Abstract
Ligand stimulation promotes downregulation of RTKs, a mechanism by which RTKs, through the ubiquitination pathway are removed from the cell surface, causing a temporary termination of RTK signaling. The molecular mechanisms governing RTK trafficking and maturation in the endoplasmic reticulum (ER)/Golgi compartments are poorly understood. VEGFR-2 is a prototypic RTK that plays a critical role in physiologic and pathologic angiogenesis. Here we demonstrate that Ring Finger Protein 121 (RNF121), an endoplasmic reticulum ubiquitin E3 ligase, is expressed in endothelial cells and regulates maturation of VEGFR-2. RNF121 recognizes newly synthesized VEGFR-2 in the ER and controls its trafficking and maturation. Over-expression of RNF121 promoted ubiquitination of VEGFR-2, inhibited its maturation resulted a significantly reduced VEGFR-2 presence at the cell surface. Conversely, the shRNA-mediated knockdown of RNF121 in primary endothelial cells reduced VEGFR-2 ubiquitination and increased its cell surface level. The RING Finger domain of RNF121 is required for its activity toward VEGFR-2, as its deletion significantly reduced the effect of RNF121 on VEGFR-2. Additionally, RNF121 inhibited VEGF-induced endothelial cell proliferation and angiogenesis. Taken together, these data identify RNF121 as a key determinant of angiogenic signaling that restricts VEGFR-2 cell surface presence and its angiogenic signaling.
Keywords: Angiogenesis, ubiquitination, VEGFR-2 (Vascular endothelial growth factor receptor 2), Receptor tyrosine kinase (RTK), endoplasmic reticulum (ER) ubiquitin E3 ligase, Ring Finger Protein 121 (RNF121)
Introduction
Transmembrane signaling by receptor tyrosine kinases (RTKs) plays a fundamental role in human health and disease owing to the central role of RTKs in the regulation of key cellular functions such as survival, proliferation, differentiation, migration and apoptosis. Dysregulation of RTK-mediated signaling is a leading cause of diseases such as cancer (1). RTK levels at the plasma membrane influence the temporal and spatial dynamics of RTK-mediated signaling (2, 3). The expression of RTKs is regulated by transcriptional and translational events. However, a plasma membrane level of RTKs is a critical determinant of their activity (4–6). Therefore, controlling the level of RTKs at the cell surface is an important mechanism by which cells moderate RTK-mediated signal transduction.
Until recently, most studies on RTKs have focused on the mechanisms of removal of RTKs from the plasma membrane through ligand-induced endocytosis and ubiquitination-dependent degradation of RTKs (7–9). Ligand-induced downregulation of RTKs impacts the number of receptors available for activation at the plasma membrane, serving as a fundamental regulatory mechanism to prevent excessive ligand-induced activation of RTKs and downstream signaling effectors (9). Although there is extensive research on the effects and mechanisms of ligand-induced downregulation of RTKs from the plasma membrane (9–11), less is known about whether receptor levels at the plasma membrane are also affected by non-transcriptional and non-translational mechanisms.
RTKs, like other secretory proteins, are subject to various quality control systems in the lumen of the endoplasmic reticulum (ER); receptor molecules that fail to fold correctly in the lumen of the ER are often targeted for degradation by the ubiquitin-proteasome system (12). Whether maturation of RTKs is subject to the ER ubiquitin-proteasome system to regulate the overall receptor levels at the cell surface remains unclear. Vascular endothelial growth factor receptor-2 (VEGFR-2) is an important RTK that regulates cellular events ranging from developmental vasculogenesis to pathological angiogenesis (13, 14). VEGF-induced activation of VEGFR-2 stimulates a complex array of signaling pathways promoting endothelial cell differentiation, proliferation, survival, and migration. VEGFR-2 activation also promotes its own endocytosis and downregulation by recruiting β-Trcp ubiquitin E3 ligase (15, 16), leading to its ubiquitination and subsequent degradation. RNF-121 was recently identified as an ER membrane specific E3 ligase RING Finger protein in C. elegans that is broadly expressed in larvae and adults and regulates distal tip cell migration (17, 18). In the present study, we demonstrate that RNF121 recruits newly synthesized VEGFR-2 at the ER and controls its maturation by ubiquitination.
RESULTS
RNF121 is expressed in endothelial cells and regulates maturation of VEGFR-2
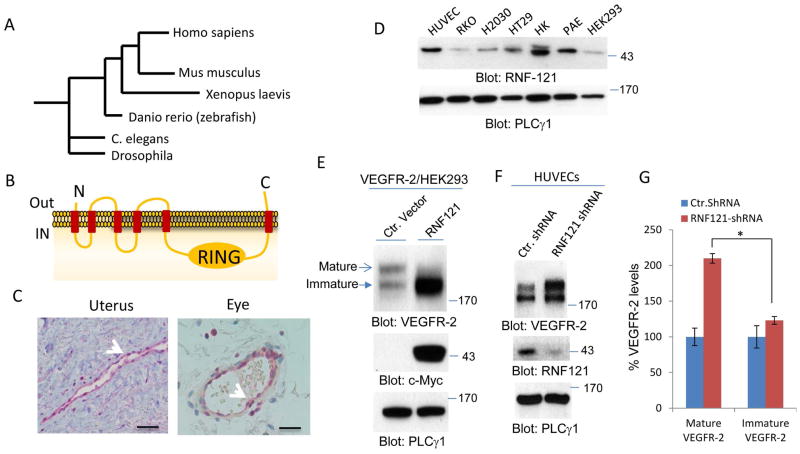
RNF121 was recently identified as an ER localized ubiquitin E3 ligase in C. elegans (17, 19). However, its cellular expression and function in mammalian cells remains largely unknown. RNF121 is highly conserved among species ranging from Drosophila and Xenopus to human (Figure 1A), suggesting an evolutionary conserved function for RNF121. RNF121 contains six putative transmembrane domains with a single RING Finger (Really Interesting New Gene) domain on C-terminus (Figure 1B). The predicted 3D structure of the RING Finger domain of RNF121 is consistent with the known structure of RING Finger domain (S. Figure 1A) and the consensus sequence of the RING Finger domain (S. Figure 1B). The RING Finger is a highly conserved protein domain that contains a Cys3HisCys4 amino acid motif and commonly found in proteins involved in protein ubiquitination (20, 21).
