Abstract
Objective
Unlike currently approved adenosine diphosphate (ADP) receptor antagonists, the new diadenosine tetraphosphate derivative GLS-409 targets not only P2Y12 but also the second human platelet ADP receptor P2Y1, and may therefore be a promising antiplatelet drug candidate. The current study is the first to investigate the in vivo antithrombotic effects of GLS-409.
Approach and Results
We studied (1) the in vivo effects of GLS-409 on agonist-stimulated platelet aggregation in anesthetized rats, (2) the antithrombotic activity of GLS-409 and the associated effect on the bleeding time in a canine model of platelet-mediated coronary artery thrombosis, and (3) the inhibition of agonist-stimulated platelet aggregation by GLS-409 versus selective P2Y1 and P2Y12 inhibition in vitro in samples from healthy human subjects before and 2 hours after aspirin intake. In vivo treatment with GLS-409 significantly inhibited ADP- and collagen-stimulated platelet aggregation in rats. Further, GLS-409 attenuated cyclic flow variation, i.e., platelet-mediated thrombosis, in vivo in our canine model of unstable angina. The improvement in coronary patency was accompanied by a non-significant 30% increase in bleeding time. Of note, GLS-409 exerted its effects without affecting rat and canine hemodynamics. Finally, in vitro treatment with GLS-409 showed effects similar to that of cangrelor and the combination of cangrelor with the selective P2Y1 inhibitor MRS 2179 on agonist-stimulated platelet aggregation in human platelet-rich plasma and whole blood before and 2 hours after aspirin intake.
Conclusions
Synergistic inhibition of both P2Y1 and P2Y12 ADP receptors by GLS-409 immediately attenuates platelet-mediated thrombosis and effectively blocks agonist-stimulated platelet aggregation irrespective of concomitant aspirin therapy.
Keywords: Antiplatelet therapy, adenosine diphosphate, P2Y12, P2Y1, aspirin
Introduction
Dual antiplatelet therapy with aspirin and an adenosine diphosphate (ADP) receptor inhibitor is a mainstay of pharmacological therapy in acute coronary syndromes (ACS).1–3 In aspirin-treated patients with non-ST-segment elevation ACS, the co-administration of the P2Y12 ADP receptor antagonist clopidogrel reduced the composite of cardiovascular death, myocardial infarction and stroke by 20% compared to placebo.4 The newer ADP receptor inhibitors prasugrel and ticagrelor yielded an even greater reduction of ischemic outcomes in ACS patients than clopidogrel at the expense of a significantly increased bleeding risk.5, 6, 7 The latter is particularly problematic in patients who cannot be treated by percutaneous coronary intervention (PCI) but must immediately undergo coronary artery bypass graft (CABG) surgery. Despite these recent advances, ischemic events like acute stent thrombosis still impair the prognosis of many ACS patients. Consequently, in order to minimize the risk of thrombotic and bleeding complications in the initial phase of ACS, there is still a need for rapidly acting and reversible but highly potent antiplatelet agents.
Currently approved ADP receptor inhibitors target only the P2Y12 receptor, but not the other platelet ADP receptor, P2Y1.8 P2Y1 activation initiates ADP-induced platelet aggregation, and is responsible for platelet shape change,9 while P2Y12 activation results in amplification and stabilization of the aggregation response. There is a complex interplay between P2Y1 and P2Y12,10 and co-activation of both is necessary for full platelet aggregation.11 P2Y1-selective antagonists have been identified,12, 13 but the lack of clinical candidates contrasts with the essential role of P2Y1 in platelet aggregation.14
Adenosine tetraphosphate (Ap4A) is a naturally occurring compound in mammalian tissues.15 In human platelets, Ap4A is stored in dense granules, and therefore released along with ADP and ATP upon platelet activation.16, 17 Ap4A and its analogs have a very short plasma half-life and are known to inhibit ADP-induced platelet activation.18, 19 Specifically, they inhibit the ADP-induced platelet release reaction, calcium mobilization, thromboxane production and platelet factor 3 activities.18 However, Ap4A also acts as a P2X1 receptor agonist, an undesirable effect for potential antithrombotic drugs.20 We recently reported that Ap4A and its derivatives synergistically inhibit both human platelet P2Y1 and P2Y12 receptors, and efficiently antagonize ADP-induced human platelet aggregation.20, 21 In a subsequent study, a series of new Ap4A analogs, with modifications in the tetraphosphate chain, and/or 2-, or N6-positions in the base were synthesized and evaluated as platelet aggregation inhibitors, and with respect to their effects on platelet P2Y1, P2Y12, and P2X1 receptors.22 The established structure-activity relations were used to design Ap4A analogs which potently inhibit human platelet aggregation by simultaneously targeting platelet P2Y1 and P2Y12 ADP receptors but, unlike Ap4A, do not activate the platelet P2X1 receptor. The new Ap4A analogs can be administered intravenously and act reversibly, and they show significantly higher plasma stability than Ap4A.22 Because of their unique and reversible mechanism of action, their route of administration, the fast onset of their antiplatelet effect, and their short plasma half-life, these new compounds may be beneficial in clinical situations where a high atherothrombotic risk and an increased bleeding risk coincide. Accordingly, they are envisioned as a future treatment option in the initial phase of ACS. However, data on the antithrombotic effect of the new Ap4A analogs in vivo as well as on agonist-induced platelet aggregation without and with concomitant aspirin treatment are missing, so far. Based on its pharmacokinetic and pharmacodynamic profile, we therefore selected compound GLS-409 from the newly discovered Ap4A derivatives for further studies on antiplatelet efficacy. Specifically, we investigated (1) the effect of GLS-409, administered in vivo to anesthetized rats, on ex vivo agonist-stimulated platelet aggregation, (2) the antithrombotic activity of GLS-409 and the associated effect on the bleeding time in a canine model of recurrent platelet-mediated coronary artery thrombosis mimicking unstable angina,23–26 and (3) the inhibition of agonist-stimulated platelet aggregation by GLS-409 versus selective P2Y1 and P2Y12 inhibition in vitro in samples from healthy human subjects before and 2 hours after aspirin intake.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Protocol 1. Rat model of in vivo treatment – ex vivo platelet aggregation
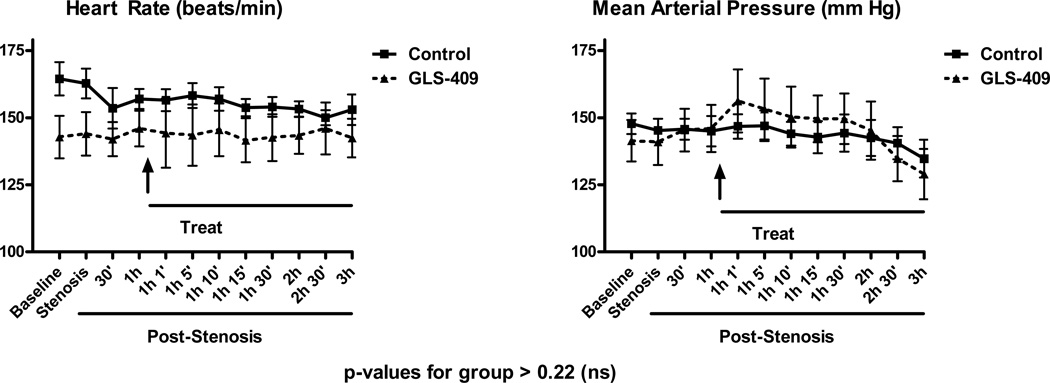
There were no significant differences in heart rate or arterial pressures in GLS-409-treated groups vs. saline controls (see Supplemental Figure I).
