Abstract
Background
TAS-102 is an oral fluoropyrimidine prodrug composed of trifluridine (FTD) and tipiracil hydrochloride (TPI) in a 1:0.5 ratio. FTD is a thymidine analog, and it is degraded by thymidine phosphorylase (TP) to the inactive trifluoromethyluracil (FTY) metabolite. TPI inhibits degradation of FTD by TP, increasing systemic exposure to FTD.
Methods
Patients with advanced solid tumors (6 M/2 F; median age 58 years; PS 0–1) were enrolled on this study. Patients in group A (N = 4) received 60 mg TAS-102 with 200 nCi [14C]-FTD, while patients in group B (N = 4) received 60 mg TAS-102 with 1000 nCi [14C]-TPI orally. Plasma, blood, urine, feces, and expired air (group A only) were collected up to 168 h, and were analyzed for 14C by accelerator mass spectrometry and analytes by LC-MS/MS.
Results
FTD: 59.8% of the 14C dose was recovered; 54.8% in urine mostly as FTY and FTD glucuronide isomers. The extractable radioactivity in the pooled plasma consisted of 52.7% FTD and 33.2% FTY. TPI: 76.8% of the 14C dose was recovered; 27.0% in urine mostly as TPI, and 49.7% in feces. The extractable radioactivity in the pooled plasma consisted of 53.1% TPI and 30.9% 6-HMU, the major metabolite of TPI.
Conclusion
Absorbed 14C-FTD was metabolized and mostly excreted in urine. The majority of 14C-TPI was recovered in feces, and the majority of absorbed TPI was excreted in urine. The current data with the ongoing hepatic and renal dysfunction studies will provide an enhanced understanding of the TAS-102 elimination profile.
INTRODUCTION
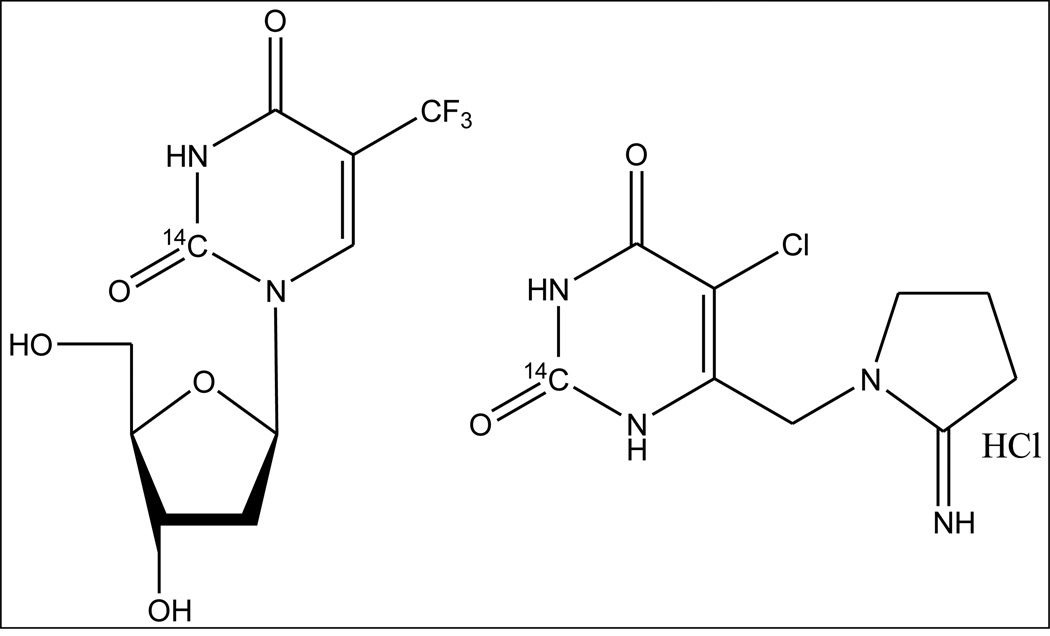
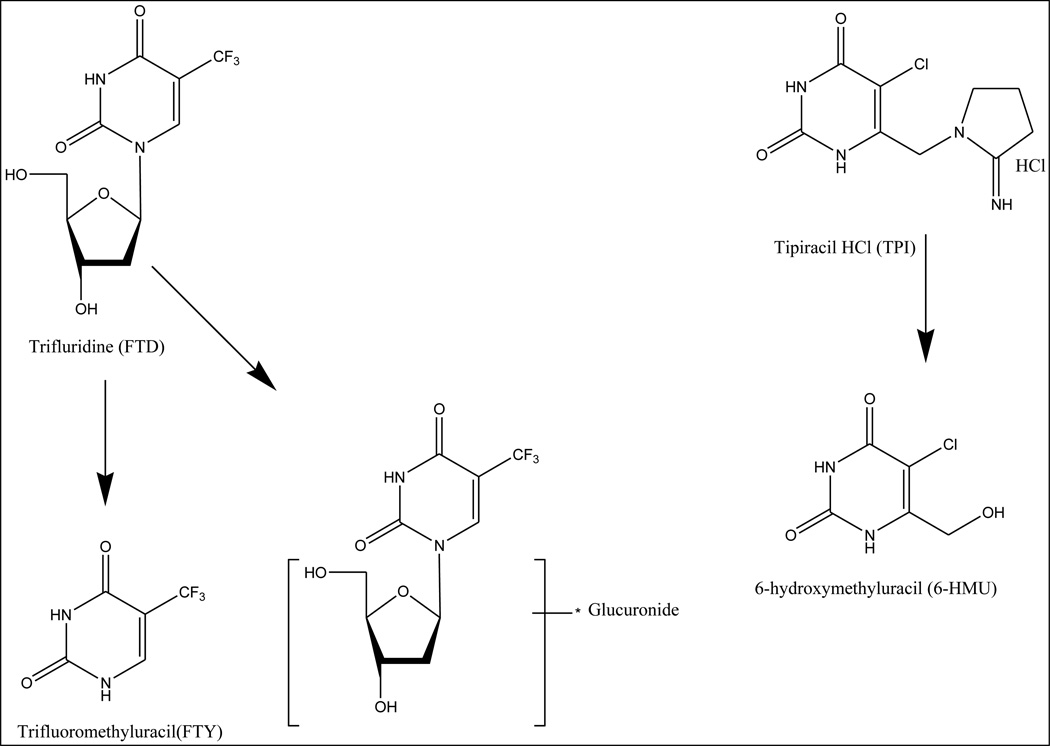
TAS-102 is a novel oral form of the combination drug consisting of trifluridine (FTD), a thymidine-based nucleoside analog, and tipiracil hydrochloride (TPI), an inhibitor of thymidine phosphorylase (TP) and these two agents are combined at a molar ratio of 1:0.5 (Figure 1). FTD is a pyrimidine analog, and its primary cytotoxic mechanism of action is mediated by DNA incorporation of its triphosphate form, leading to inhibition of DNA synthesis and function [1, 2]. In preclinical systems, TAS-102 has significant antitumor activity in 5-FU resistant human cancer cells through a mechanism involving FTD incorporation in tumor DNA [3, 4]. Oral administration of FTD has poor oral bioavailability of FTD [1]. However, concurrent oral administration of TPI with FTD significantly improves FTD oral bioavailability by inhibiting its catabolism by thymidine phosphorylase (TP), resulting in increased systemic exposure to FTD [2, 5]. The improved oral bioavailability of FTD in TAS-102 and the significant antitumor activity of TAS-102 in 5-FU resistant human cancer cells have led to the clinical development of TAS-102 [6–8].
Figure 1.
Chemical structures of 2-[14C]-FTD and 2-[14C]-TPI.
Yoshino, et al. reported the result of a randomized, placebo-controlled phase 2 trial of TAS-102 in Japanese patients with metastatic colorectal cancer (mCRC) refractory or intolerant to fluoropyrimidine, irinotecan, and oxaliplatin [9]. Patients were randomized (2:1) to either TAS-102 (N = 112) or placebo (N = 57). The primary endpoint was overall survival (OS) in the intention-to-treat population. TAS-102 improved median OS significantly (9.0 months in TAS-102 group versus 6.6 months in placebo group; hazard ratio [HR] for death, 0.56; 95% confidence interval [CI], 0.39–0.81; P = 0.0011). The RECOURSE trial was an international randomized phase 3 trial to further investigate the efficacy of TAS-102 in patients with mCRC refractory to standard therapies [10]. A total of 800 patients with chemo-refractory mCRC were randomized (2:1 ratio) to TAS-102 or placebo. The primary endpoint of this study was OS. TAS-102 demonstrated a significant improvement compared with placebo in median OS (7.1 versus 5.3 months; HR, 0.68; 95% CI, 0.58–0.81; P < 0.001). The most frequent adverse events associated with TAS-102 were neutropenia (38%), leukopenia (31%), and febrile neutropenia (4%).
