Abstract
Purpose
ASP9853 is an inhibitor of iNOS dimerization, which results in decreased NO production. Here we report preclinical pharmacology of ASP9853 and the impact of ASP9853 in combination with a taxane on tumor volume in vivo. In addition, a Phase I open-label study of ASP9853 plus docetaxel was conducted to assess this combination in patients with advanced solid tumors.
Methods
The preclinical efficacy of ASP9853 in combination with a taxane was studied in tumor-bearing mice. In the clinic, patients with solid tumors that had progressed or failed to respond to previous therapies were treated with once daily ASP9853 in combination with docetaxel once every 3 weeks to assess safety and tolerability and to determine the maximum tolerated dose (MTD) and the recommended Phase II dose (RP2D) of the combination.
Results
ASP9853 in combination with docetaxel showed greater tumor growth inhibition than docetaxel alone against non-small lung cancer xenografts. Twenty patients were treated with ASP9853 and docetaxel. Five patients experienced neutropenic dose limiting toxicities. Owing to overall toxicity that limited further dose escalation, the ASP9853 concentrations predicted for efficacy based on the preclinical data were not achieved. Due to toxicity and lack of clear efficacy, the study was terminated without determination of MTD or RP2D.
Conclusions
Inhibition of iNOS by ASP9853 in combination with docetaxel was not tolerable and resulted in the possible potentiation of neutropenia. Manipulation of the iNOS pathway, with or without chemotherapy, appears to be more complicated than initially expected.
Keywords: Phase I clinical trial, tumor, ASP9853, taxane, nitric oxide synthase dimerization, docetaxel
Introduction
Nitric oxide (NO) is involved in various physiological functions such as regulation of blood pressure, reproductive biology and immune response (for reviews see [1-3]). NO affects signal transduction pathways by interacting with metal ligands [4], or modifying protein function through nitration, nitrosylation and oxidation [5,6]. Unlike exogenous NO, which plays a major role in the cardiovascular system, inducible nitric oxide synthase, iNOS, is unlikely to impact the cardiovascular system or affect vasodilation under normal physiological conditions because of its low or absent expression [7].
Alongside physiological functions, NO has also been implicated in pathophysiological conditions including inflammation [8] and cancer [9]. For cancer, although NO released by iNOS [10,11] has been found to have dual pro- and anti-tumorigenic action depending on local concentration in vitro and in vivo [9,12-14], the effect of NO on the initiation, maintenance and progression of many tumor types is of importance to this study [9]. Studies have demonstrated prostate and colon cancer tissue contain higher iNOS expression and/or activity than benign hyperplasia tissue [15], or adjacent colonic tissue [16]. Such up-regulation of iNOS can invoke a chronic inflammatory state in tumor cells making the environment suitable for metastatic growth [17].
Alongside a role in tumorigenesis, NO reduces the effect of some cytotoxic agents, including platinum and taxanes [18-22]. The mechanisms here are not fully elucidated; iNOS inhibition may overcome chemoresistance by making tumor cells more prone to apoptosis [21], resulting in in vitro synergism. Taxanes demonstrate antitumor activity in many tumor types notably including non-small cell lung cancer (NSCLC) and advanced prostate cancer [23-25].
ASP9853 ((2E)-3-(4-chlorophenyl)-N-[(1S)-2-oxo-2-{[2-oxo-2-(4-{[6-(trifluoromethyl)-4-pyrimidinyl]oxy}-1-piperidinyl)ethyl]amino}-1-(2-pyridinylmethyl) ethyl]acrylamide dehydrate) (Supplemental Fig. 1), is an inhibitor of iNOS dimerization, which results in decreased NO production [26]. This report details the preclinical pharmacology of ASP9853, an in vivo assessment of impact on tumor reduction in combination with a taxane and the results of a Phase I open-label study of ASP9853 in combination with docetaxel in patients with advanced solid tumors. The primary objectives of the clinical study were to determine the safety and tolerability of ASP9853 in combination with docetaxel once every 3 weeks, and to establish the maximum tolerated dose (MTD) and recommended Phase II dose (RP2D). The secondary objectives were to evaluate the antitumor activity and determine the pharmacokinetic (PK) profile of ASP9853 in combination with docetaxel.