In vivo administration of GLS-409 inhibited ex vivo ADP-stimulated platelet aggregation in a dose-dependent manner, with ~complete inhibition, assessed at 10 minutes after addition of the agonist, achieved with 0.054 mg/kg bolus + 0.0018 mg/kg/min infusion and doses 10x, 100x and 300x higher than this ‘threshold’ dose (Table 1). In contrast, the effect of GLS-409 on collagen-induced platelet aggregation was modest and manifest only at 1/4, 1 and 10x the ‘threshold’ dose: i.e., the agent had no effect on collagen-stimulated aggregation at either low or high doses (Table 1).
Table 1.
Rat model: ex vivo platelet aggregation following in vivo treatment with GLS-409
| GLS-409: Dose | Platelet Aggregation (ohms): 3 µM ADP |
Platelet Aggregation (ohms): 2 µg/ml collagen |
||||
|---|---|---|---|---|---|---|
| Bolus (mg/kg) | Infusion (mg/kg/min) |
Relative to ‘Threshold’ Dose |
Maximum | End | Maximum | End |
| Control (0) | Control (0) | 0 | 20.0 ± 0.9 | 17,8 ± 1.2 | 17.2 ± 1.0 | 15.1 ± 0.9 |
| 0.000135 | 0.0000045 | 1/400 | 19.1 ± 1.7 | 16.9 ± 2.4 | 17.6 ± 0.7 | 16.0 ± 0.8 |
| 0.000675 | 0,0000225 | 1/80 | 18.6 ± 1.7 | 12.2 ± 2.2 † | 17.3 ± 0.5 | 16.8 ± 0.6 |
| 0.0027 | 0.00009 | 1/20 | 17.7 ± 1.0 | 10.6 ± 1.1 ‡ | 16.8 ± 0.8 | 14.1 ± 1.9 |
| 0.0135 | 0.00045 | ¼ | 15.4 ± 0.7 * | 6.8 ± 0.5 ‡ | 13.9 ± 0.7 * | 12.9 ± 1.1 |
| 0.054 | 0.0018 | 1 | 8.9 ± 2.0 ‡ | 2.6 ± 1.0 ‡ | 14.0 ± 0.7 * | 11.4 ± 1.3 * |
| 0.54 | 0.018 | 10 | 7.3 ± 1.4 ‡ | 1.0 ± 0.9 ‡ | 13.7 ± 0.5 * | 11.4 ± 0.5 * |
| 5.4 | 0.18 | 100 | 8.2 ± 1.2 ‡ | 3.0 ± 0.5 ‡ | 15.6 ± 0.8 | 12.7 ± 0.4 |
| 16.2 | 0.54 | 300 | 3.7 ± 0.5 ‡ | 1.3 ± 0.6 ‡ | 16.9 ± 0.8 | 15.3 ± 1.2 |
p<0.05,
p<0.01 and
p<0.001 versus corresponding value in the control group
Protocol 2. Canine Model of Recurrent Coronary Thrombosis
As in the rat model, there were no differences in heart rate or arterial pressures in the GLS-409-treated group when compared with saline controls at any time during the 3-hour observation period (all p-values for group >0.22; Figure 2).
Figure 2.
Heart rate and mean arterial pressure (mean ± SEM) in dogs randomized to receive GLS-409 and placebo (Control).
