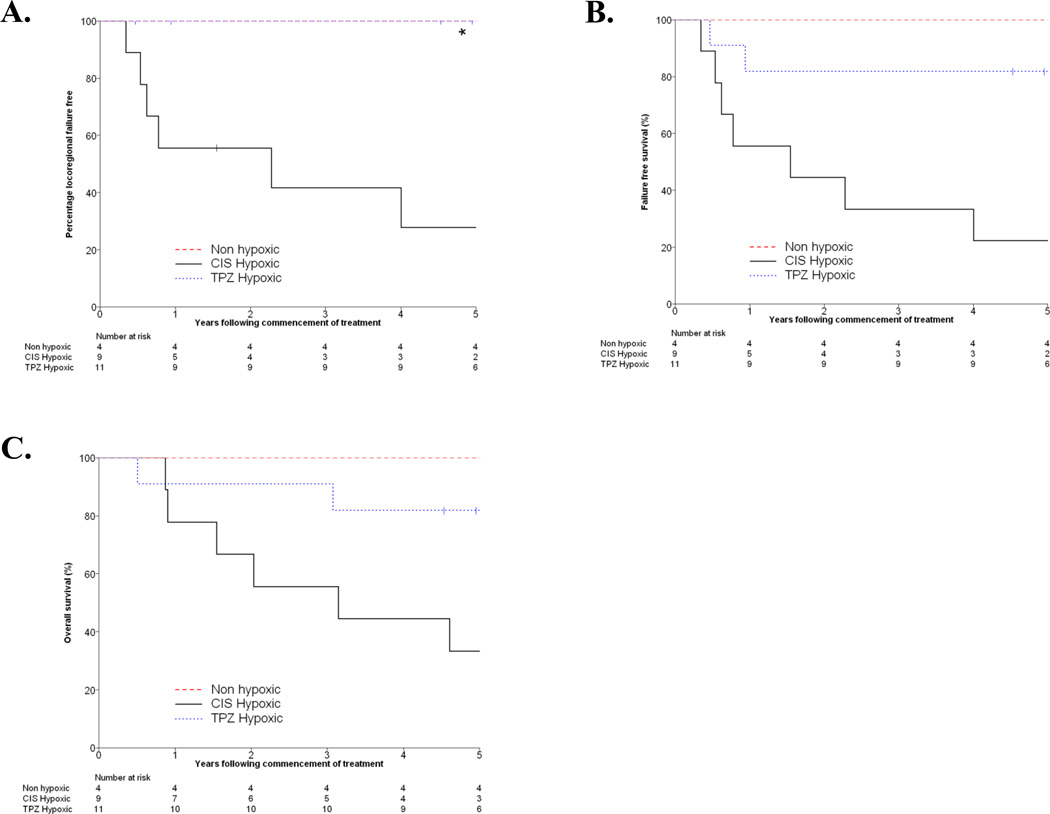
The Kaplan Meier curves for locoregional failure free, failure free survival, and overall survival for the HPV negative patients are shown in Figure 5, stratified by their quantitative FAZA hypoxia status and the treatment arm in which they were enrolled. Due to the low number of failures in the HPV positive group, parallel analysis of those patients was not possible. A significant difference in time to locoregional failure (p<0.001) and failure free survival (p=0.004) was observed between HPV negative patients treated with chemoradiotherapy and with chemoradiotherapy plus tirapazamine in the subset of patients with hypoxic tumors. However, the difference between these treatment arms in terms of overall survival was not significant (p=0.17).
Figure 5.
Kaplan-Meier curves for locoregional failure free (A), failure free survival (B), and overall survival (C). Curves are stratified into groups consisting of the patients with non-hypoxic tumors according to FAZA PET, patients with hypoxic tumors treated in the cisplatin arm, and patients with hypoxic tumors treated with tirapazamine. Identification of hypoxia was based on a hypoxic tumor volume greater than 0. The curves for the non-hypoxic and tirapazamine hypoxic groups in A overlap.
Discussion
In this study we have applied hypoxia PET imaging using the radiotracer 18F-FAZA towards patients treated with chemoradiotherapy and a hypoxic cytotoxin for tumors of the head and neck. PET imaging of hypoxia has been applied in studies of human head and neck cancer for almost two decades. The FMISO tumor:blood (T:B) ratio as well as the absolute hypoxic volume, computed using a threshold FMISO T:B ratio of 1.2, were found to be independent predictors of outcome in a group of 73 head and neck cancer patients[17]. Qualitative analysis of FMISO images has been shown to identify patients who would benefit from the addition of tirapazamine in a prior phase II TROG study [7]. Analysis of FMISO time-activity curves obtained from dynamic PET scanning of head and neck cancer patients revealed an association between curve shape and patient response to radiotherapy [15].