Figure 1. RNF121 is a highly conserved ubiquitin E3 ligase that is expressed in human blood vessels and regulates maturation of VEGFR-2.
(A) RNF121 is highly conserved in metazoans. (B) Shown is the schematic of predicted domain information of RNF121including, transmembrane domains and the RING Finger domain. “IN” is refereed to lumen of endoplasmic reticulum. (C) RNF121 is expressed in human blood vessels. Shown is the immunohistochemistry staining of RNF121 in human uterus and ocular tissues. Scale bars, 100μm. (D) Whole cell lysates extracted from various cells including endothelial cells and tumor cell lines and blotted for RNF121 and loading control PLCγ1. (E) VEGFR-2/HEK-293 cells were transfected with Myc-tagged RNF121 or control vector and whole cell lysates were probed for VEGFR-2, RNF121 and loading control protein, PLCγ1. The arrows show mature and immature forms of VEGFR-2. (F) Human primary umbilical vein endothelial cells (HUVECs) were infected with RNF121 shRNA or control shRNA and after selection with puromycin, cells were lysed and whole cell lysates were subjected to western blot analysis and probed for VEGFR-2, RNF121 and loading control protein, PLCγ1. (G) Graph is representative of three independent experiments. *P<0.05.
Our initial observation using immunohistochemistry staining showed that RNF121is expressed in human blood vessels (Figure 1C). In addition, RNF121 was detected in cell lysates of human umbilical vein endothelial cells (HUVECs), porcine aortic endothelial (PAE) cells, colon carcinoma cell lines (RKO and HT29), kidney cells (HK2 and HEK-293) and lung carcinoma cell line (H2030) (Figure 1D). Considering that VEGFR-2 is a major RTK expressed in endothelial cells and plays a central role in endothelial cell function and angiogenesis, we sought to examine possible role of RNF121 in the regulation of VEGFR-2. Co-expression of RNF121 with VEGFR-2 in HEK-293 cells unexpectedly reduced the levels of mature VEGFR-2 and resulted in the accumulation of immature VEGFR-2 (Figure 1E). VEGFR-2 is detected at two different molecular weights in SDS-PAGE followed by western blot analysis: a high molecular weight that corresponds to the mature form of VEGFR-2 and a low molecular weight VEGFR-2. The low molecular weight VEGFR-2 corresponds to newly synthesized and partially glycosylated VEGFR-2 which is not fully matured, hereafter referred as immature VEGFR-2 (Figure 1E). The presence of immature VEGFR-2 disappeared when cells was treated with the protein synthesis inhibitor, cycloheximide for 90 minutes (S. Figure 2A). However, cycloheximide treatment of cells over-expressing RNF121 did not block the accumulation of immature VEGFR-2 (S. Figure 2B), suggesting that the increase in the immature VEGFR-2 level in cells co-expressing RNF121 and VEGFR-2 is not associated with the protein synthesis of VEGFR-2.
Given that co-expression of RNF121with VEGFR-2 altered VEGFR-2 maturation, we sought to examine the effect of depletion of RNF121 on VEGFR-2. The knockdown of RNF121 in primary endothelial cells (HUVECs) by shRNA markedly increased maturation of VEGFR-2 (Figure 1F, 1G) and slightly increased. Interestingly, the level of immature VEGFR-2 was also (Figure 1F, 1G), suggesting a possible positive feedback loop mechanism, where increased maturation of VEGFR-2 results in the production of more VEGFR-2. Taken together, the data demonstrate that RNF121regulates maturation of VEGFR-2.
RNF121 regulates trafficking of VEGFR-2
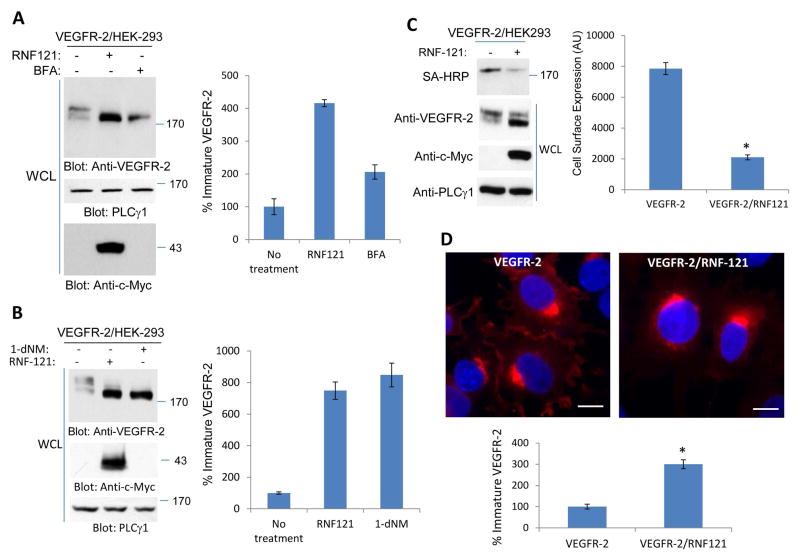
RNF121 was recently identified as an ER protein (17), suggesting that it has the potential to regulate maturation of VEGFR-2 by controlling its exit from the ER. To test role of RNF121 in the trafficking of VEGFR-2, we first tested the effect of known agents such as Brefeldin A (BFA) and 1-deoxynojirimycin (dNM) that results in the accumulation of proteins in the ER. Brefeldin A inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus indirectly by inhibiting COPI (22). Treatment of HEK-293 cells expressing VEGFR-2 with BFA and dNM, which inhibits protein glycosylation and blocks trafficking of the secretory proteins from the ER, resulted in the accumulation of immature VEGFR-2 (Figure 2A, 2B). The effect of BFA and dNM on VEGFR-2 was similar to that of RNF121effect on VEGFR-2 supporting the notion that RNF121 is involved in the exit of VEGFR-2 from the ER to Golgi complexes.