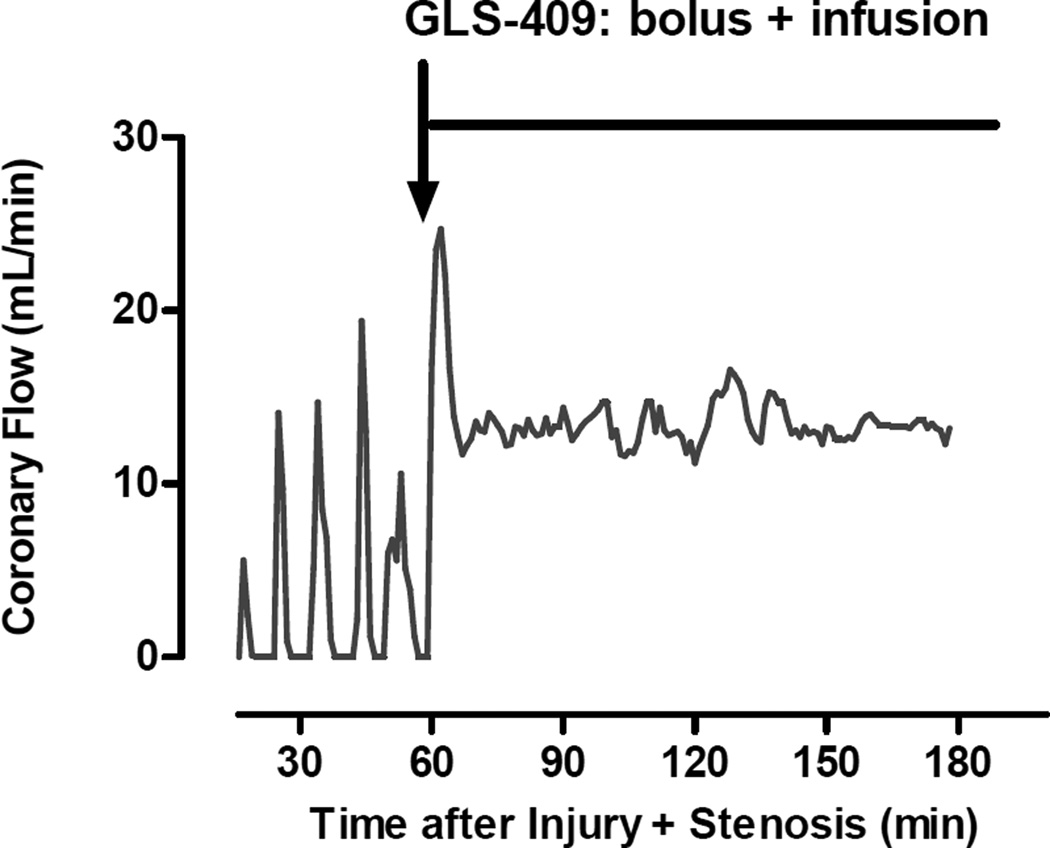
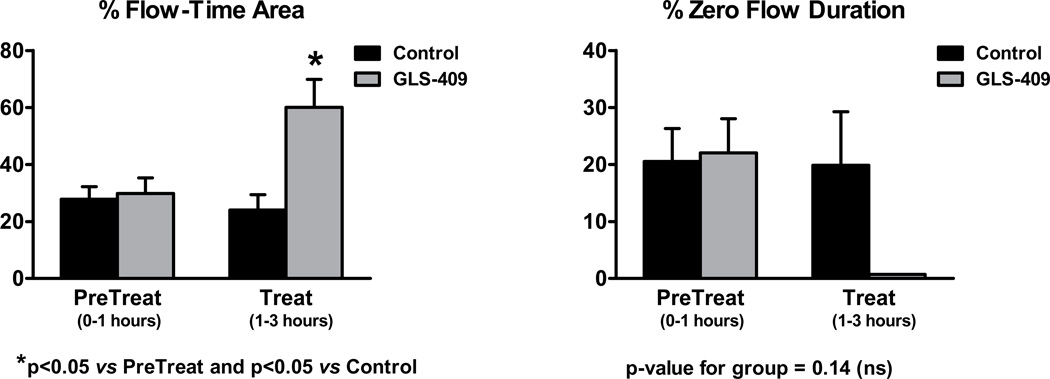
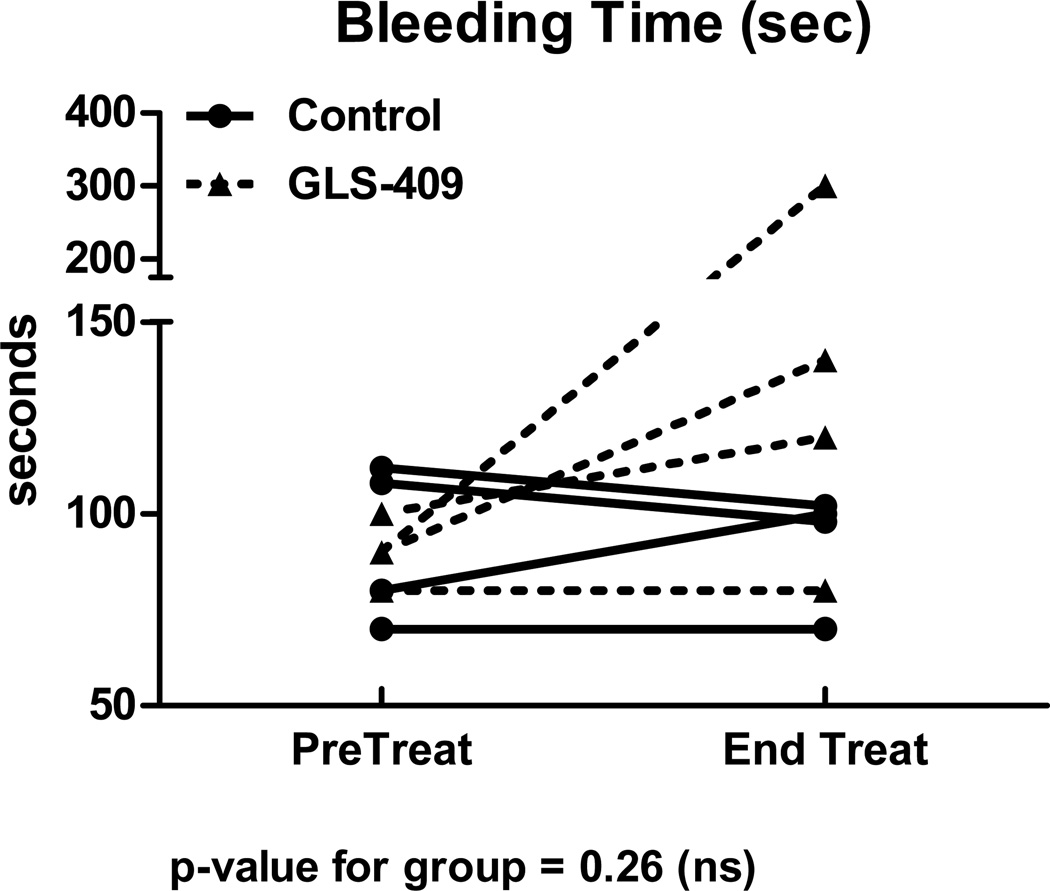
As expected, all dogs developed cyclic variations in coronary blood flow (i.e., spontaneous episodes of recurrent thrombosis) following coronary injury + stenosis (Figure 1). During the 1 hour pretreatment period, coronary patency was comparable in dogs later assigned to receive GLS-409 vs. saline: flow-time area was 30±5% vs. 28±4% and zero flow duration was 22±6% vs. 21±5%, respectively (Figure 3). In addition, template bleeding times assessed before the onset of treatment were similar in both groups (mean of 90-93 seconds; Figure 4).
Figure 1.
Original recording of coronary blood flow, measured following coronary artery injury + stenosis, for one representative dog treated with GLS-409. Before randomization and treatment, the animal displayed cyclic variations in coronary flow (CFVs) caused by the repeated, spontaneous accumulation/dislodgment of platelet-rich thrombi at the site of injury + stenosis. Administration of GLS-409 (bolus + infusion denoted by the red arrow + line) was associated with an attenuation in the development of CFVs and better maintenance of coronary patency.
Figure 3.
Percent flow-time area (left panel) and % zero flow duration (mean ± SEM), quantified before and after treatment, in control and GLS-409-treated groups of dogs.
Figure 4.
Template bleeding time (seconds) measured immediately before treatment and at the end of the 2-hour treatment period for 4 control and 4 GLS-409-treated dogs.
The saline-control group displayed no change in coronary patency or template bleeding time during the 2 hour treatment period when compared with the pretreatment phase (Figures 3 and 4). In contrast, administration of GLS-409 evoked a significant increase in flow time-area (to 60±10%; p<0.05 vs. pretreatment and p<0.05 vs. controls) together with a decrease in zero flow duration (to <1%; p-value for group = 0.14; Figures 1 and 3). The effect of GLS-409 on template bleeding time was variable, averaging 160±48 seconds at 2 hours post-treatment (p-value for group = 0.26; Figure 4).