TAS-102 has been approved in Japan and US for the treatment of mCRC and it is currently in development in Europe [11]. Although the metabolism of FTD has been reported, the metabolic fate and excretion of TAS-102 is unknown. To support the final stages of TAS-102 clinical development, the present mass balance study was aimed at determining the pharmacokinetics, metabolism, and excretory pathways of [14C]-FTD and [14C]-TPI, respectively, as the main components of TAS-102. The primary objective of this study was to determine recovery of the radioactive dose in urine, feces, and expired air. The use of Accelerator Mass Spectrometry (AMS) allowed our objectives to be achieved at low radioactive doses resulting in negligible radiation exposure [12].
MATERIALS AND METHODS
Study design
This was an open-label, single-dose study in which 8 patients with advanced solid tumors received an oral solution incorporating a light tracer dose of either 200 nCi [14C]-FTD (group A) or 1000 nCi [14C]-TPI (group B) and 60 mg TAS-102. The protocol and informed consent form were approved by the University of Pittsburgh Institutional Review Board, and each patient gave written informed consent before receiving the study medication. The radiolabeled dose and 7-day collection period was followed by a regimen of BID 35 mg/m2 of TAS-102 PO days 1 through 5 and 8 through 12 for a 28-day cycle (data not shown). Eligibility criteria most relevant to the mass balance study were: willingness and ability to undergo in-house admittance during the first 8 days, adequate hematopoietic (Hb ≥9.0 g/dL, ANC ≥1.5 × 109/L, platelets ≥100×109/L) hepatic (serum bilirubin ≤1.5 × upper limit of normal (ULN), AST and ALT ≤3 × ULN or ≤ 5 × ULN if due to underlying liver metastases) and renal (creatinine ≤1.5 mg/dL) function, an ECOG performance status of 0–1, and ability to take oral medications. Patients could not have partial or total gastrectomy, intestinal obstruction, a medical condition that jeopardized or impaired the ability to adequately collect representative excreta (e.g. patients with a bile duct stent due to bile duct stenosis), and exposure to 14C in the preceding 12 months.
Study medication
Individual vials of radiolabeled FTD contained approximately 1.32 µg, 220 nCi 2-[14C]-α,α,α-trifluorothymidine (specific activity 50 mCi/mmol). Individual vials of radiolabeled TPI contained approximately 6.16 µg, 1100 nCi 2-[14C]-5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]pyrimidine-2,4-(1H,3H)-dione monohydrochloride (specific activity 50 mCi/mmol). The [14C]-FTD and [14C]-TPI were repurified by Ricerca Biosciences (Concord, OH, USA). Radiochemical and chemical purity was >99%. On Day 1, [14C]-FTD (220 nCi, group A) or [14C]-TPI (1100 nCi, group B) was dissolved in 44 mL sterile water for injection. Forty mL was transferred into a bottle of TAS-102 powder for oral solution containing 60 mg of FTD plus 28.26 mg TPI (conforming to the 1:0.5 molar ratio of the TAS-102 combination antineoplastic drug in tablet formulation). The remaining 4 mL of the radioactive solution was used to confirm the 14C dose of each solution by liquid scintillation counting (LSC), and HPLC radiopurity (apart from an occasional, small signal at the void time no peaks other than the compounds were detected). Individual dose LSC radioactivity values were used to express recovered 14C in feces, urine, and exhaled air as percentage of dose administered.
Dose administration
The 40 mL dosing solution of 60 mg TAS-102 and [14C]-FTD (group A) or [14C]-TPI (group B) was orally administered in the morning of day 1 within 30 min after completion of a standardized breakfast, high-fat (approximately 50 percent of total caloric content of the meal), high-calorie (approximately 800 to 1000 calories) breakfast. An additional 40 mL of sterile water was used to rinse the dose container, which was then also administered to the patient. The patient subsequently drank another 160 mL of water from another container to bring the total amount ingested to approximately 240 mL. The empty dosing container was retained for quantitation of any remaining 14C (less than 0.01% of dose could be recovered by a water and methanol wash). Specifically, human thymidine kinase substrates were avoided during participation in this trial.
Sample collection
Patients remained at the clinical site from 10 h prior to the administration of study drug on Day 1 through the completion of all postdose sample collections on Day 8. Heparin anti-coagulated blood was collected at 0 (pre-dose), 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 96, 120, 144, and 168 h after dose from an indwelling IV catheter. Aliquots of whole blood (0.5 mL) were retained for AMS analysis of whole blood TRA. The remainder of the blood was centrifuged (10 min, 1500 × g, 4 °C) to prepare plasma, which was separated into aliquots for AMS analysis of plasma TRA, metabolite profiling and identification, and LC-MS/MS analysis of FTD, FTY, and TPI (JCL Bioassay USA Inc.).
On Days 1 through 8, complete urine and fecal samples were collected from all patients for measurement of TRA and metabolite profiling. Collection intervals were done prior to dosing (single sample collection) and in blocks of 24 h from 0 to 168 h post-dose. Patients were asked to void their bladder in the morning prior to dosing, from which the pre-dose urine sample was obtained. Patients who did not have at least 1 bowel movement within a 24-hour period were administered a stool softener as determined by the Investigator. Urine collections were quantitated gravimetrically assuming a density of 1 g/mL. Fecal samples were also weighted and stored at −80 °C until homogenization in an exactly determined amount of water (1 part water to feces, v/g, unless more was needed to liquefy). Approximately 15 g of homogenate was accurately weighed into a 20 mL container, frozen at −80 °C overnight, and freeze-dried (FreeZone, Labconco, Fisher Sci, Hanover Park, IL) until at a stable weight. The freeze-dried residue was pulverized in a mortar and pestle, and dispensed for AMS analysis. All samples were stored at −80 °C until analysis.
For group A ([14C]-FTD) only, expired carbon dioxide (CO2) was collected at the same time points as those used for blood sampling. Samples of expired CO2 air were obtained using two parallel Drechsler bottles containing 400 mL of 25 mM potassium hydroxide. Phenolphthalein (1 mL of 0.01%) was added to the trapping solution to indicate saturation of the trapping agent with expired CO2. The amount of time that the patient exhaled through the trapping solution at each collection time point was recorded. For each time point, a 1:1 mixture of the two solutions was prepared and stored for AMS analysis.
AMS analysis of total radioactivity (TRA) in blood, plasma, urine, feces and CO2
TRA in blood, plasma, urine, and feces was determined using AMS (Xceleron Inc., Germantown, MD) as described previously [12, 13].
Bioanalysis of unlabelled FTD, FTY, and TPI in plasma and urine
Concentrations of FTD, FTY, and TPI in plasma and urine were determined with a validated liquid chromatography assay equipped with tandem mass spectrometric detection (JCL Bioassay USA Inc.). Briefly, stable isotope internal standards for each analyte were added to 0.1 mL of each sample, calibration standard and quality control sample. For FTD and FTY analysis, the samples were extracted with 1 mL t-butyl methyl ether. Reconstituted dry residue was injected into the AB Sciex Triple Quad 5500 LC-MS/MS system. Chromatographic separation was achieved, isocratically, on a Capcell PAK C18 AQ column (2.0 × 150 mm) at a flow rate of 0.4 mL/min with detection by electrospray tandem mass spectrometry. The mobile phase contained water 0.1% acetic acid - methanol (75:25, v/v). For TPI analysis, the samples were extracted using Bond Elut PRS extraction cartridges. Reconstituted dry residue was injected into the AB Sciex 5500 LC-MS/MS system. Chromatographic separation was achieved, isocratically, on an Inertsil ODS-3 column (2.1 × 150 mm) at a flow rate of 0.35 mL/min with detection by electrospray tandem mass spectrometry. The mobile phase consisted of 10 mmol ammonium acetate in water - methanol (90:10, v/v). The standard curve ranged from 5 to 5000 ng/mL for FTD and FTY, and from 0.8 to 200 ng/mL for TPI. Precision and accuracy met standard criteria detailed in the relevant FDA guidance [14].