Materials and methods
Preclinical pharmacology
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Astellas Pharma, Inc., and the Tsukuba Research Center of Astellas Pharma, Inc., is accredited by AAALAC International. ASP9853 was synthesized at Astellas Pharma Inc. (Tokyo, Japan). Docetaxel hydrate (Taxotere® injection) was purchased from Sanofi-Aventis Pharma Ltd. (Paris, France). Athymic male nude mice (CAnN.Cg-Foxn1nu/CrlCrlj) were purchased from Charles River Laboratories, Japan, Inc. (Kanagawa, Japan). Human NSCLC cell line Calu-6 was obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured at 37°C in a 5% CO2 humidified atmosphere in RPMI1640 medium (Invitrogen Corp.; Camarillo, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum and 50 units/mL penicillin-streptomycin (GIBCO BRL; Grand Island, NY, USA).
The antitumor effect of ASP9853 was examined in athymic nude mice subcutaneously xenografted with the human NSCLC cell line Calu-6. The cells were subcutaneously inoculated into the flank of the athymic mice at 3 × 106 cells/0.1 mL/mouse. After tumors were established (reaching from 100 to 400 mm3 in volume), mice were divided into treatment groups on Day 0 to minimize the difference in mean tumor volumes of each group. After tumors were established, control (control group were untreated [as 0.5% MC did not affect tumor growth in preliminary study [data not shown]), docetaxel alone, ASP9853 suspended in 0.5% methylcellulose (0.5% MC), or ASP9853 plus docetaxel were administered. Docetaxel was administered at 10 mg/kg/day IV on Day 1, 5, 9 and ASP9853 at 100 mg/kg oral, twice daily during the study period. Tumor volume was determined by calculating the volume of an ellipsoid using the formula: length × width2 × 0.52 [27], and measured at least twice a week using a digital caliper.
Clinical study summary
The clinical trial design was a Phase I, multicenter, open-label, dose-escalation study (ClinicalTrials.gov Identifier: NCT01705483) of ASP9853 in combination with docetaxel conducted in accordance with the protocol, Good Clinical Practice, International Committee on Harmonisation and ethical principles that have their origin in the Declaration of Helsinki. An Independent Ethics Committee or Institutional Review Board reviewed the ethical, scientific, and medical appropriateness prior to study. Written informed consent was obtained from all patients before screening.
Patient population
Adult patients (aged ≥ 18 years) with histologically or cytologically confirmed incurable, locally advanced, or metastatic non-hematologic malignancies that progressed or failed to respond to therapies known to have clinical benefit, were eligible. Inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1 and a life expectancy of over 12 weeks. Adequate bone marrow, renal and hepatic function were required: absolute neutrophil count (ANC) ≥ 1500 cells/mm3, platelet count ≥ 100,000 cells/mm3, hemoglobin ≥9 g/dL, serum creatinine ≤ 1.5 × upper limit of normal (ULN) or calculated creatinine clearance ≥60 mL/min if serum creatinine was >1.5 × ULN, total bilirubin ≤ 1.5 × ULN, aspartate aminotransferase or alanine aminotransferase ≤ 3 × ULN (or <5 × ULN in subjects with liver metastases or hepatocellular carcinoma).
Exclusion criteria included three or more prior treatment regimens containing a cytotoxic agent (for example prior taxane therapy was allowed if it did not constitute three or more treatment regimens), previous anaphylactic or hypersensitivity reaction to taxanes, receipt of systemic chemotherapy within 21 days, and receipt of strong inhibitors/inducers of cytochrome P450 3A4 within 2 weeks prior to the start of study treatment (CYP3A4 is the major cytochrome P450 CYP isozyme involved in ASP9853 metabolism)..Patients were also excluded if they had central nervous system metastases or leptomeningeal involvement, peripheral neuropathy greater than Grade 1; known hepatitis B/known suspected active hepatitis C/known human immunodeficiency virus positivity; significant/uncontrolled cardiac, renal or hepatic disease, other systemic disorders or psychological condition, or electrocardiogram abnormalities.
Study design
This Phase I study followed a lead-in and dose-escalation phase design. Dose-escalation rules were determined by ASP9853 safety outcomes in each dose cohort. Hematologic dose-limiting toxicities (DLTs) were Grade 3 or 4 neutropenia with fever and/or documented infection (where fever was defined as an oral temperature ≥ 38.5°C), Grade 4 neutropenia (ANC < 500 cells/mm3) or Grade 4 thrombocytopenia (platelets < 25,000 cells/mm3 but > 10,000 cells/mm3) lasting over seven consecutive days, or Grade 3 or 4 thrombocytopenia with bleeding, or a platelet count less than 10,000 cells/mm3 at any time. Non-hematologic DLTs were any ≥Grade 3 non-hematological toxicity (excluding nausea, vomiting, diarrhea, anorexia, fatigue), nausea, vomiting and diarrhea of ≥ Grade 3 severity despite receiving optimal prophylaxis and/or treatment, any study drug-related toxicity resulting in a treatment delay of over 2 weeks, or any study drug-related toxicity resulting in discontinuation of treatment at the patient’s assigned dose level.