Protocol 3. Effect of GLS-409 versus selective P2Y1 and P2Y12 inhibition on agonist-stimulated human platelet aggregation in vitro before and 2 hours after aspirin intake
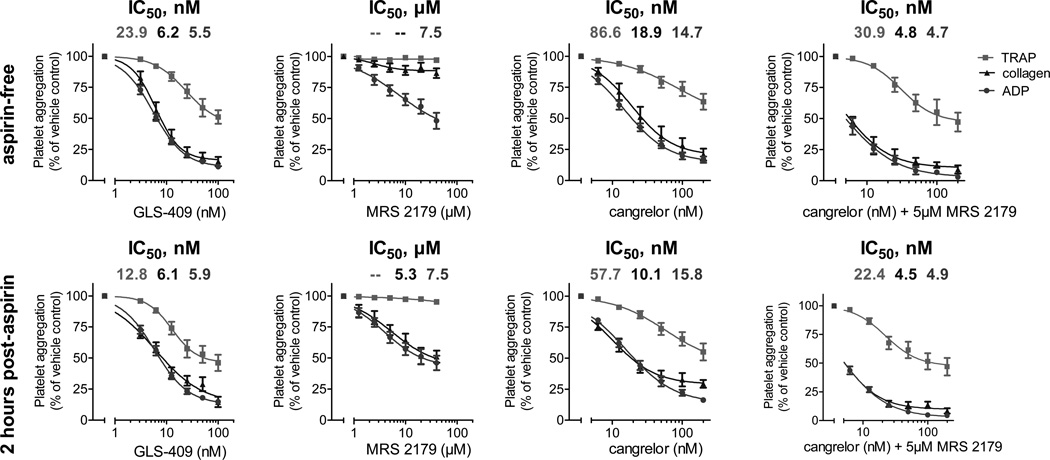
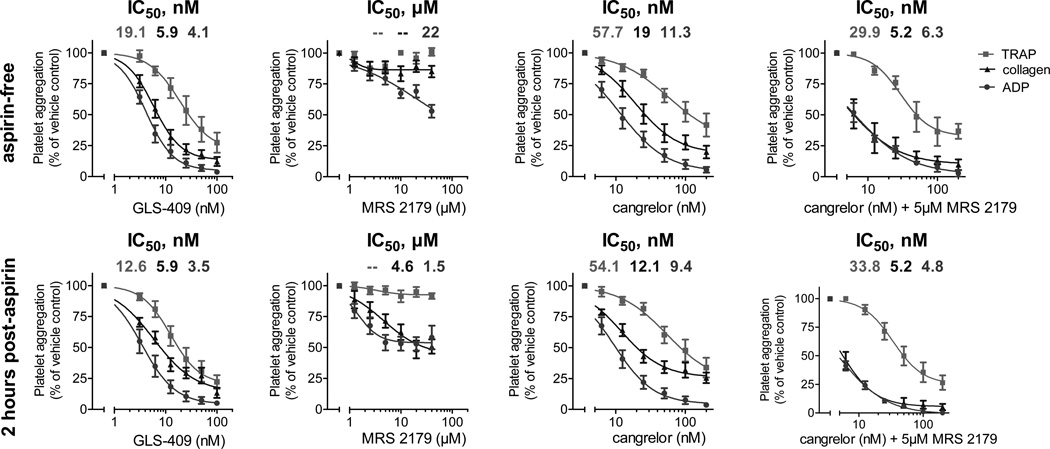
In the third component of the study, we compared the effects of GLS-409 to those of the selective P2Y1 inhibitor MRS 2179 and to those of the selective P2Y12 inhibitor cangrelor on ADP-, collagen-, and thrombin receptor-activating peptide (TRAP)-stimulated platelet aggregation in platelet-rich plasma (PRP) from 6 healthy donors before and 2 hours after the intake of 325 mg of uncoated aspirin. Moreover, we compared the effects of GLS-409 to those of the combination of cangrelor with 5 µM of MRS 2179 on ADP-, collagen-, and TRAP-stimulated platelet aggregation in PRP from 3 healthy donors pre- and post-aspirin. The inhibition of both platelet ADP receptors by GLS-409 had similar effects to those of cangrelor and the combination of cangrelor with 5 µM of MRS 2179 on ADP-, collagen- and TRAP-stimulated maximal and final platelet aggregation in PRP without and with aspirin treatment (Figures 5 and 6). GLS-409, cangrelor and the combination of cangrelor with 5 µM of MRS 2179 inhibited maximal and final platelet aggregation in response to all agonists pre- and post-aspirin more strongly than the selective P2Y1 antagonist MRS2179 (Figures 5 and 6). The IC50s for ADP-, collagen-, and TRAP-stimulated platelet aggregation were lower with GLS-409 compared to cangrelor and MRS 2179, but similar to the combination of cangrelor with 5 µM of MRS 2179 prior to and 2 hours after the intake of aspirin (Tables 2 and 3). Inhibition of ADP- and collagen-stimulated platelet aggregation by GLS-409 in PRP was not significantly altered by aspirin treatment, whereas the IC50 for TRAP-stimulated aggregation with GLS-409 decreased after the administration of aspirin to healthy donors (Tables 2 and 3). Selective inhibition of P2Y1 by MRS 2179 at the tested concentrations showed no inhibition of collagen- and TRAP-stimulated aggregation in PRP from aspirin-free healthy donors. In contrast, 2 hours after the administration of aspirin, ADP- and collagen-stimulated platelet aggregation was inhibited to a similar extent in PRP from healthy donors concomitantly treated with MRS 2179 (Figures 5 and 6).
Figure 5.
Percent of maximal human platelet aggregation (mean ± SEM) in response to adenosine diphosphate (ADP), collagen or thrombin receptor-activating peptide (TRAP) as assessed by 96-well optical aggregometry prior to and two hours after the ingestion of 325 mg aspirin in platelet-rich plasma preincubated with GLS-409, MRS 2179, cangrelor or the combination of cangrelor with 5 µM MRS 2179.
Figure 6.
Percent of final human platelet aggregation (mean ± SEM) in response to adenosine diphosphate (ADP), collagen or thrombin receptor-activating peptide (TRAP) as assessed by 96-well optical aggregometry prior to and two hours after the ingestion of 325 mg aspirin in platelet-rich plasma preincubated with GLS-409, MRS 2179, cangrelor or the combination of cangrelor with 5 µM MRS 2179.
Table 2.
