Abstract
Background
Pharmacotherapy – methadone, buprenorphine, or naltrexone – is an evidence-based treatment for opioid use disorder, but little is known about receipt of these medications among veterans involved in the justice system. The current study examines receipt of pharmacotherapy for opioid use disorder among veterans with a history of justice involvement at U.S. Veterans Health Administration (VHA) facilities compared to veterans with no justice involvement.
Methods
Using national VHA clinical and pharmacy records, we conducted a retrospective cohort study of veterans with an opioid use disorder diagnosis in fiscal year 2012. Using a mixed-effects logistic regression model, we examined receipt of pharmacotherapy in the 1-year period following diagnosis as a function of justice involvement, adjusting for patient and facility characteristics.
Results
The 1-year rate of receipt for pharmacotherapy for opioid use disorder was 27% for prison-involved veterans, 34% for jail/court-involved veterans, and 33% for veterans not justice-involved. Compared to veterans not justice-involved, those prison-involved had 0.75 lower adjusted odds (95% confidence interval [CI]: 0.65–0.87) of receiving pharmacotherapy whereas jail/court-involved veterans did not have significantly different adjusted odds.
Conclusions
Targeted efforts to increase receipt of pharmacotherapy for opioid use disorder among veterans exiting prison is needed as they have lower odds of receiving these medications.
Keywords: Opioid-related disorders, Criminal Justice, Methadone, Buprenorphine, United States Department of Veterans Affairs
1.0. Introduction
Drug overdose is the leading cause of death within 4 years of prison release, with opioids involved in 59% of these deaths (Binswanger et al., 2013). Pharmacotherapy, which is therapy using medications, is one treatment option for individuals with addictive disorders. Medications to treat opioid use disorder (OUD) – methadone, buprenorphine or naltrexone – are effective in treating OUD, retaining patients in treatment, and reducing alcohol and drug use (Amato et al., 2005; Kleber, 2008; Marsch, 1998; Mattick et al., 2009). These medications are also cost-effective (Barnett, 2009). For justice-involved adults, pharmacotherapy for OUD has been shown to reduce opioid use and incarceration (Coviello et al., 2012; Dolan et al., 2005), heroin use and illegal activity (Gryczynski et al., 2012), relapse to opioid use (Lee et al., 2015), and illegal activity (Kelly et al., 2013).
Once adults leave justice system settings, receiving pharmacotherapy can be challenging. These medications were provided or funded in only 17% of probation/parole agencies and 38–56% of drug courts (Friedmann et al., 2012; Matusow et al., 2013). Patients with a referral from the justice system had higher odds of delayed admission to methadone clinics compared to self-referred patients (Gryczynski et al., 2011). Women released from jail reported difficulty getting treatment services because of stigma related to incarceration or drug abuse (van Olphen et al., 2009). For veterans with OUD who have access to treatment through the national integrated Veterans Health Administration (VHA) system, it is unknown whether justice involvement is a barrier to pharmacotherapy for OUD.
Veterans represented about 8% of the incarcerated population in 2011–2012 (Bronson et al., 2015). After release from incarceration, veterans qualifying for services have the option of receiving treatment at VHA facilities. VHA provides treatment to more than 6 million US military veterans, approximately 35–45% of VA-eligible veterans, in over 1,700 locations and is the largest addiction treatment system in the US (http://www.va.gov/health/). Treatment for substance use disorders is offered in over 220 outpatient and residential specialty programs. Buprenorphine and naltrexone are provided through VHA pharmacy prescriptions and methadone is dispensed at 28 clinic locations across the US. Pharmacotherapy for OUD is mandated to be available and considered for every veteran for whom it is indicated (Department of Veterans Affairs, 2008).
Receipt of pharmacotherapy for OUD at VHA facilities among veterans with a history of criminal justice involvement is unknown. Therefore, the purpose of this study is to examine receipt of pharmacotherapy for OUD for justice-involved VHA patients compared to patients with no justice involvement in order to determine if targeted efforts are needed to increase access to these effective medications.