FAZA is a successor to FMISO that has been reported to exhibit improved tumor to muscle ratios, indicative of faster washout of unbound probe [18]. While FAZA has been comprehensively studied in animal models of cancer and has been shown to be predictive of the efficacy of chemoradiotherapy and hypoxiadirected therapies [19], only recently has it entered into pilot clinical trials [20, 21]. In our study, FAZA PET signals exhibited a strong association with T stage, which are larger for HPV negative tumors. This is consistent with previously noted associations between hypoxia as detected by FMISO PET and T stage [22]. Notably, the FDG and FAZA avid regions in the involved lymph nodes were larger in the HPV positive than in the HPV negative tumors. The frequent presence of cystic nodal metastases in HPV positive tumors has been previously reported [23], and may explain the lack of association between nodal hypoxia and treatment outcome that has been observed [7].
We observed a lower incidence of qualitative hypoxia in this cohort with FAZA compared to an earlier cohort imaged with FMISO [24]. While this could reflect in part differences between the cohorts, it may be due to the different tracers and/or changes and image interpretation. Locoregional failures in the HPV negative patients on the control arm in the FMISO cohort were predominantly in patients with tumors that had qualitative hypoxia, but this was not the case in the current study, with several locoregional failures in the non-hypoxic group. This suggests that a lower, more sensitive, threshold for qualitative hypoxia may be required for FAZA than for FMISO in order maintain its predictive value in identifying patients at risk of locoregional failure with standard treatment. These findings also emphasize the difficulty in applying qualitative scoring schema established with one agent to another with different uptake characteristics.
PET imaging of hypoxia has a number of limitations that remain to be overcome as it progresses toward widespread clinical adoption. There is a lack of consensus on the optimal method to present and interpret hypoxia PET data, one that has been identified as a key limitation of many emerging functional and molecular imaging methods. Hypoxia PET is limited by the inherently low signal:noise ratio (SNR) and dynamic range of the acquired images. This makes selection of a robust threshold discriminating FAZA positive and negative tumors much more difficult than for FDG, where SUVs may be as high as 20. The physical and biological basis of this poor SNR has been suggested [25], however this does not compensate for the practical difficulties imposed by it. Although qualitative score is commonly used for PET interpretation, it is potentially subject to inter-reader variability and might be less reproducible than a quantitative metric. Therefore, we performed both qualitative and quantitative analyses for these studies using investigators that were blinded to each other’s results. The spread of quantitative metrics within a single qualitatively equivalent group likely reflects the emphasis of qualitative analysis on image contrast and quantitative analysis on absolute image intensities. The hypoxic tumor volume also incorporates a volumetric dimension not included in qualitative interpretation. It is possible that both the severity and extent of tumoral hypoxia will have an influence on outcomes and therefore further work is required to determine optimum quantitative analysis methods and to establish prognostically important thresholds for these parameters.
This study was subject to several shortcomings. Because of the duration over which patients were accrued, two different scanners were used to acquire the PET data. While each scanner was routinely calibrated according to the manufacturer’s specifications, the differences in spatial resolution and imaging performance between these systems impose a source of variability across the dataset. However, we did not observe any significant differences in the quantitative and qualitative metrics between patients imaged on each system. Additionally, due to practical considerations there was an interval of between 1 and 29 days between the acquisition of the FDG and FAZA PET scans. While the majority of patients were scanned with each radiotracer within a week, longer delays were encountered for some patients that could complicate comparison of the scans because of changes in the tumor during this interval. As above, we did not observe any significant association between the imaging metrics considered and the delay between the scans. Finally, interpretation of FAZA scans relative to the total tumor volume, such as the fHTV, should ideally be performed using the gross tumor volume (GTV) identified from an anatomic CT imaging examination. In the absence of these images within this dataset, we instead relied on the FDG PET-derived MTV to identify the tumor volume. While practically convenient, this practice may underestimate the tumor volume if portions of the tumor are not FDG-avid, introducing error into the calculated metrics.
Overall, our data support the prognostic significance of hypoxia in HPC negative head and neck cancer treated with conventional radiotherapy and the potential benefit of hypoxia cytotoxins in this setting, consistent with our previous reports [7]. The subjectivity of the qualitative analysis as well as the strong relationship between the quantitative grading of hypoxia and outcome as seen in Figure 5 encourage the use of these standardized, quantitative methods in future studies. Hypoxic HPV negative tumors treated with tirapazamine exhibited significantly better locoregional failure free and overall failure free survival.