Figure 2. RNF121 regulates the cell surface levels of VEGFR-2.
(A) HEK-293 cells expressing VEGFR-2 alone or VEGFR-2 with c-Myc-RNF121 were treated with Brefeldin-A (BFA) or control vehicle and cells were lysed and whole cell lysates (WCL) were blotted for VEGFR-2, RNF121 and PLCγ1 as a loading control. (B) The same cell lines were treated with 1-deoxynojirimycin (1-dNM) and whole cell lysates were blotted for VEGFR-2, RNF121 and PLCγ1 as a loading control. (C) HEK-293 cells expressing VEGFR-2 alone or VEGFR-2 with c-Myc-RNF121 were subjected to cell surface biotinylation assay and biotinylated proteins was detected with Streptavidin-HRP (SA-HRP) as described in the Materials and Method section. Whole cell lysates were also blotted for VEGFR-2, RNF121 and PLCγ1. Shown is the quantification of the blot of cell surface biotinylation of VEGFR-2. *P<0.035. n=3 (D) Immunofluorescence staining of HEK-293 cells expressing VEGFR-2 or VEGFR-2 with c-Myc-RNF121 is shown. Cells were stained with anti-VEGFR-2 antibody. Scale bars, 10μm. (E) Graph is representative of immature VEGFR-2 from quantification of at least 20 cells per group. The Image J software was used to quantify the images. P<.005, n=3.
To examine whether RNF121-mediated accumulation of immature VEGFR-2 at the ER compartments affects the levels of VEGFR-2 at the plasma membrane, we subjected cells overexpressing RNF121 to cell surface biotinylation assay and evaluated the cell surface level of VEGFR-2. Results from the cell surface biotinylation assay showed that over-expression of RNF121 markedly reduced the cell surface level of VEGFR-2 supporting the hypothesis that overexpression of RNF121 interferes with the trafficking of VEGFR-2 from the ER and hence reduces the amount of mature VEGFR-2 at the cell surface (Figure 2C). Additionally, immunofluorescence microscopy analysis of cells overexpressing RNF121 showed that overexpression of RNF121 significantly reduced VEGFR-2 at the cell surface and resulted in the accumulation of VEGFR-2 in the perinuclear region likely in the ER/Golgi compartments (Figure 2D). Consistent with the previous report (17), RNF121 was mainly present at the ER compartments, as RNF121 was co-localized with GFP-KDEL (S. Figure 3A). The GFP-KDEL chimeric construct is commonly used as an ER marker and consists of green fluorescent protein fused to the KDEL endoplasmic reticulum retention signal downstream of the prolactin leader sequence (23). Furthermore, both RNF121 and GFP-KDEL were also co-localized with VEGFR-2 (S. Figure 3A).
Since overexpression of RNF121 significantly reduced the presence of mature VEGFR-2 at the cell surface, we investigated the effect of over-expression of RNF121 on the VEGF-induced phosphorylation of VEGFR-2 and downstream signaling proteins. The results showed that due to the reduced levels of VEGFR-2 at the cell surface, cells over-expressing RNF121 showed significantly reduced phosphorylation of VEGFR-2 (54% reduction compared to control) (S. Figure 4A, 4B). Similarly, phosphorylation of downstream signaling proteins including p38MAPK, and PLCγ1 in response to VEGF stimulation was also reduced (S. Figure 4A, 4B). The data demonstrate that RNF121 by inhibiting maturation of VEGFR-2 attenuates the VEGF-induced tyrosine phosphorylation of VEGFR-2 and activation of downstream signaling proteins.
RNF121 interacts with the cytoplasmic domain of immature VEGFR-2
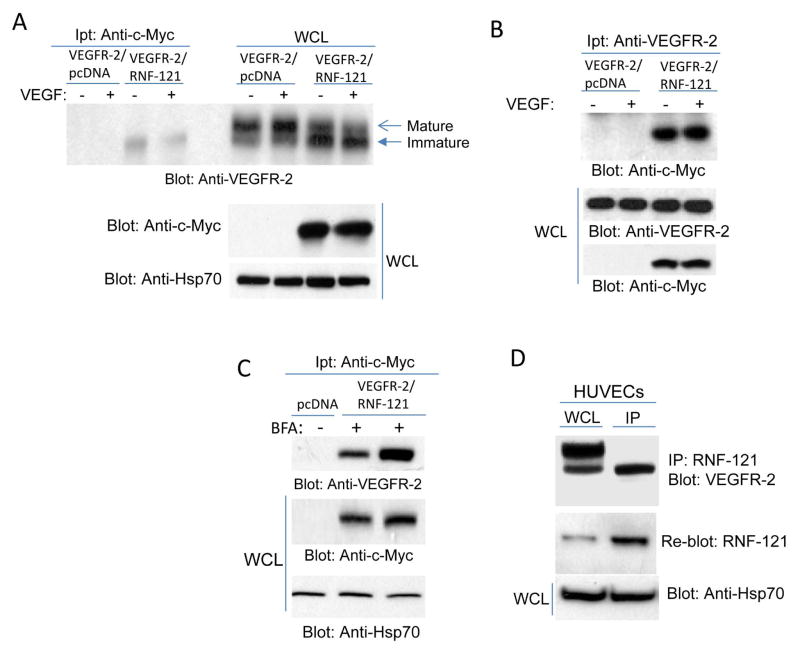
Given the observed effect of RNF121 in the maturation of VEGFR-2, we decided to test the whether RNF121 physically interacts with VEGFR-2. To demonstrate binding of RNF-121 to VEGFR-2, cell lysates derived from HEK-293 cells co-expressing VEGFR-2 and RNF121 were subjected to a co-immunoprecipitation assay. RNF121 was co-immunoprecipitated only with the immature VEGFR-2 and not with the mature cell surface VEGFR-2 (Figure 3A, 3B). Treatment of cells with BFA, which blocks the exit of newly made proteins from the ER to Golgi complexes, also did not prevent the binding of RNF121 with VEGFR-2 (Figure 3C). These data demonstrate that RNF121 binds to immature VEGFR-2 at the ER compartments. To further demonstrate the recognition of immature VEGFR-2 by endogenous RNF121, we used non-transfected primary human endothelial cells, HUVECs, and show that the endogenous RNF121 binds to non-transfected immature VEGFR-2 (Figure 3D).