2.0. Material and Methods
Using national VHA clinical/administrative records, we conducted a retrospective cohort study of all VHA users who received an OUD diagnosis (abuse or dependence, excluding in remission; International Classifications of Diseases [ICD]-9th Edition-CM codes 304.0x, 304.7x, or 305.5x) during an outpatient or inpatient visit in fiscal year 2012.
2.1. Measures
2.1.1. Outcome
Receipt of pharmacotherapy for OUD was defined as having a methadone clinic outpatient visit with a concurrent OUD diagnosis and/or receiving at least one pharmacy prescription for buprenorphine or naltrexone during the one-year period after a veteran’s first OUD diagnosis in fiscal year 2012.
2.1.2. Justice involvement
Prison-involved veterans were defined by a clinic code (591) indicating contact with the VHA Health Care for Reentry Veterans program. Jail/court-involved veterans were defined by a clinic code (592) indicating contact with the Veterans Justice Outreach program (Blue-Howells et al., 2013). Both groups included veterans on probation or parole. All other patients were coded as not justice-involved. Although there may have been veterans with justice involvement in the not justice-involved group, less than 3% of veterans are justice-involved (Blue-Howells et al., 2013) and it is therefore likely only a small number of veterans were misclassified.
2.1.3. Patient characteristics
Demographic variables included gender, age, ethnicity/race (Hispanic, non-Hispanic: American Indian/Alaskan Native, Asian, Black, White; based on the US Bureau of Census categories), marital status (single, married, separated/divorced, widowed), urban or rural residence (living in an urban or rural area), and homeless status (drawn from a homeless indicator variable, utilization of services for homeless veterans, and ICD-9 codes for housing and homelessness). Military characteristics included service in Iraq or Afghanistan and service-connected disability rating. The demographic and service-connected disability characteristics were coded the same day the veteran received her/his first OUD diagnosis, or from the next available record. Service in Iraq or Afghanistan was drawn from the Iraq/Afghanistan Roster, which is a list of all veterans who served in those conflicts. Patient health characteristics included co-occurring psychiatric disorder (depression, post-traumatic stress disorder, anxiety, bipolar, schizophrenia, other psychosis, or personality disorders), co-occurring substance use disorder (not including tobacco use disorder), and the Deyo comorbidity index (Deyo et al., 1992), a sum of up to 17 comorbid medical diagnoses, during the one year period after the index OUD diagnosis.
2.1.4. Facility characteristics
Facility characteristics were from the 2012 Drug and Alcohol Program Survey of VHA substance use disorder treatment programs (100% response rate, N=129 facilities). Facility characteristics included availability of the following substance use disorder-related services: weekend, weeknight, women-specific, Iraq/Afghanistan veteran, or dual diagnosis services, psychiatric medications, and pharmacotherapy for smoking or for alcohol use disorder. Other facility characteristics included housing support, abstinence required for program admittance, services for patients who used substances after admittance, waiting list size and length, number of patients with substance use disorders at the facility, number of evidence-based practices available, ratio of substance use disorder clinic staff to 100 patients diagnosed with substance use disorder, the ratio of clinic staff who can prescribe substance use disorder medications to 1000 patients diagnosed with substance use disorders, and presence of a methadone clinic.
2.2. Analyses
The rate of receipt of pharmacotherapy for OUD was calculated as the number of patients who received pharmacotherapy for OUD within one-year of diagnosis divided by number of patients diagnosed with an OUD overall and by facility, stratified by justice-involved status. A mixed-effects logistic regression model using SAS PROC GLIMMIX tested whether justice-involvement was associated with odds of receiving pharmacotherapy for OUD. All patient and facility characteristics were entered into the model simultaneously as main effects and a random effect for facility was included to account for patient clustering by facility. We then trimmed all non-significant (p ≥ .05) covariates resulting in the final model. American Indian/Alaskan Native and Asian veterans were excluded from the mixed effects model because of small sample sizes.