IC50s and 95% confidence intervals for adenosine diphosphate (ADP)-, collagen-, and thrombin receptor-activating peptide (TRAP)-stimulated maximal platelet aggregation with GLS-409, MRS 2179, cangrelor and the combination of cangrelor with 5µM MRS 2179 in platelet-rich plasma from healthy donors prior to and 2 hours after the intake of 325 mg aspirin.
| no aspirin | 2 hours post aspirin | |||||
|---|---|---|---|---|---|---|
| Agent | ADP | Collagen | TRAP | ADP | collagen | TRAP |
| GLS-409 (nM) | 5.5 4.8 – 6.2 |
6.2 5 – 7.6 |
23.9 11.1 – 51.4 |
5.9 5 – 6.9 |
6.1 3.8 – 9.7 |
12.8 8.3 – 19.7 |
| MRS 2179 (µM) | 7.5 1.4 – 39.6 |
indefinable indefinable |
indefinable indefinable |
3.8 1.9 – 7.7 |
5.3 1.7 – 16.3 |
indefinable indefinable |
| cangrelor (nM) | 14.7 12.2 – 17.8 |
18.9 12.7 – 28 |
86.6 7.9 – 943.9 |
15.8 12.8 – 19.5 |
10.1 7.6 – 13.4 |
57.7 16.5 – 201.9 |
| cangrelor + MRS 2179 (nM) | 4.7 3.6 – 6.2 |
4.8 3.4 – 6.6 |
30.9 18.2 – 52.5 |
4.9 4.3 – 5.6 |
4.5 3.7 – 5.5 |
22.4 14 – 35.7 |
Table 3.
IC50s for adenosine diphosphate (ADP)-, collagen-, and thrombin receptor-activating peptide (TRAP)-stimulated final platelet aggregation with GLS-409, MRS 2179, cangrelor and the combination of cangrelor with 5µM MRS 2179 in platelet-rich plasma from healthy donors prior to and 2 hours after the intake of 325 mg aspirin.
| no aspirin | 2 hours post aspirin | |||||
|---|---|---|---|---|---|---|
| Agent | ADP | collagen | TRAP | ADP | collagen | TRAP |
| GLS-409 (nM) | 4.1 3.4 – 5 |
5.9 4.6 – 7.4 |
19.1 8.7 – 42.2 |
3.5 2.5 – 4.7 |
5.9 3.9 – 8.9 |
12.6 7.8 – 20.6 |
| MRS 2179 (µM) | 22 indefinable |
indefinable indefinable |
indefinable indefinable |
1.5 0.9 – 2.3 |
4.6 1.6 – 12.9 |
indefinable indefinable |
| cangrelor (nM) | 11.3 8.3 – 15.4 |
19 12.6 – 28.6 |
57.7 16.7 – 200 |
9.4 7.6 – 11.7 |
12.1 9.1 – 16.1 |
54.1 20.1 – 145.6 |
| cangrelor + MRS 2179 (nM) | 6.3 5 – 7.9 |
5.2 3 – 9.2 |
29.9 18.9 – 47.2 |
4.8 4.2 – 5.6 |
5.2 4.2 – 6.6 |
33.8 23.2 – 49.4 |
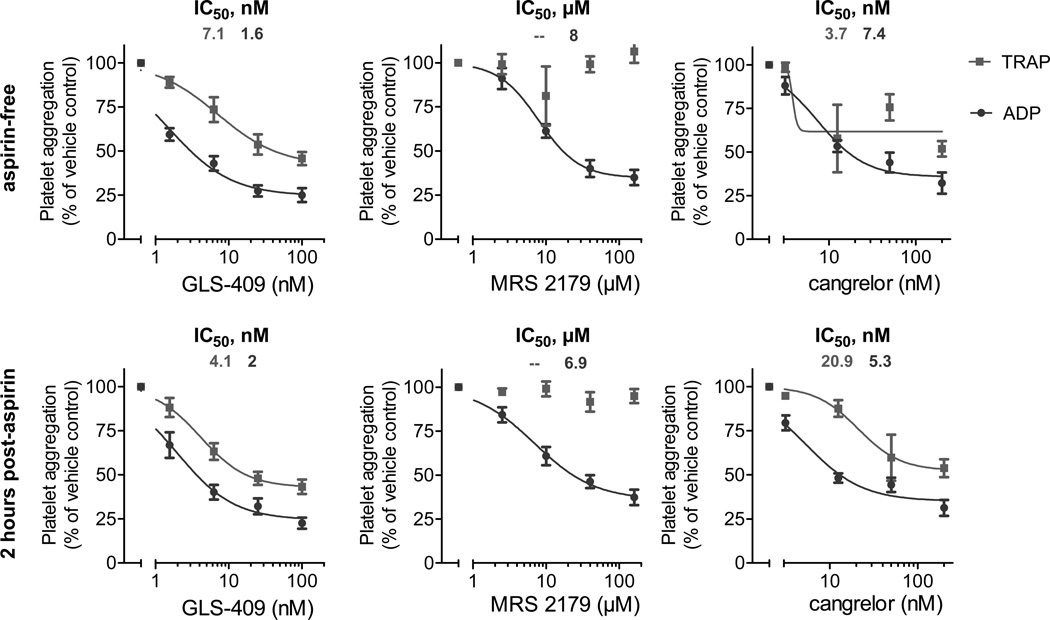
Finally, we compared the effects of GLS-409 to those of the selective P2Y1 inhibitor MRS 2179 and to those of the selective P2Y12 inhibitor cangrelor on ADP- and TRAP-stimulated platelet aggregation in whole blood from 6 healthy donors pre- and post-aspirin intake. GLS-409 showed effects similar to those of cangrelor on ADP- and TRAP-stimulated platelet aggregation, and both had more pronounced effects than MRS 2179 on agonist-stimulated platelet aggregation (Figure 7). Inhibition of ADP-stimulated aggregation by GLS-409 in whole blood was not significantly altered by aspirin treatment, whereas the IC50 for TRAP-stimulated aggregation with GLS-409 decreased after the administration of aspirin to healthy donors (Table 4). Similar to the results in PRP, selective inhibition of P2Y1 by MRS 2179 at the tested concentrations showed no inhibition of TRAP-stimulated platelet aggregation pre- and post-aspirin treatment (Figure 7).
Figure 7.
Percent of maximal human platelet aggregation (mean ± SEM) in response to adenosine diphosphate (ADP) or thrombin receptor-activating peptide (TRAP) as assessed by impedance aggregometry prior to and two hours after the ingestion of 325 mg aspirin in whole blood preincubated with GLS-409, MRS 2179, or cangrelor. No curve could be fitted to the inhibition of TRAP-stimulated platelet aggregation by MRS 2179.