Pharmacokinetic Analyses
The pharmacokinetic analyses of the concentration versus time data were performed by non-compartmental methods using Phoenix WinNonlin Professional (Certara USA, Inc., Princeton, NJ). Similarly, pseudopharmacokinetic parameters were calculated for plasma radioactivity.
Metabolic profiling of plasma, urine, and feces
Plasma pooled samples were prepared by pooling samples with ≥ 5% of the Cmax concentration across time-points based on an AUC approach [15] and then across individual subjects. A volume of 2 mL [14C]-FTD plasma pool was diluted (1:1, v/v) with 0.5% formic acid and then extracted with 6 mL acetonitrile. The supernatant was aspirated and the acetonitrile step repeated twice, followed by another extraction with 6 mL of water:acetonitrile (1:1, v/v). Combined supernatants were dried down and reconstituted in 0.1% formic acid before injection. A volume of 2 mL [14C]-TPI plasma pool was diluted (0.85:1, v/v) with 0.5% formic acid and then extracted with 6 mL acetonitrile. The supernatant was aspirated and the acetonitrile step repeated twice. Combined supernatants were dried down and reconstituted in 0.1% formic acid before injection.
Homogenized feces pooled samples (0–96 h for [14C]-FTD, and 0–120 h for [14C]-TPI) were prepared by mixing a constant proportion, by feces weight, of the total excreted over the relevant individual collection periods so as to account for at least 90% of total 14C excreted by the relevant route, followed by an across subject pool. A 300 mg sample of [14C]-FTD feces homogenate pool was diluted with 400 µL 0.5% formic acid, then extracted with 900 µL acetonitrile. The supernatant was aspirated and the acetonitrile step repeated twice, followed by another extraction with 1.2 mL of water / acetonitrile (1:1, v/v). Combined supernatants were dried down and reconstituted in 0.1% formic acid before injection. A 300 mg sample of [14C]-TPI feces homogenate pool was diluted with 400 µL 0.5% formic acid, then extracted with 900 µL acetonitrile. The supernatant was aspirated and the acetonitrile step repeated twice. Combined supernatants were dried down and reconstituted in 0.1% formic acid before injection.
A 0–96 h urine pooled sample was prepared by mixing a constant proportion, by urine volume excreted, of the total excreted over the relevant individual collection period so as to account for at least 95% of total 14C excreted by the relevant route, followed by an across subject pool. Pooled urine samples were diluted (1:1, v/v) with Mobile Phase A of the respective HPLC profiling method, before injection.
Profiling and collection of fractions was performed on an Agilent 1200 Series HPLC system equipped with a fraction collector. The [14C]-FTD pools were chromatographed on a Imtakt Scherzo SM-C18 column (2.0×150 mm, 3 µm) at 40 °C and a flow rate of 0.2 mL/min, with the UV detector set at 254 nm/266 nm. Mobile phase consisted of A: 5 mM ammonium acetate; and B: 50 mM ammonium acetate / methanol (50:50, v/v). The HPLC gradient was increased from 0% to 100% B over 20 min, decreased to 0% B at 20.1 min, and equilibrated for 12 min. All injections for profiling were 50 µL. HPLC eluent fractions were collected every 10 s for 32 min, and for plasma profiling, the fractions of 4 replicate injections of pool extracts were combined to enhance sensitivity. The [14C]-TPI pools were chromatographed on a Phenomenex Synergi Polar-RP column (150×4.6 mm, 4um, 80A) at 40 °C and a flow rate of 0.5 mL/min, with the UV detector set at 276 nm. Mobile phase consisted of A: 20 mM ammonium acetate (pH 5.5); and B: acetonitrile. The HPLC gradient was increased from 0% to 3% B over 20 min, increased to 20% B at 20.1 min and held for 5 min, decreased to 0% B at 25.1 min, and equilibrated for 5 min. All injections for profiling were 50 µL. HPLC eluent fractions were collected every 12 s for 30 min, and for plasma profiling, the fractions of 3 replicate injections of pool extracts were combined to enhance sensitivity.
Fractionation runs were bracketed by injection of a mixture of non-radiolabeled reference standards: FTD, FTY, 5-C-dUrd, 5-CU, and orotic acid for FTD profiling and TPI, 6-HMU, and uracil for TPI profiling. Individual or pooled fractions were subjected to AMS analysis in order to generate a radiochromatographic profile, as described previously [13]. A threshold of 0.5% of the total 14C recovered in the each profile was set. All individual HPLC fractions measured by AMS with 14C content above this threshold were assigned to a peak.
Structural metabolite identification
Structural identification of metabolites was performed on selected HPLC fractions collected from urine profiling. This was performed on an AB Sciex Triple Quad 5500 mass spectrometer (AB SCIEX, Foster City, CA) equipped with a Turbo Ion Spray source. Samples were infused directly and analysed in negative ion mode (DP −100, EP −10, IS −4500 V) full scan (Q1) mode from m/z 60 to 600 followed by product ion scans with collision energy adjusted between −10 and −40 V.
Extractable fraction
To evaluate the extraction efficiency of 14C, FTD plasma pools were prepared across subjects for the following time points: pre-dose, 2, 8, 24, 96, and 168 h. A plasma aliquot of 200 µL was mixed with 600 µL of acetonitrile, followed by vortex-mixing for 10 min, and centrifugation at 3210×g for 10 min at 4°C. The supernatant was collected and combined with the supernatants of one more repeat extraction.
RESULTS
To evaluate the pharmacokinetics, excretory pathways, and metabolism of [14C]-FTD and [14C]-TPI, respectively, as components of TAS-102, we performed a mass balance study in 8 patients.
Subject Disposition
Eight patients (6 male and 2 female) with a median age of 58 years (range 45–68), median weight of 102.5 kg (range 62.3–148.8) were enrolled. All patients had advanced cancer for which no standard anti-cancer therapy was available. The primary tumors were colon cancer (N=6) and rectal cancer (N=2). All 8 patients completed the mass balance portion of the study with no reported adverse events that were greater than CTCAE grade 3/4 or that were considered to be related to TAS-102.