3.0. Results
In fiscal year 2012, there were 48,689 VHA patients diagnosed with OUD: 8% of prison-involved veterans (n=1,105), 13% of jail/court-involved veterans (n=4,333), and 1% of veterans not justice-involved (n=43,251). Of 1,105 prison-involved veterans, 301 (27%) received pharmacotherapy for OUD within one year – 186 (17%) had a methadone clinic visit, 167 (15%) received buprenorphine, and 46 (4%) received naltrexone (not mutually exclusive). Of 4,333 veterans who were jail/court-involved, 1,501 (34%) received pharmacotherapy within one year – 693 (16%) received methadone, 913 (21%) received buprenorphine, and 299 (7%) received naltrexone. Among 43,251 veterans not justice-involved, 14,265 (33%) received pharmacotherapy – 7,895 (18%) received methadone, 7,994 (18%) received buprenorphine, and 1,262 (3%) received naltrexone.
There was wide variation across VHA facilities in receipt of pharmacotherapy for OUD (Figure 1). The range was 0% to 100% for prison-involved veterans, 0% to 80% for jail/court-involved veterans, and 1% to 68% for veterans not justice-involved. Within facility, the difference in rate of receipt between prison-involved veterans and veterans not justice-involved ranged from −65% to +95%. For jail/court-involved veterans compared to veterans not justice-involved the rate of receipt differed from −24% to +44%.
Figure 1.
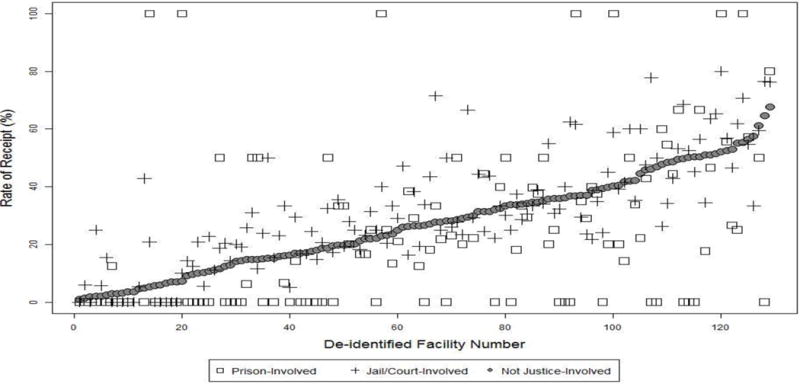
Rate of receipt of pharmacotherapy for opioid use disorder by facility for veterans with prison involvement, jail/court involvement, or without justice involvement.
The results from the mixed-effects logistic regression model indicated that veterans who were prison-involved, but not those who were jail/court-involved, had lower adjusted odds of receiving pharmacotherapy for OUD compared to veterans not justice-involved (Table 1). Other patient characteristics associated with lower adjusted odds of receiving pharmacotherapy included female, Black/African American, rural residence, homeless, a 50% or great service-connected disability rating, and a co-occurring mental health disorder.
Table 1.
Multivariable Model of Receipt of Pharmacotherapy for Opioid Use Disorder Among Veterans in Fiscal Year 2012
| Characteristics | OR | 95% CI |
|---|---|---|
| Patient characteristics | ||
| Justice-involved status (ref: Not justice-involved) | ||
| Prison-involved | 0.75 | 0.65, 0.87 |
| Jail/court-involved | 0.93 | 0.86, 1.00 |
| Women (ref: Men) | 0.82 | 0.75, 0.90 |
| Age (ref: < 35) | ||
| 35–44 | 0.64 | 0.59, 0.70 |
| 45–54 | 0.48 | 0.44, 0.51 |
| 55+ | 0.47 | 0.44, 0.50 |
| Ethnicity/Race (ref: non-Hispanic White) | ||
| Hispanic | 1.13 | 1.03, 1.25 |
| Non-Hispanic Black/African American | 0.89 | 0.84, 0.94 |
| Marital status (ref: Married) | ||
| Single | 1.11 | 1.05, 1.17 |
| Divorced/Separated | 1.06 | 1.00, 1.12 |
| Widowed | 1.02 | 0.90, 1.15 |
| Rural residence (ref: Urban) | 0.73 | 0.69, 0.77 |
| Homeless (ref: Housed) | 0.58 | 0.53, 0.63 |
| Service-connected disability rating (ref: None) | ||
| < 50% | 0.96 | 0.91, 1.00 |
| ≥ 50% | 0.82 | 0.78, 0.88 |
| Co-occurring mental health diagnosis | 0.92 | 0.87, 0.97 |
| Co-occurring substance use disorder diagnosis | 1.32 | 1.26, 1.39 |
| Deyo medical co-morbidity index | 0.89 | 0.87, 0.90 |
| Facility characteristics | ||
| Medication for psychiatric disorders | 2.06 | 1.29, 3.31 |
| Ave. months to residential bed admittance | 0.73 | 0.55, 0.98 |
| Ratio of staff to 100 patients | 0.96 | 0.93, 0.99 |
| Presence of methadone clinic | 2.56 | 1.78, 3.70 |
Note. N = 46,289. Cases with missing data (n = 1,410; 3%) were excluded from the mixed effects logistic regression model.