Table 4.
IC50s for adenosine diphosphate (ADP)- and thrombin receptor-activating peptide (TRAP)-stimulated maximal platelet aggregation with GLS-409, MRS 2179 and cangrelor in whole blood from healthy donors prior to and 2 hours after the intake of 325 mg aspirin.
| no aspirin | 2 hours post aspirin | |||
|---|---|---|---|---|
| Agent | ADP | TRAP | ADP | TRAP |
| GLS-409 (nM) | 1.6 1.1 – 2.3 |
7.1 2.9 – 17.3 |
2 1.3 – 3.1 |
4.1 2.6 – 6.5 |
| MRS 2179 (µM) | 8 5.5 – 11.5 |
indefinable indefinable |
6.9 3.9 – 12.2 |
Indefinable indefinable |
| cangrelor (nM) | 7.4 4.8 – 11.3 |
3.7 indefinable |
5.3 3.6 – 7.7 |
20.9 8.5 – 51.4 |
Discussion
Our study is the first to investigate the effects of synergistic P2Y1 and P2Y12 receptor inhibition by a single compound (GLS-409) on agonist-stimulated platelet aggregation in rats and on recurrent coronary artery thrombosis in a canine model of unstable angina. In addition, we studied the antiplatelet effect of GLS-409 versus selective P2Y1 and P2Y12 receptor antagonists on agonist-stimulated platelet aggregation in PRP and whole blood from healthy human donors in vitro pre- and 2 hours post-aspirin intake. In vivo treatment with GLS-409 significantly inhibited ADP- and collagen-stimulated platelet aggregation in rats. Furthermore, GLS-409, administered intravenously after coronary stenosis and injury, evoked an immediate and significant attenuation of platelet-mediated thrombosis in vivo in our canine model of unstable angina. The improvement in coronary patency was accompanied by a non-significant 30% increase in the median bleeding time, an observation that must be interpreted with caution given the small n-value of 4 per group and variability in the data. GLS-409 showed a rapid biphasic plasma clearance with a first phase half-life of 9.7 minutes and a second phase half-life of 58 minutes. Of note, GLS-409 exerted its effects in vivo without affecting rat or canine hemodynamics, respectively. Finally, in vitro treatment with GLS-409 showed effects similar to that of cangrelor and the combination of cangrelor with MRS 2179 on agonist-stimulated platelet aggregation in human PRP and whole blood. The inhibition of ADP- and collagen-stimulated platelet aggregation by GLS-409 in PRP, and the inhibition of ADP-stimulated platelet aggregation in whole blood were not altered by aspirin treatment, whereas the IC50s for TRAP-stimulated platelet aggregation in PRP and whole blood with GLS-409 decreased after the administration of aspirin to healthy donors.
P2Y1 is widely expressed in different tissues, where it is involved in the control of various functions.27 Therefore, it is of utmost importance for antiplatelet drug candidates inhibiting P2Y1 to avoid adverse effects associated with non-platelet P2Y1 targeting. Since GLS-409 inhibited agonist-stimulated human platelet aggregation at levels at which P2Y1 was not significantly inhibited in our study, such side effects seem unlikely with this agent. Specifically, the IC50 for ADP-stimulated platelet aggregation with the P2Y1 inhibitor MRS 2179 was significantly higher compared to GLS-409. Moreover, selective blockage of P2Y1 by MRS 2179 did not affect platelet activation by TRAP and, in line with a previous publication by Mangin et al.,28 inhibited collagen-stimulated platelet aggregation only in samples from aspirin-treated healthy donors. These findings suggest that P2Y1 inhibition alone may not effectively prevent agonist-stimulated platelet aggregation at acceptable plasma concentrations.
Zhang et al. recently investigated the crystal structures of human P2Y1 in complex with a nucleotide antagonist MRS 2500 and with a non-nucleotide antagonist BPTU, thereby revealing two disparate ligand-binding sites of the receptor.29 Although MRS 2500 and BPTU bind to distinct sites in P2Y1, both ligands stabilize the receptor in similar inactive conformations. Nevertheless, future studies are needed to determine if nucleotide and non-nucleotide antagonists exert similar antiplatelet effects at P2Y1. Because GLS-409 acts as a nucleotide antagonist at P2Y1, we chose another nucleotide antagonist (MRS 2179) as a comparator for our experiments in aspirin-free and aspirin-treated samples from healthy volunteers.
Activation of P2Y12 by ADP inhibits adenylyl cyclase through activation of the Gαi2 G-protein subtype.30 Thus, P2Y12 activation counteracts the antiplatelet effects of prostacyclin, which inhibits platelet function by increasing the levels of cyclic adenosine monophosphate through activation of adenylyl cyclase. Consequently, P2Y12 receptor inhibitors exert their antithrombotic effects in part by fostering the antiplatelet potency of prostacyclin.31 By inhibiting prostacyclin synthesis of endothelial cells, concomitant aspirin therapy may attenuate the antiplatelet effects of P2Y12 inhibition. Indeed, it has been speculated that the combination of ticagrelor with high-dose aspirin at many study sites in North America in the PLATO trial may have been responsible for the less pronounced effects of ticagrelor on the reduction of adverse ischemic events compared to study sites in the rest of the world.32 Since ADP receptor antagonists are typically used in conjunction with aspirin in ACS,3 it is important to show that concomitant aspirin therapy does not reduce the antiplatelet potency of new drugs targeting P2Y12. We therefore assessed the inhibition of agonist-stimulated human platelet aggregation by GLS-409 in samples drawn before and 2 hours after the intake of 325 mg of uncoated aspirin. In our study, inhibition of ADP-stimulated platelet aggregation by GLS-409 was similar in PRP and whole blood, and inhibition of collagen-stimulated platelet aggregation by GLS-409 was similar in PRP before and 2 hours after aspirin intake. Furthermore, the IC50s for TRAP-stimulated platelet aggregation in PRP and whole blood with GLS-409 decreased after pretreatment with aspirin. These findings suggest that the strong antiplatelet effects of GLS-409 are retained in aspirin-treated subjects.