Conclusion
In summary, in this study we have characterized PET images acquired using the metabolic radiotracer FDG and the hypoxia radiotracer FAZA in patients with head and neck cancer being treated as part of the TROG 02.02 trial of chemoradiotherapy and tirapazamine. A significant association between FAZA PET signals and tumor T-stage was observed. Locoregional failure free and failure free survival for HPV negative tumors identified as hypoxic by PET imaging and quantitative analysis was improved for patients treated with tirapazamine relative to those treated with cisplatin. These findings will need to be validated in future larger studies, testing hypoxic cell radiosensitizers or hypoxia activated prodrugs.
Acknowledgements
This work was funded by the National Institutes of Health and the National Cancer Institute of the United States (R01 CA118582, P01 CA67166) and by the National Health and Medical Research Council of Australia.
Funding:
Footnotes
Compliance with Ethical Standards
Informed consent:
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest:
E.E. Graves declares that he has no conflict of interest.
R.J. Hicks declares that he has no conflict of interest.
D. Binns declares that he has no conflict of interest.
M. Bressel declares that he has no conflict of interest.
Q.T. Le declares that she has no conflict of interest.
L. Peters declares that he has no conflict of interest.
R. Young declares that he has no conflict of interest.
D. Rischin declares that he has no conflict of interest.
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OCA. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 2.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Höckel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991;51:6098–6102. [PubMed] [Google Scholar]
- 4.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 5.Adam MF, Gabalski EC, Bloch DA, Oehlert JW, Brown JM, Elsaid AA, et al. Tissue oxygen distribution in head and neck cancer patients. Head Neck. 1999;21:146–153. doi: 10.1002/(sici)1097-0347(199903)21:2<146::aid-hed8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 8.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989–2995. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 12.Peters LJ, O'Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 13.Reischl G, Ehrlichmann W, Bieg C, Solbach C, Kumar P, Wiebe LI, et al. Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation. Appl Radiat Isot. 2005;62:897–901. doi: 10.1016/j.apradiso.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Graves EE, Quon A, Loo BW. RT_Image: An Open-Source Tool for Investigating PET in Radiation Oncology. Technol Cancer Res Treat. 2007;6:111–121. doi: 10.1177/153303460700600207. [DOI] [PubMed] [Google Scholar]
- 15.Thorwarth D, Eschmann SM, Holzner F, Paulsen F, Alber M. Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol. 2006;80:151–156. doi: 10.1016/j.radonc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 16.La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–1341. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran JG, Schwartz DL, O'Sullivan J, Peterson LM, Ng P, Scharnhorst J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piert M, Machulla HJ, Picchio M, Rieschl G, Ziegler S, Kumar P, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46:106–113. [PubMed] [Google Scholar]
- 19.Beck R, Roper B, Carlsen J, Huisman M, Lebschi J, Andratschke N, et al. Pretreatment 18F-FAZA PET Predicts Success of Hypoxia-Directed Radiochemotherapy Using Tirapazamine. J Nucl Med. 2007;48:973–980. doi: 10.2967/jnumed.106.038570. [DOI] [PubMed] [Google Scholar]
- 20.Souvatzoglou M, Grosu AL, Roper B, Krause BJ, Beck R, Reischl G, et al. Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: a pilot study. Eur J Nucl Med Mol Imaging. 2007;34:1566–1575. doi: 10.1007/s00259-007-0424-3. [DOI] [PubMed] [Google Scholar]
- 21.Postema EJ, McEwan AJ, Riauka TA, Kumar P, Richmond DA, Abrams DN, et al. Initial results of hypoxia imaging using 1-alpha-D: -(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole (18F-FAZA) Eur J Nucl Med Mol Imaging. 2009;36:1565–1573. doi: 10.1007/s00259-009-1154-5. [DOI] [PubMed] [Google Scholar]
- 22.Rischin D, Fisher R, Peters L, Corry J, Hicks R. Hypoxia in head and neck cancer: studies with hypoxic positron emission tomography imaging and hypoxic cytotoxins. Int J Radiat Oncol Biol Phys. 2007;69:S61–S63. doi: 10.1016/j.ijrobp.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 24.Trinkaus ME, Hicks RJ, Young RJ, Peters LJ, Solomon B, Bressel M, et al. Correlation of p16 status, hypoxic imaging using [18F]-misonidazole positron emission tomography and outcome in patients with loco-regionally advanced head and neck cancer. J Med Imaging Radiat Oncol. 2014;58:89–97. doi: 10.1111/1754-9485.12155. [DOI] [PubMed] [Google Scholar]
- 25.Carlin S, Humm JL. PET of hypoxia: current and future perspectives. J Nucl Med. 2012;53:1171–1174. doi: 10.2967/jnumed.111.099770. [DOI] [PubMed] [Google Scholar]