4.0. Discussion
Addressing OUD is especially important among justice-involved veterans due to the higher prevalence rate of this disorder observed among veterans exiting (8%) or in jails or courts (13%) relative to other veterans (1%) and the higher risk of mortality among veterans exiting prison, with overdose the leading cause of death (Wortzel et al., 2012). Compared to the overall rate of methadone and buprenorphine treatment in the general U.S. population of 22–28% between 2008 and 2012 (SAMHSA, 2013), a similar percentage of prison-involved veterans (27%) and somewhat higher percentage of jail/court-involved veterans (34%) and veterans not justice-involved (33%) received pharmacotherapy. The wide variation across and within facilities by justice status indicates that at some facilities justice-involved veterans have more difficulty getting pharmacotherapy whereas facilities are viable locations to seek pharmacotherapy.
Although future studies with qualitative methodologies are needed to explain these findings, previous research provides potential directions. These medications are often unavailable in criminal justice settings or only provided in detoxification (Friedmann et al., 2012; Nunn et al., 2009). Although efforts are underway at VHA to increase receipt of these medications, educating staff who conduct outreach with justice-involved veterans about the use of these medications may increase receipt. VHA is currently providing training for prison physicians on trauma-informed psychotherapy. A similar training program on pharmacotherapy for substance use disorders for correctional physicians along with education of their administrators may help increase use of these medications prior to veterans’ release from prison.
Other patient factors were associated with pharmacotherapy for OUD. Female veterans had lower odds of receiving pharmacotherapy for OUD than males. Previous research indicated that women have higher odds of receiving pharmacotherapy for alcohol use disorder at VHA compared to men (Harris et al., 2012), suggesting that there may be gender-related barriers to these medications. Black/African American veterans also had lower odds of receiving pharmacotherapy for OUD compared to White veterans, which is consistent with other research indicating that ethnic minority veterans had lower odds of receiving pharmacotherapy for depression compared to White veterans (Quinones et al., 2014). With the overrepresentation of the Black/African American population among veterans in the justice system (Tsai et al., 2013), targeted efforts to improve receipt of pharmacotherapy are needed for these veterans. Veterans with a 50% or greater service-connected disability rating, a co-occurring mental health disorder, or more comorbid medical conditions had lower odds of receiving pharmacotherapy for OUD. For veterans on other medications, there may be concerns about drug interactions with pharmacotherapy for OUD (McCance-Katz et al., 2010). Also, other health problems may be perceived as more pressing, such as addressing acute mental health issues. Finally, veterans in rural areas had lower odds of receiving pharmacotherapy, which may be due to limited methadone treatment or a lack of physicians registered to prescribe buprenorphine (Sigmon, 2014).
4.1. Limitations
This retrospective study was limited to veterans who received treatment at VHA facilities; findings may not generalize to veterans who do not use VHA care. Some veterans may use community treatment and we were unable to capture these visits. We were also unable to delve into some factors that may have contributed to the lower rate of pharmacotherapy among prison-involved veterans, such as return to incarceration. Although we controlled for patient and facility characteristics, there may be other differences between justice-involved veterans and veterans without justice involvement that were unaccounted for in available data. For example, adults released from prison often have limited housing and employment options and limited social, financial, and community support (Binswanger et al., 2012; Geller and Curtis, 2011; Nally et al., 2014; Re-entry Policy Council, 2005). Studies that match veterans on these factors may provide future insights into access to pharmacotherapy.