Figure 3. RNF121 selectively binds to immatureVEGFR-2.
(A) VEGFR-2/HEK293 cells were transfected with an empty vector or with Myc-RNF121. After 48 hours of transfection, cells were starved for overnight followed by stimulation with VEGF for 10 minutes (+) or left unstimulated (-). Cells were lysed and whole cell lysates was immunoprecipitated with anti-c-Myc antibody followed by immunoblotting with anti-VEGFR-2 antibody. An aliquot of whole cell lysates (WCL) of the same lysates was also subjected to western blot analysis as a control and blotted for RNF121 and loading control. (B) Cell lysates derived from VEGFR-2/HEK-293 co-expressing empty vector or RNF121 prepared as panel A and cell lysates were immunoprecipitated with anti-VEGFR-2 antibody followed by immunoblotting with anti-c-Myc antibody. (C) VEGFR-2/HEK293 cells co-expressing empty vector or Myc-RNF121 were treated with vehicle (-) or with Brefeldin A (BFA) (+) and cell lysates was subjected to immunoprecipitation using anti-c-Myc antibody followed by immunoblotting with anti-VEGFR-2 antibody. Of note, the immunoprecipitated proteins shown in panel B and C were resolved on 12% SDS-PAGE to probe for RNF121 and hence VEGFR-2 is detected only as a single band. Whole cell lysates from the same group were blotted for RNF121 using anti-c-Myc antibody and loading control, HSP70. (D) Cell lysates derived from primary human endothelial cells (HUVECs) were subjected to immunoprecipitation assay using RNF121 antibody followed by immunoblotting with anti-VEGFR-2 antibody. The same membrane was re-probed for RNF121. The whole cell lysates was also blotted for HSP70 as a loading control. All the western blots shown in figure 3 are presentative of at least three independent experiments.
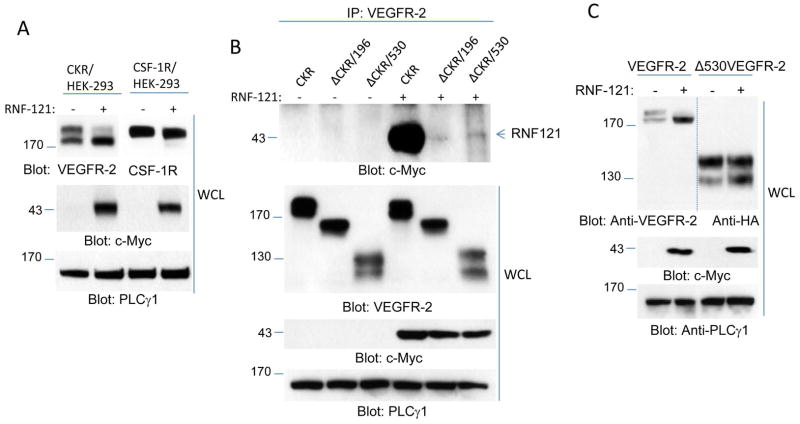
To gain further insight into the binding of RNF121 with VEGFR-2, we used a previously established chimeric VEGFR-2 (CKR), in which the extracellular domain of VEGFR-2 was replaced with the extracellular domain of human colony stimulating growth factor-1 receptor (CSF-1R)(24). Similar to VEGFR-2, co-expression of RNF121 with CKR reduced cell surface expression of mature CKR but not that of CSF-1R (Figure 4A), indicating that RNF121 regulates maturation of VEGFR-2 through recognition of its cytoplasmic domain. Co-immunoprecipitation experiments also showed that RNF121 interacts with CKR and partial deletion of the cytoplasmic domain of CKR (196 amino acids) or complete deletion of the cytoplasmic domain (530 amino acids) abolished binding of RNF121 to CKR (Figure 4B). Deletion of 530 amino acids from the cytoplasmic domain of VEGFR-2 also rendered VEGFR-2 resistant to the effect of RNF121 (Figure 4C), indicating that the presence of the cytoplasmic domain is critically important for the effect of RNF121 on VEGFR-2.
Figure 4. RNF121 binds to cytoplasmic domain of VEGFR-2.
(A) HEK293 cells co-expressing chimeric VEGFR-2 (CKR) with empty vector (-) or RNF121 (+) and HEK293 cells co-expressing CSF-1 receptor (CSF-1R) with empty vector (-) or RNF121 (+) were lysed and whole cell lysates (WCL) was subjected to immunoblotting for VEGFR-2, CSF-1R, and RNF121 (anti-c-Myc antibody) or loading control, PLCγ1. (B) HEK-293 cells expressing CKR, or C-terminus truncated CKR (ΔCKR/196, and ΔCKR530, whereas 196 amino acids and 530 amino acids were deleted, respectively) were transfected with empty vector or with myc-RNF121. Cells after 48 hours transfection were lysed and whole cell lysates was subjected to immunoprecipitation using anti-VEGFR-2 followed by immunoblotting with anti-c-Myc antibody for RNF121. Whole cell lysates from the same group of cells were blotted for VEGFR-2, RNF121 and loading control. (C) HEK-293 cells expressing wild type VEGFR-2 or C-terminus truncated VEGFR-2 (ΔVEGFR-2/530) were transfected with RNF121 or control vector. Cells were lysed after 48 hours and whole cell lysates was blotted for VEGFR-2, RNF121 and loading control protein. Blots (A–C) are representative of at least three independent experiments.