Synergistic inhibition of P2Y1 and P2Y12 by GLS-409 is envisioned as an antiplatelet strategy for the initial phase of ACS. When patients suffer an ACS, they are in a prothrombotic state initiated by the rupture of an atherosclerotic plaque with subsequent coronary artery stenosis and occlusion. In this situation, rapid platelet inhibition is critical to prevent further prothrombotic actions. Moreover, most patients with ACS currently undergo PCI with implantation of a drug-eluting stent. This procedure is associated with a further increased risk of ischemic events, e.g., acute stent thrombosis, and therefore requires a fast, strong and consistent antiplatelet therapy. In contrast to the oral antiplatelet agents prasugrel and ticagrelor, GLS-409 is administered intravenously, thereby avoiding the need for intestinal absorption. Consequently, GLS-409 is able to immediately exert its antiplatelet effect which may be associated with a further reduction of thrombotic events in the initial phase of ACS compared to oral ADP receptor antagonists. Alternatively, GLS-409 could serve as additional intravenous treatment for high-risk patients who are already on prasugrel or ticagrelor.
On the other hand, some patients develop serious bleeding complications while being treated with antithrombotic therapy. Other patients are not eligible for PCI and stent implantation due to their coronary artery anatomy, and require emergency CABG surgery. In these cases, the fast restoration of platelet function would be of great importance. Since GLS-409 has a much shorter plasma half-life than prasugrel and ticagrelor,22, 33 its use may result in a significant reduction of serious bleeding events compared to the clinically approved, oral P2Y12 inhibitors in the above-mentioned patient populations.
In contrast to prasugrel, ticagrelor and cangrelor, GLS-409 targets not only P2Y12 but also the second platelet ADP receptor P2Y1, and may therefore provide a more complete inhibition of ADP-induced human platelet aggregation. Our findings indicate that because of the synergistic nature of this inhibition of platelet aggregation, therapeutic inhibition may be achieved at lower concentrations of drug and lower blockade of P2Y1 and P2Y12 than with agents that only block P2Y1 or P2Y12. One may speculate that a more comprehensive inhibition of ADP-induced platelet aggregation at lower drug concentrations will be associated with an improved benefit/risk ratio. However, large randomized clinical trials are needed to reveal the potential benefits of the presently-described combined inhibition of P2Y1 and P2Y12 compared to the currently FDA-approved solely P2Y12 blockers. Of particular interest would be a clinical study comparing GLS-409 to the intravenous P2Y12 receptor antagonist cangrelor in the initial phase of ACS since the latter yielded heterogeneous results in large clinical trials. While cangrelor was not superior to an oral loading dose of 600 mg clopidogrel in reducing the composite of death from any cause, myocardial infarction, or ischemia-driven revascularization at 48 hours in ACS patients undergoing PCI,34 it significantly reduced the composite of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis at 48 hours compared to clopidogrel in patients undergoing urgent or elective PCI.35 In both studies, the use of cangrelor was not associated with a significantly increased risk of severe bleeding compared to clopidogrel. Moreover, the use of periprocedural cangrelor during PCI was not superior to placebo in reducing the composite of death from any cause, myocardial infarction, or ischemia-driven revascularization at 48 hours, but significantly reduced the secondary end points of stent thrombosis and death from any cause, albeit at a significantly increased bleeding risk.36 It remains to be established if, due to its unique mechanism of action, GLS-409 can provide additional reduction of the ischemic risk beyond intravenous P2Y12 blockade at an acceptable bleeding risk.
Our data demonstrate the antithrombotic efficacy of GLS-409 in vivo, and show that it rapidly and potently inhibits agonist-stimulated human platelet aggregation in vitro with and without concomitant aspirin therapy. Moreover, GLS-409 did not affect hemodynamics in our rat and canine model, and was only associated with a moderate non-significant increase in median bleeding time in our canine model, while showing a fast plasma clearance. These findings, together with the previous data on its high plasma stability and reversibility of its antiplatelet effects,22 suggest that GLS-409 could become a meaningful addition to the current pharmacological armamentarium in ACS. Presently, GLS-409 is undergoing late stage pre-clinical evaluation with a particular focus on the transition from GLS-409 to clinically-approved, oral P2Y12 inhibitors with a goal of initiating human clinical testing in near future.
In conclusion, synergistic inhibition of both P2Y1 and P2Y12 ADP receptors by GLS-409 immediately attenuates platelet-mediated thrombosis and effectively blocks agonist-stimulated platelet aggregation irrespective of concomitant aspirin therapy. GLS-409 is therefore a promising antiplatelet drug candidate, in particular for the initial phase of ACS.
Supplementary Material
Significance.
Our study is the first to investigate the effects of synergistic P2Y1 and P2Y12 ADP receptor inhibition by a single compound (GLS-409) on agonist-stimulated platelet aggregation in rats and on recurrent coronary artery thrombosis in a canine model of unstable angina. In addition, we studied the antiplatelet effect of GLS-409 versus selective P2Y1 and P2Y12 receptor antagonists on agonist-stimulated platelet aggregation in PRP and whole blood from healthy human donors in vitro pre- and 2 hours post-aspirin intake. Our results show that in vivo treatment with GLS-409 significantly inhibits ADP- and collagen-stimulated platelet aggregation in rats and immediately attenuates platelet-mediated thrombosis in dogs. GLS-409 exerted these effects without affecting rat or canine hemodynamics. Finally, GLS-409 effectively blocks agonist-stimulated platelet aggregation irrespective of concomitant aspirin therapy. GLS-409 is therefore a promising antiplatelet drug candidate, in particular for the initial phase of ACS.
Acknowledgments
Sources of funding
Funding was provided by NIH SBIR grants R44HL088828 and R44TR000983 to Ivan Yanachkov. Karin Przyklenk was supported in part by NIH R01HL072684.
Disclosures
Ivan B. Yanachkov, Milka Yanachkova and George E. Wright are employees of GLSynthesis, Inc. Alan D. Michelson has received grant support from Bristol-Myers Squibb, GLSynthesis, Lilly/Daiichi Sankyo and Pfizer, and served on data monitoring committees of Lilly/Daiichi Sankyo clinical trials. Andrew L. Frelinger has received grant support from Bristol-Myers Squibb, GLSynthesis, Lilly/Daiichi Sankyo and Pfizer. Karin Przyklenk serves on the scientific advisory board of Infarct Reduction Technologies, Inc.