4.2. Conclusions
Less than a third of justice-involved veterans received pharmacotherapy for OUD and there was wide variation across facilities. Increasing pharmacotherapy for OUD is an important quality improvement target, particularly for veterans exiting prison. In addition, targeted efforts to improve treatment access for women and veterans in rural areas may also be needed.
Highlights.
We examined rates of receipt for pharmacotherapy for opioid use disorder among veterans who were prison-involved, jail/court-involved, or who were not justice-involved.
Veterans prison-involved had lower odds of receiving pharmacotherapy for opioid use disorder compared to veterans not justice-involved.
Veterans jail/court-involved had the same odds of receiving pharmacotherapy for opioid use disorder compared to veterans not justice-involved.
Rates of receipt varied widely within facilities between justice-involved veterans and veterans not justice-involved.
Acknowledgments
Role of Funding Source
This work was supported by the Department of Veterans Affairs (VA) Substance Use Disorder Quality Enhancement Research Initiative (SUD-QLP59-045). Dr. Finlay was supported by a Department of Veterans Affairs Health Services Research & Development (VA HSR&D) Career Development Award (CDA 13-279). Dr. Timko was funded as a Senior Research Career Scientist (RCS 00-001) in VA HSR&D. Dr. Harris was funded as a Research Career Scientist (RCS 14-132) in VA HSR&D. A portion of this work was also supported by the VA National Center on Homelessness Among Veterans.
The views expressed in this article are those of the authors and do not necessarily reflect the position nor policy of the Department of Veterans Affairs (VA) or the United States government. The VA had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
Dr. Finlay was the lead in conceptualizing, analyzing, and writing this manuscript. All other authors contributed to conceptualizing and writing the manuscript.
Conflict of Interest
No conflict declared.
Contributor Information
Andrea K. Finlay, Email: Andrea.Finlay@va.gov.
Alex H.S. Harris, Email: Alexander.Harris2@va.gov.
Joel Rosenthal, Email: Joel.Rosenthal@va.gov.
Jessica Blue-Howells, Email: Jessica.Blue-Howells@va.gov.
Sean Clark, Email: Sean.Clark2@va.gov.
Jim McGuire, Email: jimfmcguire@yahoo.com.
Christine Timko, Email: ctimko@stanford.edu.
Susan M. Frayne, Email: Susan.Frayne@va.gov.
David Smelson, Email: David.Smelson@umassmed.edu.
Elizabeth Oliva, Email: Elizabeth.Oliva@va.gov.
Ingrid Binswanger, Email: Ingrid.A.Binswanger@kp.org.
References
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28:321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Barnett PG. Comparison of costs and utilization among buprenorphine and methadone patients. Addiction. 2009;104:982–992. doi: 10.1111/j.1360-0443.2009.02539.x. [DOI] [PubMed] [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159:592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Nowels C, Corsi KF, Glanz J, Long J, Booth RE, Steiner JF. Return to drug use and overdose after release from prison: a qualitative study of risk and protective factors. Addiction science & clinical practice. 2012;7:3. doi: 10.1186/1940-0640-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue-Howells JH, Clark SC, van den Berk-Clark C, McGuire JF. The US Department of Veterans Affairs Veterans Justice Programs and the sequential intercept model: case examples in national dissemination of intervention for justice-involved veterans. Psych Serv. 2013;10:48–53. doi: 10.1037/a0029652. [DOI] [PubMed] [Google Scholar]
- Bronson J, Carson A, Noonan M, Berzofsky M. Veterans in prison and jail 2015 [Google Scholar]
- Coviello DM, Cornish JW, Lynch KG, Boney TY, Clark CA, Lee JD, Friedmann PD, Nunes EV, Kinlock TW, Gordon MS, Schwartz RP, Nuwayser ES, O’Brien CP. A multisite pilot study of extended-release injectable naltrexone treatment for previously opioid-dependent parolees and probationers. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2012;33:48–59. doi: 10.1080/08897077.2011.609438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Veterans Affairs. VHA Handbook 1160.1. US Department of Veterans Affairs, Veterans Health Administration; Washington, DC: 2008. Uniform Mental Health Services in VA Medical Centers and Clinics. [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction. 2005;100:820–828. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Gordon M, Schwartz R, Kinlock T, Knight K, Flynn PM, Welsh WN, Stein LA, Sacks S, O’Connell DJ, Knudsen HK, Shafer MS, Hall E, Frisman LK, Mat Working Group Of, C.J.D Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2012;33:9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A, Curtis MA. A Sort of Homecoming: Incarceration and the housing security of urban men. Soc Sci Res. 2011;40:1196–1213. doi: 10.1016/j.ssresearch.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski J, Kinlock TW, Kelly SM, O’Grady KE, Gordon MS, Schwartz RP. Opioid agonist maintenance for probationers: patient-level predictors of treatment retention, drug use, and crime. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse. 2012;33:30–39. doi: 10.1080/08897077.2011.616816. [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Schwartz RP, Salkever DS, Mitchell SG, Jaffe JH. Patterns in admission delays to outpatient methadone treatment in the United States. J Subst Abuse Treat. 2011;41:431–439. doi: 10.1016/j.jsat.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AHS, Oliva EM, Bowe T, Humphreys KN, Kivlahan DR, Trafton JA. Pharmacotherapy of alcohol use disorders by the Veterans Health Administration: Patterns of receipt and persistence. Psychiatric Services. 2012;63:679–685. doi: 10.1176/appi.ps.201000553. [DOI] [PubMed] [Google Scholar]
- Kelly SM, O’Grady KE, Jaffe JH, Gandhi D, Schwartz RP. Improvements in outcomes in methadone patients on probation/parole regardless of counseling early in treatment. Journal of addiction medicine. 2013;7:133–138. doi: 10.1097/ADM.0b013e318284a0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303–2305. doi: 10.1001/jama.2008.648. [DOI] [PubMed] [Google Scholar]
- Lee JD, McDonald R, Grossman E, McNeely J, Laska E, Rotrosen J, Gourevitch MN. Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction. 2015;110:1008–1014. doi: 10.1111/add.12894. [DOI] [PubMed] [Google Scholar]
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93:515–532. doi: 10.1046/j.1360-0443.1998.9345157.x. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3 doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- Matusow H, Dickman SL, Rich JD, Fong C, Dumont DM, Hardin C, Marlowe D, Rosenblum A. Medication assisted treatment in US drug courts: Results from a nationwide survey of availability, barriers and attitudes. Journal of Substance Abuse Treatment. 2013;44:473–480. doi: 10.1016/j.jsat.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4–16. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nally JM, Lockwood S, Ho T, Knutson K. Post-release recidivism and employment among different types of released offenders: A 5-year follow-up study in the United States. International Journal of Criminal Justice Sciences. 2014;9:16–34. [Google Scholar]
- Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105:83–88. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones AR, Thielke SM, Beaver KA, Trivedi RB, Williams EC, Fan VS. Racial and ethnic differences in receipt of antidepressants and psychotherapy by veterans with chronic depression. Psychiatric Services. 2014;65:193–200. doi: 10.1176/appi.ps.201300057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re-entry Policy Council. Report of the Re-entry Policy Council: Charting the safe and successful return of prisoners to the community. New York, NY: 2005. Downloaded from www.reentrypolicy.org. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Survey of Substance Abuse Treatment Services (N-SSATS): 2012. Data on Substance Abuse Treatment Facilities. BHSIS Series S-66, HHS Publication No. (SMA) 14-4809. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA psychiatry. 2014;71:359–360. doi: 10.1001/jamapsychiatry.2013.4450. [DOI] [PubMed] [Google Scholar]
- Tsai J, Rosenheck RA, Kasprow WJ, McGuire JF. Risk of incarceration and clinical characteristics of incarcerated veterans by race/ethnicity. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1777–1786. doi: 10.1007/s00127-013-0677-z. [DOI] [PubMed] [Google Scholar]
- van Olphen J, Eliason MJ, Freudenberg N, Barnes M. Nowhere to go: how stigma limits the options of female drug users after release from jail. Substance abuse treatment, prevention, and policy. 2009;4:10. doi: 10.1186/1747-597X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortzel HS, Blatchford P, Conner L, Adler LE, Binswanger IA. Risk of death for veterans on release from prison. J Am Acad Psychiatry Law. 2012;40:348–354. [PMC free article] [PubMed] [Google Scholar]