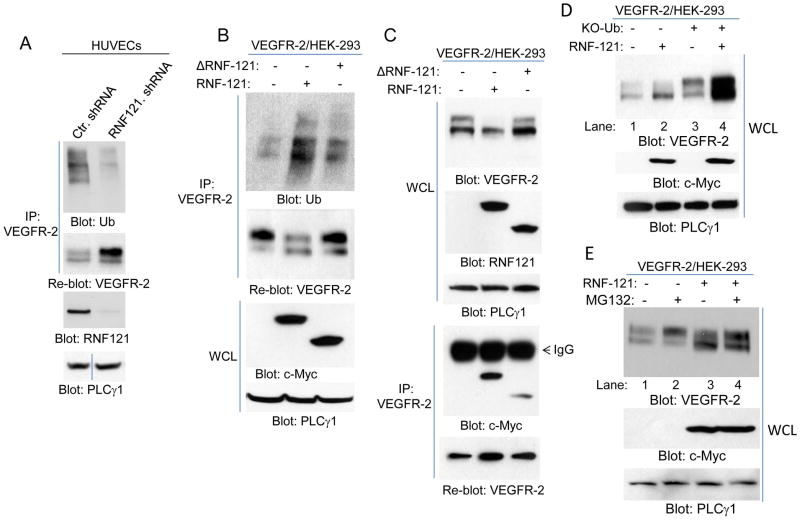
RNF121 ubiquitination of VEGFR-2 regulates its maturation
Considering that RNF121 was originally described as an ubiquitin E3 ligase (17), we sought to examine the hypothesis that RNF121 E3 ligase activity is important for its ability to regulate maturation of VEGFR-2. The shRNA mediated knocked down of RNF121 in endothelial cells showed that VEGFR-2 is moderately ubiquinated in endothelial cells, and its ubiquitination was significantly inhibited in the RNF121knockdown cells (Figure 5A), indicating that RNF121 is involved in the ubiquitination of VEGFR-2. Conversely, over-expression of RNF121 increased the baseline ubiquitination of VEGFR-2 and deletion of the RING Finger domain partially reduced its ability to ubiquitinate VEGFR-2 (Figure 5B), indicating that the RING domain is important for RNF121 to mediated ubiquitination of VEGFR-2. More importantly, deletion of the RING Finger domain also led to the reversal of its ability to inhibit maturation of VEGFR-2 (Figure 5C). The deletion of the RING domain did not inhibit the binding of RNF121 with VEGFR-2 (though the deletion appeared to slightly reduce the binding of RNF121 with VEGFR-2) (Figure 5C). Taken together, the data demonstrate that inhibiting RNF121 E3 ligase activity abrogates it effect on VEGFR-2, demonstrating that RNF121 mediated ubiquitination of VEGFR-2 regulates maturation of VEGFR-2.
Figure 5. RNF121 ubiquitinates VEGFR-2.
(A) Human primary endothelial cells (HUVECs) were infected with control shRNA or RNF121 shRNA. Cells were lysed and whole cell lysates (WCL) was subjected to immunoprecipitation assay using anti-VEGFR-2 antibody followed by immunoblotting with anti-ubiquitin antibody (Ub). The same membrane was blotted for VEGFR-2. An aliquot of whole cell lysates from the same group were blotted for RNF121 and for loading control protein, PLCγ1. (B) HEK293 cells expressing VEGFR-2 transfected with control vector, myc-RNF121 or RING Finger domain truncated RNF121 (ΔRNF121). After 48 hours, cells were lysed and whole cell lysates were subjected to immunoprecipitation using anti-VEGFR-2 antibody followed by immunoblotting with anti-ubiquitin antibody. Aliquot of whole cell lysates were also blotted for RNF121 and loading control protein, PLCγ1. Also shown is a graph quantification of ubiquitination of VEGFR-2 by RNF121 or ΔRNF121. It is representative of three independent experiments. *p< 0.05. (C) HEK293 cells expressing VEGFR-2 transfected with control vector, Myc-RNF121 or RING domain truncated RNF121 as panel B, and whole cell lysates (WCL) were blotted for VEGFR-2 and RNF121. Whole cell lysates from the same group were subjected to immunoprecipitation using anti-VEGFR-2 antibody followed by immunoblotting with anti-c-Myc antibody for RNF121. All the blots presented here are representative of at least three independent experiments.
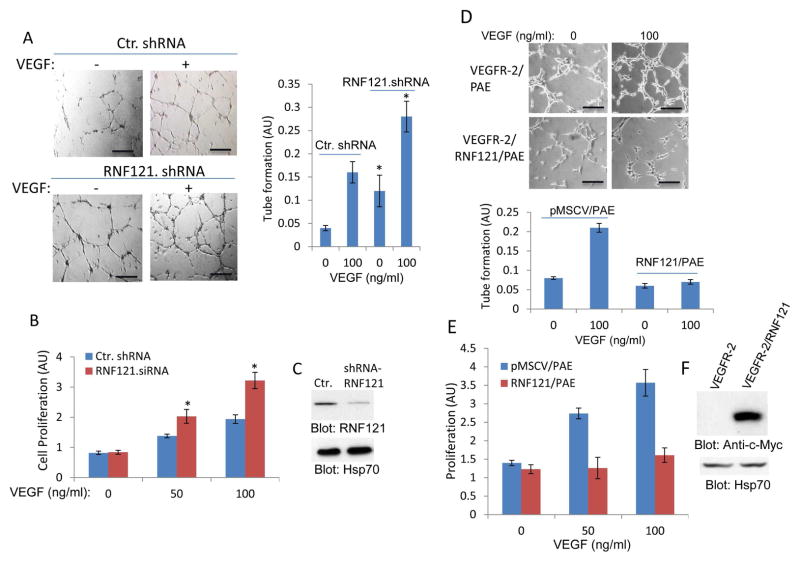
RNF121 activity inhibits VEGF-induced angiogenesis
Activation of VEGFR-2 in endothelial cells stimulates cell proliferation and capillary tube formation (25). To examine whether RNF121 mediated inhibition of maturation of RN121 affects the angiogenic effect of VEGF in endothelial cells, we silenced RNF121 expression by shRNA in human primary endothelial cells (HUVECs) and subjected them to in vitro matrigel angiogenesis and proliferation assays. The results showed that the knockdown of RNF121 in HUVECs significantly increased the VEGF-induced capillary tube formation and proliferation (Figure 6A, 6B). In contrast, over-expression of RNF121 in porcine aortic endothelial (PAE) cells inhibited both VEGF-induced angiogenesis and proliferation (Figure 6D, 6E). The data demonstrate that RNF121 negatively regulates the VEGF-induced angiogenic responses in endothelial cells, as RNF121 knockdown and over-expression increased and decreased capillary tube formation and proliferation of endothelial cells, respectively.