Plasma Pharmacokinetics (PK)
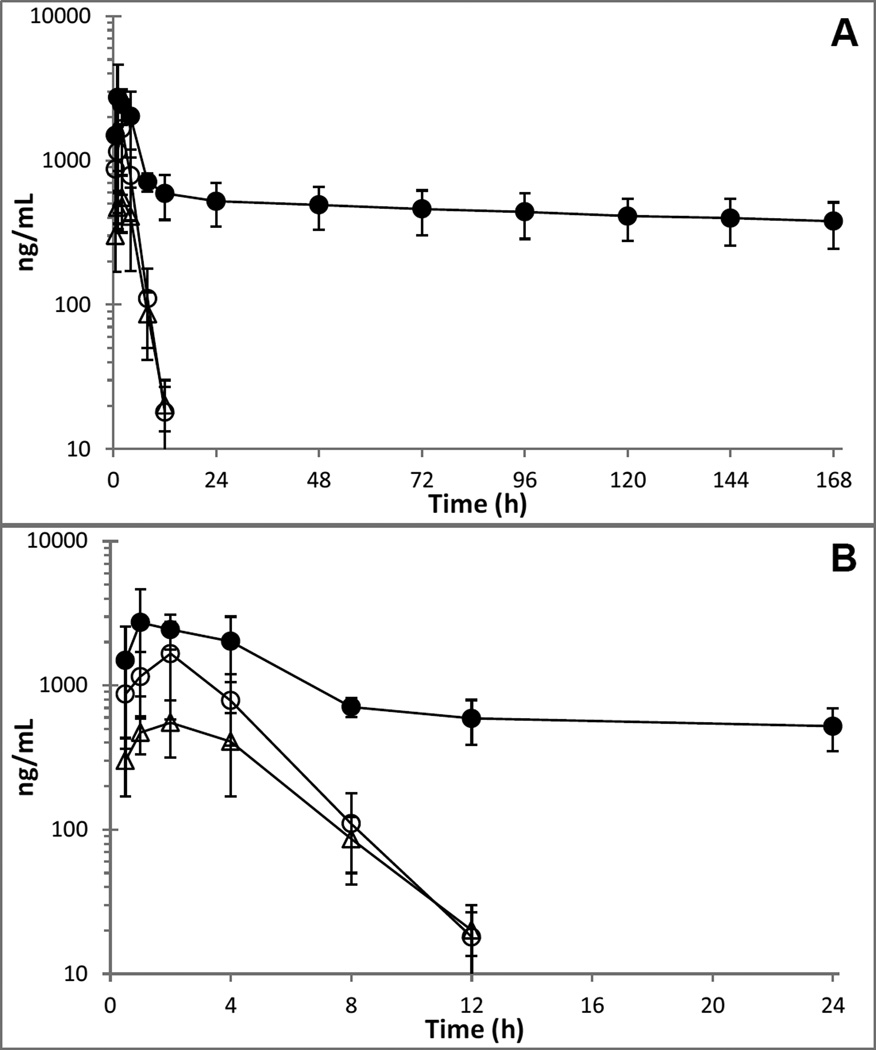
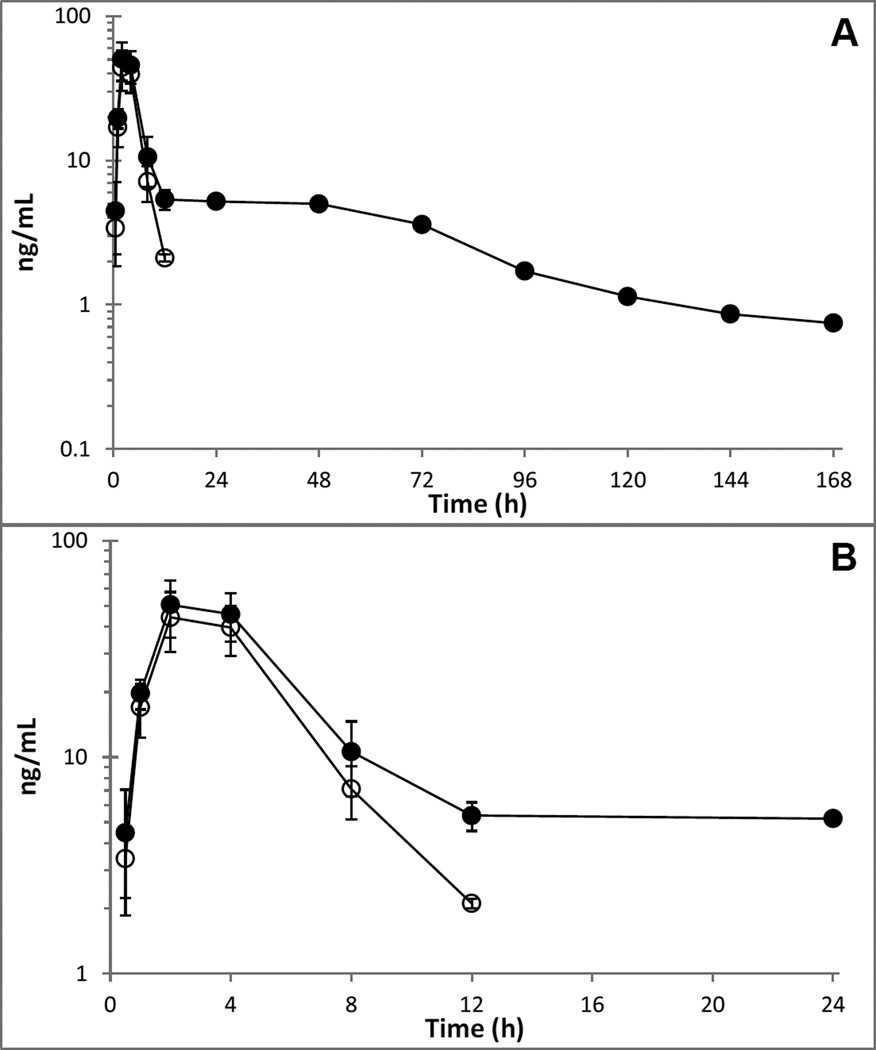
Plasma concentration versus time profiles of total radioactivity (AMS), parent compounds and metabolite are shown in Figure 2 and Figure 3, respectively, while PK parameters are listed in Table 1.
Figure 2.
Mean (±SD) plasma concentration-time curves of [14C]-FTD derived total radioactivity (●, ng-FTD-equivalents/mL), unchanged FTD (○), and FTY (Δ) in 4 patients.
Figure 3.
Mean (±SD) plasma concentration-time curves of [14C]-TPI derived total radioactivity (●, ng-TPI-equivalents/mL), and unchanged TPI (○) in 4 patients.
Table 1.
Plasma pharmacokinetic parameters of [14C]-FTD and [14C]-TPI in cancer patients after administration of a dose of 60 mg TAS-102 (60 mg FTD plus 28.26 mg TPI).
| Radioactivity (FTD-equiv.) | FTD | FTY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Cmax (µg/mL) |
Tmax (h) |
AUClast1 (µg*h/mL) |
t½2 (h) |
Cmax (µg/mL) |
Tmax (h) |
AUClast1 (µg*h/mL) |
t½ (h) |
AUClast ratio3 |
Cmax (µg/mL) |
AUClast1 (µg*h/mL) |
t½ (h) |
AUClast ratio3,4 |
| 1 | 1.67 | 2.0 | 62.8 | 234 | 1.01 | 2.0 | 4.24 | 1.4 | 0.068 | 0.451 | 2.71 | 2.0 | 0.071 |
| 2 | 2.23 | 2.0 | 64.5 | 332 | 1.07 | 2.0 | 3.57 | 1.2 | 0.055 | 0.510 | 2.21 | 1.8 | 0.056 |
| 3 | 3.06 | 0.5 | 92.4 | 383 | 1.52 | 0.5 | 5.76 | 1.6 | 0.062 | 0.453 | 1.74 | 2.2 | 0.031 |
| 4 | 5.42 | 0.9 | 125 | 313 | 3.27 | 1.9 | 10.5 | 1.5 | 0.084 | 0.892 | 4.29 | 1.7 | 0.056 |
| Mean | 3.09 | 1.3 | 86.2 | 316 | 1.72 | 1.6 | 6.03 | 1.4 | 0.067 | 0.577 | 2.74 | 1.9 | 0.051 |
| SD | 1.65 | 0.8 | 29.4 | 62 | 1.06 | 0.7 | 3.14 | 0.2 | 0.012 | 0.212 | 1.11 | 0.2 | 0.017 |
| Radioactivity (TPI-equiv.) | TPI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pt | Cmax (µg/mL) |
Tmax (h) |
AUClast1 (µg*h/mL) |
t½ (h) |
Cmax (µg/mL) |
Tmax (h) |
AUClast1 (µg*h/mL) |
t½ (h) |
AUClast ratio3 |
| 5 | 0.049 | 4.0 | 0.868 | 68.0 | 0.045 | 4.0 | 0.189 | −5 | 0.22 |
| 6 | 0.045 | 2.0 | 0.424 | 31.8 | 0.036 | 2.0 | 0.160 | 2.3 | 0.38 |
| 7 | 0.070 | 2.0 | 0.481 | 16.5 | 0.056 | 2.0 | 0.249 | 1.9 | 0.52 |
| 8 | 0.052 | 2.0 | 0.937 | 73.4 | 0.056 | 2.0 | 0.244 | 1.8 | 0.26 |
| Mean | 0.054 | 2.5 | 0.678 | 47.4 | 0.048 | 2.5 | 0.211 | 2.0 | 0.34 |
| SD | 0.011 | 1.0 | 0.262 | 27.7 | 0.009 | 1.0 | 0.043 | 0.2 | 0.13 |
AUClast represents ≥28% ([14C]-FTD), ≥99% (FTD), ≥97% (FTY), ≥84% ([14C]-TPI), ≥96% (TPI) of AUCinf, respectively.
Half-life could not be estimated accurately because of the relatively short observation time (168 h) relative to the estimates of the half-life.
AUClast ratio is calculated by dividing AUClast of the respective compound (FTD, FTY or TPI) by AUClast total radioactivity.
Corrected for difference in molecular weight using a conversion factor = 296.20 (FTD)/180.08 (FTY) = 1.645.