Abbreviations
- ACS
acute coronary syndrome
- ADP
adenosine diphosphate
- Ap4A
adenosine tetraphosphate
- CABG
coronary artery bypass graft
- PCI
percutaneous coronary intervention
- PRP
platelet-rich plasma
- TRAP
thrombin receptor-activating peptide
References
- 1.Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 2004;24:1980–1987. doi: 10.1161/01.ATV.0000145980.39477.a9. [DOI] [PubMed] [Google Scholar]
- 2.Patrono C, Andreotti F, Arnesen H, et al. Antiplatelet agents for the treatment and prevention of atherothrombosis. Eur Heart J. 2011;32:2922–2932. doi: 10.1093/eurheartj/ehr373. [DOI] [PubMed] [Google Scholar]
- 3.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2015 [PubMed] [Google Scholar]
- 4.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 6.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 7.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 8.Cattaneo M. P2Y12 receptor antagonists: a rapidly expanding group of antiplatelet agents. Eur Heart J. 2006;27:1010–1012. doi: 10.1093/eurheartj/ehi851. [DOI] [PubMed] [Google Scholar]
- 9.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 10.Hardy AR, Jones ML, Mundell SJ, Poole AW. Reciprocal cross-talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood. 2004;104:1745–1752. doi: 10.1182/blood-2004-02-0534. [DOI] [PubMed] [Google Scholar]
- 11.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N(6)-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate is a selective high affinity P2Y(1) receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathieu R, Baurand A, Schmitt M, Gachet C, Bourguignon JJ. Synthesis and biological activity of 2-alkylated deoxyadenosine bisphosphate derivatives as P2Y(1) receptor antagonists. Bioorg Med Chem. 2004;12:1769–1779. doi: 10.1016/j.bmc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 14.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther. 2005;108:180–192. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Rapaport E, Zamecnik PC. Presence of diadenosine 5',5''' -P1, P4-tetraphosphate (Ap4A) in mamalian cells in levels varying widely with proliferative activity of the tissue: a possible positive"pleiotypic activator". Proc Natl Acad Sci U S A. 1976;73:3984–3988. doi: 10.1073/pnas.73.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luthje J, Ogilvie A. The presence of diadenosine 5',5'''-P1,P3-triphosphate (Ap3A) in human platelets. Biochem Biophys Res Commun. 1983;115:253–260. doi: 10.1016/0006-291x(83)90997-x. [DOI] [PubMed] [Google Scholar]
- 17.Jankowski J, Jankowski V, Laufer U, van der Giet M, Henning L, Tepel M, Zidek W, Schluter H. Identification and quantification of diadenosine polyphosphate concentrations in human plasma. Arterioscler Thromb Vasc Biol. 2003;23:1231–1238. doi: 10.1161/01.ATV.0000075913.00428.FD. [DOI] [PubMed] [Google Scholar]
- 18.Chan SW, Gallo SJ, Kim BK, Guo MJ, Blackburn GM, Zamecnik PC. P1,P4-dithio-P2,P3-monochloromethylene diadenosine 5',5'''-P1,P4-tetraphosphate: a novel antiplatelet agent. Proc Natl Acad Sci U S A. 1997;94:4034–4039. doi: 10.1073/pnas.94.8.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamecnik PC, Kim B, Gao MJ, Taylor G, Blackburn GM. Analogues of diadenosine 5',5'''-P1,P4-tetraphosphate (Ap4A) as potential anti-platelet-aggregation agents. Proc Natl Acad Sci U S A. 1992;89:2370–2373. doi: 10.1073/pnas.89.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang H, Yanachkov IB, Michelson AD, Li Y, Barnard MR, Wright GE, Frelinger AL., 3rd Agonist and antagonist effects of diadenosine tetraphosphate, a platelet dense granule constituent, on platelet P2Y1, P2Y12 and P2X1 receptors. Thromb Res. 2010;125:159–165. doi: 10.1016/j.thromres.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang H, Yanachkov IB, Dix EJ, Li YF, Barnard MR, Wright GE, Michelson AD, Frelinger AL., 3rd Modified diadenosine tetraphosphates with dual specificity for P2Y1 and P2Y12 are potent antagonists of ADP-induced platelet activation. J Thromb Haemost. 2012;10:2573–2580. doi: 10.1111/jth.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanachkov IB, Chang H, Yanachkova MI, Dix EJ, Berny-Lang MA, Gremmel T, Michelson AD, Wright GE, Frelinger AL., 3rd New highly active antiplatelet agents with dual specificity for platelet P2Y1 and P2Y12 adenosine diphosphate receptors. Eur J Med Chem. 2016;107:204–218. doi: 10.1016/j.ejmech.2015.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hata K, Whittaker P, Kloner RA, Przyklenk K. Brief antecedent ischemia attenuates platelet-mediated thrombosis in damaged and stenotic canine coronary arteries: role of adenosine. Circulation. 1998;97:692–702. doi: 10.1161/01.cir.97.7.692. [DOI] [PubMed] [Google Scholar]
- 24.Linden MD, Barnard MR, Frelinger AL, Michelson AD, Przyklenk K. Effect of adenosine A2 receptor stimulation on platelet activation-aggregation: differences between canine and human models. Thromb Res. 2008;121:689–698. doi: 10.1016/j.thromres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Przyklenk K, Frelinger AL, 3rd, Linden MD, Whittaker P, Li Y, Barnard MR, Adams J, Morgan M, Al-Shamma H, Michelson AD. Targeted inhibition of the serotonin 5HT2A receptor improves coronary patency in an in vivo model of recurrent thrombosis. J Thromb Haemost. 2010;8:331–340. doi: 10.1111/j.1538-7836.2009.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folts J. An in vivo model of experimental arterial stenosis, intimal damage, and periodic thrombosis. Circulation. 1991;83:IV3–IV14. [PubMed] [Google Scholar]
- 27.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 28.Mangin P, Ohlmann P, Eckly A, Cazenave JP, Lanza F, Gachet C. The P2Y1 receptor plays an essential role in the platelet shape change induced by collagen when TxA2 formation is prevented. J Thromb Haemost. 2004;2:969–977. doi: 10.1111/j.1538-7836.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Gao ZG, Zhang K, et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature. 2015;520:317–321. doi: 10.1038/nature14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK. Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol. 2003;64:1210–1216. doi: 10.1124/mol.64.5.1210. [DOI] [PubMed] [Google Scholar]
- 31.Cattaneo M, Lecchi A. Inhibition of the platelet P2Y12 receptor for adenosine diphosphate potentiates the antiplatelet effect of prostacyclin. J Thromb Haemost. 2007;5:577–582. doi: 10.1111/j.1538-7836.2007.02356.x. [DOI] [PubMed] [Google Scholar]
- 32.Mahaffey KW, Wojdyla DM, Carroll K, Becker RC, Storey RF, Angiolillo DJ, Held C, Cannon CP, James S, Pieper KS, Horrow J, Harrington RA, Wallentin L. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544–554. doi: 10.1161/CIRCULATIONAHA.111.047498. [DOI] [PubMed] [Google Scholar]
- 33.Floyd CN, Passacquale G, Ferro A. Comparative pharmacokinetics and pharmacodynamics of platelet adenosine diphosphate receptor antagonists and their clinical implications. Clin Pharmacokinet. 2012;51:429–442. doi: 10.2165/11630740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 35.Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 36.Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361:2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.