Figure 6. RNF121 inhibits VEGF-induced angiogenesis.
(A) Human primary umbilical vein endothelial cells (HUVECs) that were infected with control shRNA or with RNF121 shRNA were plated on the matrigel coated 24-well plates and stimulated with VEGF or control medium containing 2% FBS. Capillary tube formation of HUVECs viewed under microscope after 12 hours and pictures were taken with camera attached to microscope. Scale bars, 100 μm. Capillary tube formation of HUVECs was quantified using NIH Image J program. Graph is representative of capillary tube formation of HUVECs infected with control and RNF121 shRNA (three randomly selected fields per group were used for quantification). *p< 0.05, n=3 compared to control cells expressing control shRNA. (B) HUVECs infected with control shRNA or with RNF121 shRNA were subjected to proliferation assay using MTT solution as described in the Materials and Method section. *p< 0.05, n=3 compared to control cells without VEGF stimulation. (C) Shown is the silencing effect of RNF121 shRNA on the expression of RNF121. (D) PAE cells expressing VEGFR-2 alone or co-expressing VEGFR-2 with RNF121 were subjected to matrigel angiogenesis assay as panel A and tube formation was quantified in a similar manner. Scale bars, 100 μm. (E) Proliferation of cells was measured by using MTT assay as panel A. (F) Ectopic expression of RNF121 in PAE cells is shown. Experiments (D–E) are representative of three independent experiments.
Discussion
Our study demonstrates for the first time a novel role for RNF121 as a key negative regulator of VEGFR-2. RNF121 selectively binds to newly synthesized immature VEGFR-2 in the ER and restricts its presence at the cell surface. Knockdown and overexpression studies in endothelial cells strongly support the negative role of RNF121 in angiogenesis. The ability to stimulate angiogenesis represents the most critical step in tumor growth and metastasis (26). To grow and metastasize, tumor cells activate a highly complex process called the “angiogenic switch” that leads to increased expression of proteins with pro-angiogenic activities and suppresses expression of proteins with anti-angiogenesis function (26, 27). Activation of VEGFR-2 by VEGF ligands on the endothelial cells is a principal mechanism of tumor activated angiogenic switch (26, 28). The data presented in this manuscript demonstrates that RNF121 by regulating trafficking and maturation of VEGFR-2 reduces its presence at the cell surface leading to reduced VEGF-induced phosphorylation of VEGFR-2.
The role of RNF121 in mammalian cells and its possible link to human pathology are yet to be fully determined. However, recent studies and the data presented in this manuscript point to RNF121 as an important player in controlling key cellular processes. RNF121 was originally identified as an ER ubiquitin E3 ligase in C. elegans as a negative regulator of β-integrin and was upregulated in response to the unfolded protein response (UPR) pathway (17). Consistent with the possible role of RNF121 in the UPR pathway, we also found that treatment of human cells with ER stress inducing agents such as tunicamycin, and oxidative stress inducing agent, hydrogen peroxide upregulates expression of RNF121 (S. Figure 6A, 6B). In agreement with its role in the regulation of cellular processes in C. elegans, a recent study also suggests that RNF121 controls distal tip cell migration (18) and NF-κB signaling (19). Consistent with our observation, while the current manuscript was in preparation, Ogino et al., 2015 reported that RNF121 also regulates maturation of voltage-gated sodium channel (29).
The steady state level of VEGFR-2 at the plasma membrane is tightly regulated by ligand-induced internalization and ubiquitination-dependent degradation (9, 13). This evolutionary mechanism is thought to be a central negative regulatory system to restrict excessive signaling by cell surface receptors (9, 30). The finding that ER resident RNF121 regulates the surface levels of VEGFR-2 independent of VEGF ligands-suggests a significant role for RNF121 in controlling the steady state of VEGFR-2 levels at the plasma membrane and its angiogenic signaling. Similar observation was recently reported for EGF receptor, ErbB3 whether the ER localized RING finger E3 ubiquitin ligase, Nrdp1 regulates cell surface expression of ErbB3 (31). Interestingly, expression of Nrdp1 was reported to be reduced in the breast carcinomas (32), suggesting that over-expression of ErbB3 in breast carcinomas, in part, may be associated with dysregulation of Nrdp1. In this regard, our study may bear relevance to pathological angiogenesis such as cancer, where VEGFR-2 expression and signaling is often upregulated (14, 33, 34). However, further research, and more analyses of cancer specimens, will be necessary to address the hypothesis that this negative regulatory system in pathological angiogenesis is unleashed to meet tumors’ demand for robust angiogenic VEGF signaling.