Too few points to determine terminal half-life
After a single dose of TAS-102, FTD and [14C]-FTD associated total radioactivity were rapidly absorbed with a Tmax of 1.2–1.4 h, and approximately half of the total radioactivity Cmax was accounted for by unchanged FTD. While FTD and its metabolite FTY were eliminated with a similar half-life of approximately 1.4–1.9 h and undetectable by 24 h, total radioactivity exhibited a slow terminal elimination phase with a mean half-life of approximately 300 h and plasma levels of total radioactivity detected up to the last sample of the seven day study period. The ratio of the FTD and FTY AUClast to the total radioactivity AUClast suggested that FTD and FTY accounted for 6.7% and 5.1% of plasma radioactivity. The [14C]-FTD associated total radioactivity blood:plasma ratio progressed from slightly below (0.64) to slightly above unity (1.4) over the course of the study, and the PK parameters of total radioactivity in blood (not shown) were generally similar to those in plasma. The mean (SD) AUClast blood:plasma ratio was 1.18 (0.14).
TPI and 14C-TPI associated total radioactivity were rapidly absorbed with a Tmax of 2.4 h. TPI was eliminated with a half-life of 2.0 h and was undetectable by 24 h, while total radioactivity followed a slower terminal elimination phase with a half-life of approximately 40 h and plasma levels of total radioactivity were detectable up to the last sample of the study period. TPI accounted for 32 % of plasma total radioactivity. The [14C]-TPI associated total radioactivity blood:plasma ratio progressed from slightly below (0.69) to slightly above unity (1.5) over the course of the study, and the PK parameters of total radioactivity in blood (not shown) were generally similar to those in plasma. The mean (SD) AUClast blood:plasma ratio was 0.85 (0.31).
Mass balance
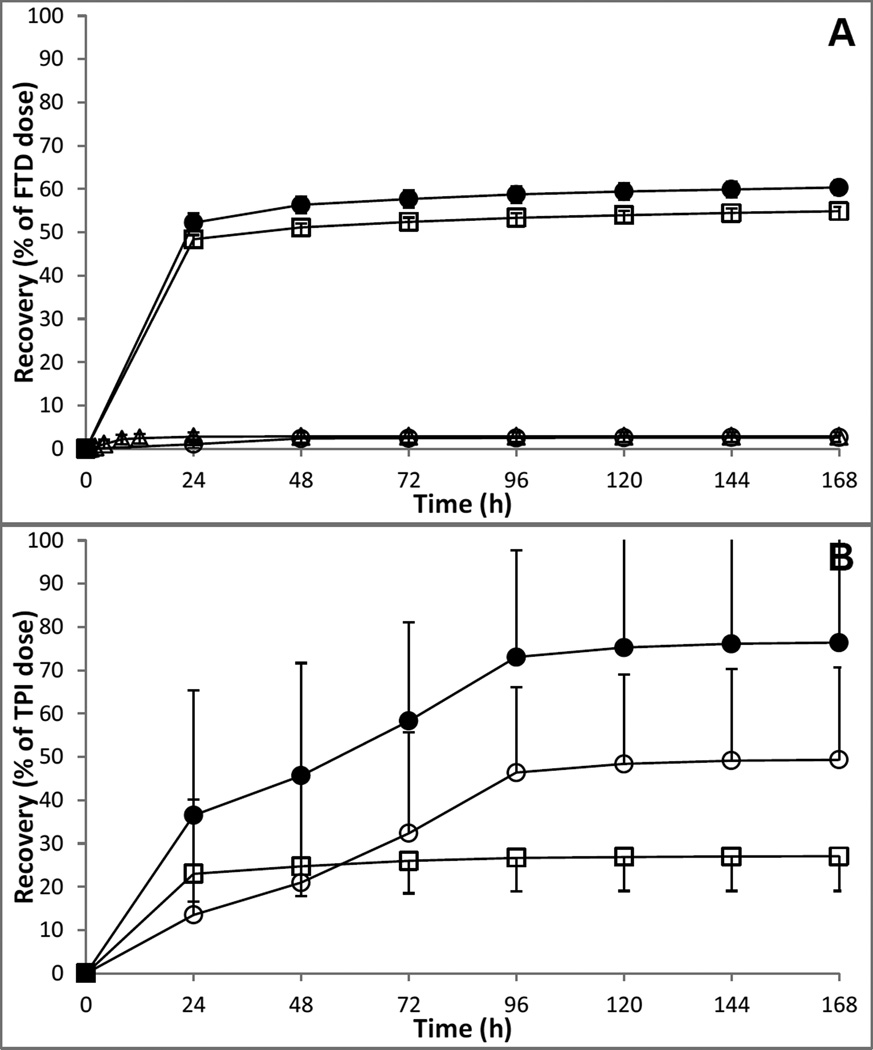
The mean (SD) cumulative excretion of radioactivity in urine, feces, expired air, and total cumulative excretion is depicted in Figure 4, and individual values are provided in Table 2.
Figure 4.
Mean (SD) urinary (□), fecal (○), expired air (Δ) and total (●) cumulative excretion (0–168 h) of [14C]-FTD related material (A) or [14C]-TPI related material (B) as part of TAS-102 in cancer patients (4 per cohort).
Table 2.
Urinary, fecal, and expired air recovery (0–168 h) of total radioactivity in cancer patients.
| Recovery (% of dose) | ||||
| [14C]-FTD | Total radioactivity | |||
| Pt | Urine | Feces | Expired Air | Total |
| 1 | 57.3 | 3.01 | 1.67 | 62.0 |
| 2 | 54.1 | 2.65 | 2.07 | 58.8 |
| 3 | 54.3 | 2.12 | 2.09 | 58.5 |
| 4 | 53.4 | 2.82 | 3.65 | 59.9 |
| Mean | 54.8 | 2.65 | 2.37 | 59.8 |
| SD | 1.7 | 0.38 | 0.88 | 1.6 |
| [14C]-TPI | Total radioactivity | |||
| Pt | Urine | Feces | Expired Air | Total |
| 5 | 24.5 | 62.4 | - | 86.9 |
| 6 | 18.8 | 17.5 | - | 36.3 |
| 7 | 27.0 | 58.3 | - | 85.3 |
| 8 | 37.9 | 60.8 | - | 98.7 |
| Mean | 27.0 | 49.7 | - | 76.8 |
| SD | 8.0 | 21.6 | - | 27.7 |
On average, approximately 60% of [14C]-FTD related radioactivity was excreted over the 7-day period with the majority of 55% being excreted in urine, and less than 3% excreted in both feces and expired air. Most of the urinary excretion and loss of radioactivity through expired air occurred within the first 24 h, whereas excretion in feces occurred mostly in the first 48 h.
Approximately 77% of [14C]-TPI related radioactivity was excreted over the 7-day period with the majority of 50% being excreted in feces, and 27% excreted in urine. Most of the urinary excretion occurred within the first 24 h, whereas excretion in feces occurred gradually over the course of the first 96 h.