Materials and Methods
Plasmids, shRNA and antibodies
Human RNF121 cDNA was from Open Biosystems (MHS1010-73812, accession # BC009672). RNF121 cDNA through PCR amplification was cloned into retroviral vector, pMSCV and c-Myc tag was added to its C-terminus for detection purposes. RNF121 was also cloned into pCDNA.cMyc vector for transient transfection purposes. The same plasmid was used to generate a RING Finger truncated RNF121 (ΔRNF121). All the plasmids were sequenced for their identities. Human RNF121 shRNA was a pool of four retroviral shRNAs (RHS1764-9392133, RHS1764-939238, RHS1764-9685188 and RHS1764-9718984) from Dharmacon Inc. RNF121 shRNA virus and retroviral expression of RNF121 and other plasmids were prepared as described.(24, 35). The polyclonal rabbit anti-RNF121 antibody was developed against amino acids (231–247). The specificity of anti-RNF121 antibody was confirmed using a blocking peptide corresponding to amino acid sequences (231–247) of human RNF121 (S. Figure 5).
Bioinformatics analysis
Ensembl Genome Browser (http://www.ensembl.org) was used to obtain genomic information of RNF121 such as determining the chromosomal location, exons, etc. The SUPERFAMILY annotation program (http://supfam.cs.bris.ac.uk/SUPERFAMILY) was used to generate phylogenetic trees and other structural/domain information. Phyre 3D modeling program was used to predict the 3D structure of the RING domain of RNF121(36).
Cell surface biotinylation assay
HEK 293 cells stably expressing VEGFR-2 were transfected with empty vector or RNF121. After 48 hours of transfection, cells were subjected to cell surface biotinylation according to manufacturer’s instructions (Pierce, Inc.). Briefly, cells were biotinylated for 30 minutes at 4°C and the reaction was stopped with quenching buffer. Cells were lysed and whole cell lysates were immunoprecipitated with anti-VEGFR-2 antibody followed by blotting with streptavidin-HRP (SA-HRP).
Cell culture
PAE, HEK-293, HT29, RKO, HK2, and H2030 cells were grown in Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and penicillin and streptomycin. HUVECs were grown in HUVEC growth medium. All cells were incubated at 37°C, 5% CO2 in a humidified chamber. PAE and HEK-293 cells expressing VEGFR-2 and chimeric VEGFR-2 (CKR) were previously described (24, 37).
Cell Proliferation Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT) assay was used to measure cell proliferation as described. In brief, cells (5×104) were seeded in 24-well plates (quadruple wells/group). Cells were washed with PBS and incubated with serum-free medium with or without VEGF as indicated in the figure legends. MTT assay results were processed as recommended by the manufacturer.
Capillary tube formation/in vitro matrigel angiogenesis assay
Matrigel (BD Biosciences) was diluted with an equal volume of DMEM and used to coat 24-well plates as previously described (37). HUVECs or PAE cells were trypsinized, re-suspended in DMEM containing 2% FBS, and counted, and 2 × 105 cells were seeded onto the solidified Matrigel mixture. Cells were allowed to seed for 2 hours before addition of VEGF. Tube formation was allowed to take place overnight, and images were obtained with a Leica inverted microscope attached to camera. For each group three fields were selected blindly and pictures were taken. Images were quantified using NIH Image J program.
Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting was performed as described (38). In brief, cells were washed with H/S buffer [25 mM Hepes (pH 7.4) and 150 mM NaCl] and then lysed in EB lysis buffer [10 mM tris-HCl, 10% glycerol, 5 mM EDTA (pH 7.4), 50 mM NaCl, 50 mM NaF, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 2 mM sodium orthovanadate, and aprotinin (20 mg/ml)]. Where appropriate, cells were serum-starved overnight before lysis, and stimulated with VEGF (100 ng/ml) for 10 min at 37°C. Cell lysates were subjected to immunoprecipitation using a desired antibody or cell lysates, boiled at 95°C for 5 min in Laemmli sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene difluoride (PVDF) membranes, and immunoblotted for proteins of interest.
Immunohistochemistry and immunofluorescence microscopy
Immunohistochemistry staining was performed as per the manufacturer’s instruction using the EXPOSE Rabbit specific HRP/DAB detection IHC kit (Abcam, Cambridge, USA). Immunofluorescence microcopy: HEK-293 cells expressing Myc-tagged RNF121 or co-expressed with GFP-KDEL were grown in chamber slides. Slides were fixed in ice-cold methanol for 10 minutes at room temperature after washing once with TBS buffer. Slides were blocked with 1% BSA in TBS buffer followed by incubation with the desired antibody as indicated in the figure legends. Slides were then incubated with the mouse FITC-conjugated secondary antibody in 1% BSA with TBS for 1 hour. The secondary antibody solution was removed and washed with TBS and slides were mounted with mounting media with DAPI. Nikon Deconvolution Wide-Field Epifluorescence System was used to pictures (Imaging core facility Boston University).
Statistical analyses
Western blots and in vitro angiogenesis and proliferation assays were quantified using Image J software. Statistical analysis was performed by two-tailed t test. All the experiments presented in this manuscript are repeated at least three times.
Supplementary Material
Synopsis Statement.
Ring Finger Protein 121 (RNF121) is an endoplasmic reticulum (ER) ubiquitin E3 ligase that binds to and ubiquitinates the newly synthesized immature vascular endothelial growth factor receptor-2 (VEGFR-2). RNF-121 ubiquitination of VEGFR-2 inhibits the trafficking of immature VEGFR-2 from the ER complex to the Golgi apparatus. This restricts the expression of VEGFR-2 at the cell surface and minimizes the VEGF angiogenic signaling in endothelial cells.
Acknowledgments
Funding: This work was supported in part through grants from the NIH (R21CA191970 and R21CA193958 to N.R.).
Footnotes
Conflict of Interest: The authors declare no conflict of interest. The authors thank Kevin B. Chandler for his edits and comments on the manuscript.
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Kholodenko BN. Cell-signalling dynamics in time and space. Nature reviews Molecular cell biology. 2006;7(3):165–176. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harbor perspectives in biology. 2013;5(5):a017459. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nature reviews Cancer. 2012;12(8):553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 5.Jura N, Zhang X, Endres NF, Seeliger MA, Schindler T, Kuriyan J. Catalytic control in the EGF receptor and its connection to general kinase regulatory mechanisms. Molecular cell. 2011;42(1):9–22. doi: 10.1016/j.molcel.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorini M, Alimandi M, Fiorentino L, Sala G, Segatto O. Negative regulation of receptor tyrosine kinase signals. FEBS letters. 2001;490(3):132–141. doi: 10.1016/s0014-5793(01)02116-0. [DOI] [PubMed] [Google Scholar]
- 7.Acconcia F, Sigismund S, Polo S. Ubiquitin in trafficking: the network at work. Experimental cell research. 2009;315(9):1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 8.d’Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6(6):429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 9.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nature reviews Molecular cell biology. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaengel K, Betsholtz C. Endocytosis regulates VEGF signalling during angiogenesis. Nature cell biology. 2013;15(3):233–235. doi: 10.1038/ncb2705. [DOI] [PubMed] [Google Scholar]
- 11.Grandal MV, Madshus IH. Epidermal growth factor receptor and cancer: control of oncogenic signalling by endocytosis. Journal of cellular and molecular medicine. 2008;12(5A):1527–1534. doi: 10.1111/j.1582-4934.2008.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medinas DB, Hetz C. Protein homeostasis: Modeling UPR adaptive responses. Nature chemical biology. 2014;10(11):879–880. doi: 10.1038/nchembio.1653. [DOI] [PubMed] [Google Scholar]
- 13.Rahimi N. The ubiquitin-proteasome system meets angiogenesis. Molecular cancer therapeutics. 2012;11(3):538–548. doi: 10.1158/1535-7163.MCT-11-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine reviews. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 15.Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Molecular and cellular biology. 2011;31(10):2010–2025. doi: 10.1128/MCB.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaik S, Nucera C, Inuzuka H, Gao D, Garnaas M, Frechette G, Harris L, Wan L, Fukushima H, Husain A, Nose V, Fadda G, Sadow PM, Goessling W, North T, et al. SCF(beta-TRCP) suppresses angiogenesis and thyroid cancer cell migration by promoting ubiquitination and destruction of VEGF receptor 2. The Journal of experimental medicine. 2012;209(7):1289–1307. doi: 10.1084/jem.20112446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darom A, Bening-Abu-Shach U, Broday L. RNF-121 is an endoplasmic reticulum-membrane E3 ubiquitin ligase involved in the regulation of beta-integrin. Molecular biology of the cell. 2010;21(11):1788–1798. doi: 10.1091/mbc.E09-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacevic I, Ho R, Cram EJ. CCDC-55 is required for larval development and distal tip cell migration in Caenorhabditis elegans. Mechanisms of development. 2012;128(11–12):548–559. doi: 10.1016/j.mod.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zemirli N, Pourcelot M, Dogan N, Vazquez A, Arnoult D. The E3 ubiquitin ligase RNF121 is a positive regulator of NF-inverted question markB activation. Cell communication and signaling : CCS. 2014;12(1):72. doi: 10.1186/s12964-014-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102(5):549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 21.Borden KL, Freemont PS. The RING finger domain: a recent example of a sequence-structure family. Current opinion in structural biology. 1996;6(3):395–401. doi: 10.1016/s0959-440x(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 22.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. The Journal of cell biology. 1992;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dayel MJ, Hom EF, Verkman AS. Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophysical journal. 1999;76(5):2843–2851. doi: 10.1016/S0006-3495(99)77438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. The Journal of biological chemistry. 2000;275(22):16986–16992. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- 25.Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Frontiers in bioscience : a journal and virtual library. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 27.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer research. 2005;65(10):3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 28.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nature reviews Cancer. 2010;10(7):505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 29.Ogino K, Low SE, Yamada K, Saint-Amant L, Zhou W, Muto A, Asakawa K, Nakai J, Kawakami K, Kuwada JY, Hirata H. RING finger protein 121 facilitates the degradation and membrane localization of voltage-gated sodium channels. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(9):2859–2864. doi: 10.1073/pnas.1414002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dikic I, Giordano S. Negative receptor signalling. Current opinion in cell biology. 2003;15(2):128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 31.Fry WH, Simion C, Sweeney C, Carraway KL., 3rd Quantity control of the ErbB3 receptor tyrosine kinase at the endoplasmic reticulum. Molecular and cellular biology. 2011;31(14):3009–3018. doi: 10.1128/MCB.05105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen L, Cao Z, Wu X, Ingalla ER, Baron C, Young LJ, Gregg JP, Cardiff RD, Borowsky AD, Sweeney C, Carraway KL., 3rd Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth. Cancer research. 2006;66(23):11279–11286. doi: 10.1158/0008-5472.CAN-06-2319. [DOI] [PubMed] [Google Scholar]
- 33.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Heidenreich R, Murayama T, Silver M, Essl C, Asahara T, Rocken M, Breier G. Tracking adult neovascularization during ischemia and inflammation using Vegfr2-LacZ reporter mice. Journal of vascular research. 2008;45(5):437–444. doi: 10.1159/000126106. [DOI] [PubMed] [Google Scholar]
- 35.Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Molecular biology of the cell. 2012;23(9):1646–1656. doi: 10.1091/mbc.E11-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nature protocols. 2009;4(3):363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 37.Hartsough EJ, Meyer RD, Chitalia V, Jiang Y, Marquez VE, Zhdanova IV, Weinberg J, Costello CE, Rahimi N. Lysine methylation promotes VEGFR-2 activation and angiogenesis. Science signaling. 2013;6(304):ra104. doi: 10.1126/scisignal.2004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivasan S, Meyer RD, Lugo R, Rahimi N. Identification of PDCL3 as a novel chaperone protein involved in the generation of functional VEGF receptor 2. The Journal of biological chemistry. 2013;288(32):23171–23181. doi: 10.1074/jbc.M113.473173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.