FTD metabolic profiling
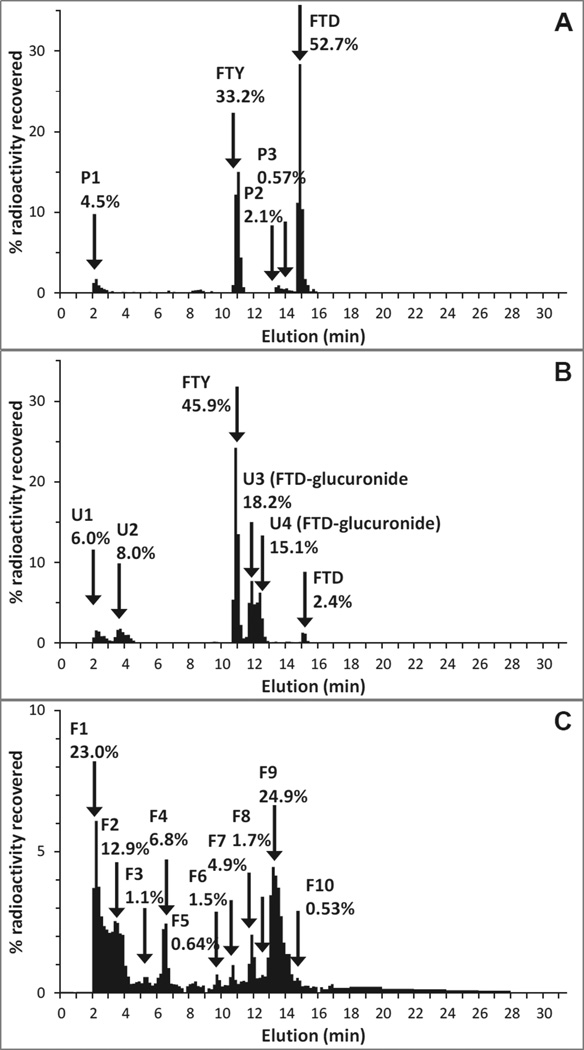
There was clear evidence of the formation of an un-extractable fraction of radioactivity in pooled plasma samples from the [14C]-FTD dosing group. The fraction of extractable radioactivity in the time point-based pools decreased from greater than 90% at 2 h to less than 10% at 24 h and less than 1% at 96 h. The extraction efficiency of the overall pooled plasma sample was poor at 6.7%, and several extraction methods using acid or hydrophilic solvents did not improve the recovery. The radiochromatogram of the extractable fraction suggested that the extractable TRA consisted of 52.7% FTD and 33.2% FTY, and no other peaks greater than 5%, see Figure 5A. The radiochromatogram of pooled urine showed a total of 14% of radioactivity in unknown front peaks, 45.9% FTY, 2.4% FTD, and 33.3% in two metabolite peaks (U3 and U4) that were selected for structural elucidation, see Figure 5B. The molecular ion peaks of U3 and U4 were m/z 471 as [M−H]− and product ions m/z 291 (2’-deoxyribose-glucuronide moiety) and m/z 179 (trifluorothymine moiety) were consistent with FTD-deoxyribose-glucuronide isomers. The radiochromatogram of pooled feces (90.6% extraction efficiency) showed multiple components at trace levels (considering the low % of dose excreted in feces in the first place), none of which were identifiable as FTD or FTY, see Figure 5C. A full accounting of routes and chemical species is provided in Table 3.
Figure 5.
Radiochromatograms of (A) pooled plasma (0–168 h), (B) pooled urine (0–96 h), and (C) pooled feces (0–120 h) following a single oral administration of [14C]-FTD. Percentage radioactivity recovered represents the radioactivity in each fraction relative to the total radioactivity of the sample injected.
Table 3.
Radioactivity as a percentage of dose by route and assigned chemical species.
| [14C]-FTD | FTD | FTY | FTD-gluc U3 |
FTD-gluc U4 |
CO2 | Other | SUM | Assigned | Unaccounted |
|---|---|---|---|---|---|---|---|---|---|
| Plasma1 | 52.7 | 33.2 | 7.21 | 100.0 | 85.9 | ||||
| Urine2 | 1.32 | 25.2 | 10.0 | 8.27 | 10.1 | 54.8 | 44.7 | ||
| Feces2 | 2.65 | 2.65 | 0.00 | ||||||
| Air2 | 2.37 | 2.37 | 2.37 | ||||||
| SUM2 | 1.32 | 25.2 | 10.0 | 8.27 | 2.37 | 12.7 | 59.8 | 47.1 | 40.2 |
| [14C]-TPI | TPI | 6-HMU | Other | SUM | Assigned | Unaccounted | |||
|---|---|---|---|---|---|---|---|---|---|
| Plasma1 | 53.1 | 30.9 | 16.0 | 100.0 | 84.0 | ||||
| Urine2 | 21.4 | 3.78 | 1.86 | 27.0 | 25.2 | ||||
| Feces2 | 24.0 | 17.0 | 8.65 | 49.7 | 41.1 | ||||
| SUM2 | 45.4 | 20.9 | 10.5 | 76.8 | 66.3 | 23.2 | |||
The values are shown as % of total radioactivity recovered in extractable fraction.
The values are shown as % of dose.
TPI metabolic profiling
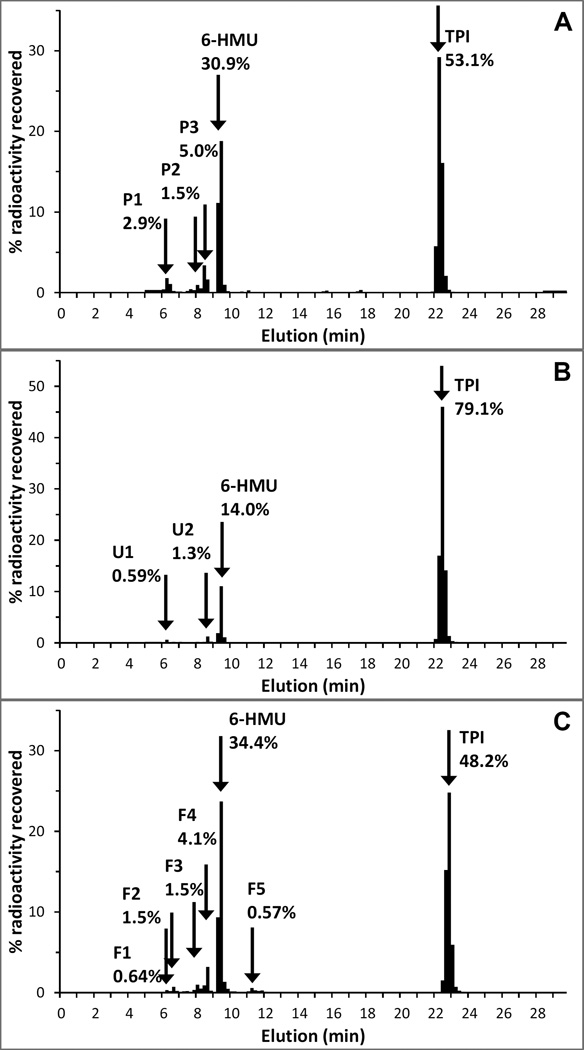
The radiochromatogram of the extractable plasma fraction (83.5% extraction efficiency) suggested that the extractable radioactivity consisted of 53.1% TPI and 30.9% 6-HMU, and no other peaks greater than 5%, see Figure 6A. The radiochromatogram of pooled urine showed 79.1% TPI, and 14.0% 6-HMU, see Figure 6B. The radiochromatogram of pooled feces (106% extraction efficiency) showed 48.2% TPI, 34.4% 6-HMU, and a number of components that were less than 5%, see Figure 6C. A full accounting of routes and chemical species is provided in Table 3.
Figure 6.
Radiochromatograms of (A) pooled plasma (0–96 h), (B) pooled urine (0–96 h), and (C) pooled feces (0–120 h) following a single oral administration of [14C]-TPI. Percentage radioactivity recovered represents the radioactivity in each fraction relative to the total radioactivity of the sample injected.
DISCUSSION
The present study aimed to define the PK, excretory pathways, and metabolism of [14C]-FTD and [14C]-TPI, respectively, as components of TAS-102. The FTD, FTY, and TPI plasma PK parameters observed in the present study were similar to those reported previously, taking into account that in our study, patients received a flat 60 mg TAS-102 dose as opposed to a BSA-scaled dose [16].
The vast majority of the [14C]-FTD plasma radioactivity Cmax (2.80 µg-eq/mL) can be attributed to FTD (1.52 µg/mL) and FTY (0.552 µg/mL = 0.908 µg-eq/mL as FTD). The plasma FTD radioactivity half-life was longer than even the duration of sampling, which prevents an accurate estimate of this value. However, this value is consistent with the moderate recovery of radioactivity in the excreta, and it suggests covalent binding of an FTD-related species to plasma proteins.
FTD-related radioactivity is predominantly eliminated in urine (54.8%), with minor contributions from feces (2.65%) and expired air (2.37%), for a total recovery of 59.8% of dose, with 47.1% of dose assigned to defined chemical species. The early and rapid urinary excretion is consistent with the short half-life of plasma FTD and FTY, small polar compounds likely excreted in urine. FTY, the expected primary metabolite of FTD catabolism, is the major component in urine at 45.9% of urinary radioactivity, or 25.2% of dose. The low fraction of urine radioactivity accounted for by FTD suggests that FTD, though well absorbed, is extensively metabolized to FTY and FTD glucuronides, despite concomitant dosing of TPI, an inhibitor of the primary metabolic route leading to FTY. Previous PK data suggested that repeated dosing with TAS-102 results in a 2.6-fold increased FTD exposure on day 12 relative to day 1, possibly because of a more extensive inhibition of tissue thymidine phosphorylase by TPI after several days of dosing. However, this is unlikely to result in a major change in the metabolic fate of [14C]-FTD in the context of chronic dosing, because the urinary excretion route of FTD would still be relatively minor after repeated administration [16]. Although natural pyrimidines have well-characterized biochemical anabolic, and catabolic pathways, none of which include glucuronidation, the glucuronidation of the sugar moiety of pyrimidine drugs has been reported previously in 1-(2-deoxy-β-D-ribofuranosyl)-2,4-difluoro-5-iodobenzene (5-IDFPdR), the capecitabine metabolite 5'-DFCR, stavudine, and zidovudine, which is 85% glucuronidated [17–22]. Perhaps the co-administration of TPI, which reduced the activity of the catabolic enzyme TP, allows glucuronidation to become a quantitatively more relevant metabolic pathway. We did not observe 5-carboxyuracil (5-CU), an inactive metabolite of FTD downstream of FTY, which has been reported as a metabolite in plasma and urine after intravenous dose of FTD [23], possibly due to limited resolution of co-eluting polar metabolites in earlier studies employing thin layer chromatography.
The limited amount of radioactivity excreted in feces suggests good absorption of FTD in the presence of TPI. Because the 2-position of the pyrimidine ring may be released as CO2 in the course of endogenous thymidine catabolism, we evaluated 14CO2 expiration [24, 25]. The amount of radioactivity excreted in expired air was low at 2.4% of dose. Our parallel Drechsler bottle setup would turn colour (i.e. titrate the amount of potassium hydroxide) in approximately 5 min, which equates to 176 mg CO2/min captured. An average resting rate of 8.86 mmol/kg/h has been reported [26], and this amount is equivalent to 649 mg CO2/min for a 100 kg person. This finding suggests that the efficiency of capturing 14CO2 was approximately 25%, suggesting that up to approximately 10% of the TAS-102 dose may have been converted to 14CO2. Even assuming such an increased estimate of the percentage of the FTD dose excreted as CO2, our results suggest that the FTD pyrimidine ring suffered limited metabolism, in large part due to the presence of TPI, which inhibits TP, the first step in the thymidine catabolic pathway. Interestingly, earlier preclinical studies report the absence of generation of 14CO2 after dosing [14C]-FTD to mice, dogs, and monkeys [27, 28].
Another contributing factor to the moderately low percentage of the FTD dose accounted for in excreta is the amount covalently bound in blood. The radioactivity plasma half-life estimated at 311 h is, therefore, partly dependent on albumin half-life, which is approximately 3 weeks, or 500 h [29]. Assuming a blood volume of 6 L, and an approximate blood concentration of 500 ng-FTD-equivalents/mL at 168 h (an analytically robust value at >20× LLQ of AMS), the blood compartment of a patient would still contain 3 mg, or 5% of the dose. In all likelihood, additional drug-related material is bound to the protein fraction of other organs. Rogers et al. also reported that both monkey and dog blood and tissues contained radioactive material that was un-extractable and shown to be associated mostly with the protein fraction. In vitro, up to 30–40% of FTY was shown to react to blood and plasma constituents over 24 h, suggesting that part or all of the binding reaction is non-enzymatic [27]. Therefore, the un-extractable radioactivity is not a human-specific observation. Covalent protein adduct formation of drug-related material is not uncommon, especially with the nonsteroidal anti-inflammatory drugs (NSAIDs) [30–37]. The non-extractable nature of drug-related radioactivity in plasma indicates that it is no longer available for free diffusion into tissues, which has been interpreted as a detoxification reaction, though this may not be completely innocuous because the haptenated proteins may be antigenic [38]. Skin or liver injury are known as the typical immune-mediated delayed toxicity caused by modified proteins, however, these adverse events have not been observed, to date, in phase 2 or phase 3 clinical trials of TAS-102 [9, 10].
Preclinically, IV dosing of [14C]-FTD without TPI resulted in rapid excretion of 85–94% in 0–24 h mouse urine, 70–90% in 0–24 h dog urine, and 60–80% of the dose in 0–24 h monkey urine, with most of the chemical species shown to have an intact pyrimidine ring [27]. Neither monkey nor dog expired air showed relevant radioactivity levels. In humans, Dexter et al. reported the clinical pharmacology of IV dosing of FTD. Approximately 94% of radioactivity was excreted in urine within 48 h by patients who received at least 6 mg/kg. Approximately 90% of the accumulated radioactivity recovered in the urine was mainly in the form of FTY, which shows that the FTD was rapidly degraded [23]. These data suggest that the administered FTD was degraded to the major metabolite, FTY, and that most of the radioactivity is excreted in urine, with limited metabolism of pyrimidine ring. The co-administration of TPI did not markedly change the excretory routes of [14C]-FTD, since the excretion profile of [14C]-FTD is generally similar to IV dosing of FTD in regard to the major elimination pathway.
The mean Cmax of [14C]-TPI related radioactivity (0.053 µg-eq/mL in plasma) was comparable to the TPI Cmax (0.047 µg/mL), suggesting that the TPI that gets absorbed from the GI tract does not undergo much metabolism upon first pass, while the plasma radioactivity at time points later than 24 h consists mostly of TPI metabolites. The somewhat sigmoidal shape of the plasma radioactivity profile may be explained by two of the patients exhibiting broad secondary peaks at 48 to 72 hours after dosing, suggesting enterohepatic recycling or enterobacterial metabolism of TPI, and two patients not having detectable radioactivity levels beyond 48 and 72 h.
TPI-related radioactivity is predominantly excreted in feces (49.7%), with a considerable contribution from urine (27.0%), for a total recovery of 76.8% of dose, with 66.3% of dose assigned to defined chemical species, with 20.9% of dose assigned to 6-HMU. Closer evaluation of the data suggests that these recoveries are likely an underestimate. In patient 6, the recovery in feces of 17.5% was approximately one-third of that in the other patients, resulting in an overall recovery of 36.3% (18.8% in urine). No significant deviations had taken place in the sample collections, but the patient did have rather poor faecal output throughout the sample collection period, ranging from 44 to 91 g/day, in contrast to other patients’ output between 110 to 300 g/day. The other 3 patients displayed overall recoveries of > 85%. The early urinary excretion limited to the 0–24 h interval coincides with the presence of TPI in plasma, which suggests that the urinary components are likely TPI and proximal metabolites. The amount of radioactivity excreted in urine suggests a lower bound to an estimate of bioavailability of TPI at 27%.
In summary, we have characterized for the first time in humans the elimination pathways and metabolic fate of TAS-102 (see Figure 7). Approximately 60% of the [14C]-FTD dose was recovered, largely in urine in the form of FTY, and FTD glucuronides. The major elimination pathway of FTD is metabolism, with the major metabolite of FTD in the extractable fraction of plasma and urine being FTY. Approximately 76% of the [14C]-TPI dose was recovered, mostly in feces as TPI and 6-HMU suggesting incomplete absorption of TPI. Most of the absorbed TPI was excreted in urine, and no other metabolites greater than 5% of total radioactivity were observed in plasma or urine. The current data with the ongoing hepatic and renal dysfunction studies will provide a better understanding of the TAS-102 dispositional profile.
Figure 7.
Proposed metabolism of FTD and TPI as components of TAS-102 in humans.
Acknowledgments
We thank the patients and their family members for their dedication and commitment to this clinical study. We thank the nursing staff of the University of Pittsburgh Clinical Translational Research Center for their invaluable assistance. This work was supported by Taiho Oncology, Inc., and NIH/NCRR/CTSA Grant UL1 RR024153. This project used the UPCI Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30CA047904. We would like to thank the following individuals and organizations for their input in the various stages of this project: Manuel Aivado, Brian Kiesel, Milind Narurkar, Robert Parise, Mark Seymour, Dennis Swanson, Tracy Topp, Erin Sternberg, and JCL Bioassay USA Inc. The authors were responsible for all content and editorial decisions and received no honoraria related to the development of this publication. All authors contributed to the research, writing, and reviewing of all drafts of this publication, and all authors approved the final draft prior to submission.
REFERENCES
- 1.Tanaka N, et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep. 2014;32(6):2319–2326. doi: 10.3892/or.2014.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temmink OH, et al. Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci. 2007;98(6):779–789. doi: 10.1111/j.1349-7006.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emura T, et al. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med. 2004;13(4):545–549. [PubMed] [Google Scholar]
- 4.Emura T, et al. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol. 2004;25(3):571–578. [PubMed] [Google Scholar]
- 5.Emura T, et al. Potentiation of the antitumor activity of alpha, alpha, alpha-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int J Oncol. 2005;27(2):449–455. [PubMed] [Google Scholar]
- 6.Overman MJ, et al. Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest. 2008;26(8):794–799. doi: 10.1080/07357900802087242. [DOI] [PubMed] [Google Scholar]
- 7.Overman MJ, et al. Phase 1 study of TAS-102 administered once daily on a 5-day-per-week schedule in patients with solid tumors. Invest New Drugs. 2008;26(5):445–454. doi: 10.1007/s10637-008-9142-3. [DOI] [PubMed] [Google Scholar]
- 8.Hong DS, et al. Phase I study to determine the safety and pharmacokinetics of oral administration of TAS-102 in patients with solid tumors. Cancer. 2006;107(6):1383–1390. doi: 10.1002/cncr.22125. [DOI] [PubMed] [Google Scholar]
- 9.Yoshino T, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 10.Mayer RJ, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 11.Kotani D, Fukuoka S, Yoshino T. [Efficacy of TAS-102] Gan To Kagaku Ryoho. 2015;42(1):1–5. [PubMed] [Google Scholar]
- 12.Beumer JH, et al. Human mass balance study of the novel anticancer agent ixabepilone using accelerator mass spectrometry. Invest New Drugs. 2007;25(4):327–334. doi: 10.1007/s10637-007-9041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham RA, et al. A single dose mass balance study of the Hedgehog pathway inhibitor vismodegib (GDC-0449) in humans using accelerator mass spectrometry. Drug Metab Dispos. 2011;39(8):1460–1467. doi: 10.1124/dmd.111.039339. [DOI] [PubMed] [Google Scholar]
- 14.Guidance for Industry-Bioanalytical Method Validation. U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation; and Research Center for Veterinary Medicine; 2001. U.S. Department of Health and Human Services Food and Drug Administration. [Google Scholar]
- 15.Hamilton RA, Garnett WR, Kline BJ. Determination of mean valproic acid serum level by assay of a single pooled sample. Clin Pharmacol Ther. 1981;29(3):408–413. doi: 10.1038/clpt.1981.56. [DOI] [PubMed] [Google Scholar]
- 16.Doi T, et al. Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer. 2012;107(3):429–434. doi: 10.1038/bjc.2012.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalili P, et al. Pharmacokinetics and metabolism of the novel synthetic C-nucleoside, 1-(2-deoxy-beta-D-ribofuranosyl)-2,4-difluoro-5-iodobenzene: a potential mimic of 5-iodo-2'-deoxyuridine. Biopharm Drug Dispos. 2002;23(3):105–113. doi: 10.1002/bdd.301. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, et al. Disposition of [1'-(14)C]stavudine after oral administration to humans. Drug Metab Dispos. 2010;38(4):655–666. doi: 10.1124/dmd.109.030239. [DOI] [PubMed] [Google Scholar]
- 19.Desmoulin F, et al. A glucuronidation pathway of capecitabine occurs in rats but not in mice and humans. Drug Metab Lett. 2007;1(2):101–107. doi: 10.2174/187231207780363615. [DOI] [PubMed] [Google Scholar]
- 20.Desmoulin F, et al. Metabolism of capecitabine, an oral fluorouracil prodrug: (19)F NMR studies in animal models and human urine. Drug Metab Dispos. 2002;30(11):1221–1229. doi: 10.1124/dmd.30.11.1221. [DOI] [PubMed] [Google Scholar]
- 21.Nicolas F, et al. Comparative metabolism of 3'-azido-3'-deoxythymidine in cultured hepatocytes from rats, dogs, monkeys, and humans. Drug Metab Dispos. 1995;23(3):308–313. [PubMed] [Google Scholar]
- 22.Good SS, et al. Isolation and characterization of an ether glucuronide of zidovudine, a major metabolite in monkeys and humans. Drug Metab Dispos. 1990;18(3):321–326. [PubMed] [Google Scholar]
- 23.Dexter DL, et al. The clinical pharmacology of 5-trifluoromethyl-2'-deoxyuridine. Cancer Res. 1972;32(2):247–253. [PubMed] [Google Scholar]
- 24.Shields AF, et al. Analysis of 2-carbon-11-thymidine blood metabolites in PET imaging. J Nucl Med. 1996;37(2):290–296. [PubMed] [Google Scholar]
- 25.Beumer JH, Beijnen JH, Schellens JH. Mass balance studies, with a focus on anticancer drugs. Clin Pharmacokinet. 2006;45(1):33–58. doi: 10.2165/00003088-200645010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Ghoos Y, et al. Measurement of 13C-glucose oxidation rate using mass spectrometric determination of the CO2: Ar ratio and spirometry. Biomed Environ Mass Spectrom. 1988;15(8):447–451. doi: 10.1002/bms.1200150806. [DOI] [PubMed] [Google Scholar]
- 27.Rogers WI, et al. The fate of 5-trifluoromethyl-2'-deoxyuridine in monkeys, dogs, mice, and tumor-bearing mice. Cancer Res. 1969;29(4):953–961. [PubMed] [Google Scholar]
- 28.Heidelberger C, Boohar J, Kampschroer B. Fluorinated Pyrimidines. Xxiv. In Vivo Metabolism of 5-Trifluoromethyluracil-2-C-14 and 5-Trifluoromethyl-2'-Deoxyuridine-2-C-14. Cancer Res. 1965;25:377–381. [PubMed] [Google Scholar]
- 29.Andersen JT, et al. Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J Biol Chem. 2014;289(19):13492–13502. doi: 10.1074/jbc.M114.549832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond TG, et al. Mass spectrometric characterization of circulating covalent protein adducts derived from a drug acyl glucuronide metabolite: multiple albumin adductions in diclofenac patients. J Pharmacol Exp Ther. 2014;350(2):387–402. doi: 10.1124/jpet.114.215079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng X, et al. Detection of drug bioactivation in vivo: mechanism of nevirapine-albumin conjugate formation in patients. Chem Res Toxicol. 2013;26(4):575–583. doi: 10.1021/tx4000107. [DOI] [PubMed] [Google Scholar]
- 32.Ariza A, et al. Protein haptenation by amoxicillin: high resolution mass spectrometry analysis and identification of target proteins in serum. J Proteomics. 2012;77:504–520. doi: 10.1016/j.jprot.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, et al. Characterization of HKI-272 covalent binding to human serum albumin. Drug Metab Dispos. 2010;38(7):1083–1093. doi: 10.1124/dmd.110.032292. [DOI] [PubMed] [Google Scholar]
- 34.Shipkova M, et al. Pharmacokinetics and protein adduct formation of the pharmacologically active acyl glucuronide metabolite of mycophenolic acid in pediatric renal transplant recipients. Ther Drug Monit. 2002;24(3):390–399. doi: 10.1097/00007691-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Dickinson RG. Bile duct ligation promotes covalent drug-protein adduct formation in plasma but not in liver of rats given zomepirac. Life Sci. 2000;68(5):525–537. doi: 10.1016/s0024-3205(00)00958-9. [DOI] [PubMed] [Google Scholar]
- 36.Georges H, et al. Glycation of human serum albumin by acylglucuronides of nonsteroidal anti-inflammatory drugs of the series of phenylpropionates. Life Sci. 1999;65(12):PL151–PL156. doi: 10.1016/s0024-3205(99)00371-9. [DOI] [PubMed] [Google Scholar]
- 37.Benet LZ, et al. Predictability of the covalent binding of acidic drugs in man. Life Sci. 1993;53(8):PL141–PL146. doi: 10.1016/0024-3205(93)90279-c. [DOI] [PubMed] [Google Scholar]
- 38.Casarett and Doull's Toxicology: The Basic Science of Poisons. 7th. New York: McGraw-Hill; 2008. p. 1309. [Google Scholar]