If any of the above DLTs were clearly related to docetaxel, then docetaxel administration could be interrupted or modified and ASP9853 treatment continued. If the docetaxel toxicity did not resolve to ≤ Grade 1 or baseline severity after omission, then ASP9853 was also interrupted until resolution of the toxicity to ≤ Grade 1 or baseline severity.
ASP9853 MTD was defined as the highest dose level tested in which no more than one of a minimum of six subjects experienced a DLT during Cycle 1. DLTs were graded according to the National Cancer Institute Common Toxicity Criteria version 4.0.
Treatment for each patient began with a 7-day lead-in period before Cycle 1 only, in which ASP9853 was administered as a single agent once daily on Days -7 to -1. Following completion of the 7-day lead-in period in Cycle 1, and for all subsequent 21-day cycles (Cycles ≥2), ASP9853 was dosed once daily continuously in combination with docetaxel administered as an IV infusion on Day 1 of each 21-day cycle.
An accelerated dose-escalation phase was initiated in single-subject cohorts based on the three accelerated escalation rules shown in Supplemental Table 1. The traditional 3+3 dose escalation phase was followed according to whether the patients experienced none, one, or two or more ASP9853 DLTs (Supplemental Table 1).
Dosing in the cohorts were as follows: cohort 1: ASP9853 25 mg; docetaxel 60 mg/m2; cohort 2: ASP9853 25 mg; docetaxel 75 mg/m2; cohort 3: ASP9853 37.5 mg; docetaxel 60 mg/m2; cohort 4: ASP9853 50 mg; docetaxel 60 mg/m2. All patients were assessed for toxicities before being allowed to continue to subsequent cycles. Patients who continued therapy beyond Cycle 1 continued to receive ASP9853 at the dose assigned to their cohort until they discontinued from the study.
The planned ASP9853 RP2D was the dose level determined by the Sponsor and Investigators to be suitable for Phase 2 testing based on overall observed tolerability of study treatment (same or lower than MTD).
Study procedures
Imaging was performed at screening (baseline) and minimally every two cycles from Cycle 1, Day 1 (window of +1 week).
Blood samples were taken for ASP9853 PK assessment on the following days: Cycle 1: Days -7, -1, and 1 (Cohort 3 only, Days -6, -2, and 1) pre-dose and at 0.25, 0.5, 1, 2, 3, 4, 6, 8, and 24 hours following ASP9853 dose; Days 8 and 15 pre-dose and Cycles ≥2: Day 1 pre-dose. For docetaxel PK assessment samples were taken on Cycle 1, Day 1 pre-dose and at 0.5, 1 (at end of docetaxel infusion), 1.5, 2, 3, 4, 6, 8, and 24 hours (immediately prior to ASP9853 dose on Day 2).
For ASP9853, PK parameters assessed included: area under the plasma concentration-time curve (AUC) at 24 hours (AUC24), maximum concentration (Cmax), time to attain Cmax (tmax) and apparent terminal elimination half-life (t1/2). For docetaxel, PK parameters included AUC24, Cmax, tmax, and t1/2.
Statistical analyses
Preclinical sets
Values were expressed as the mean ± the standard error of the mean (SEM). The unpaired Student’s t-test was used for comparison in tumor volume between docetaxel alone and the combination with ASP9853 at the end of study (Day 41). P values of less than 5% were considered to be significant. GraphPad Prism software (version 5, La Jolla, CA, USA) was used for data processing.
Clinical sets
No formal hypotheses were tested in this study, analyses were descriptive and exploratory. The safety set (SAF) was defined as all patients who received at least one dose of study drug. The full analysis set (FAS) contained all enrolled patients.
Pharmacokinetic profiles of ASP9853 and docetaxel were assessed in all patients who received at least one dose of study drug and provided drug concentration values for at least one time point.
Antitumor activity was based on the Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1 [28].
Results
ASP9853 preclinical antitumor effect
ASP9853 alone had no antitumor activity in the Calu-6 NSCLC xenograft model. However, the combination of ASP9853 with docetaxel produced significantly greater tumor growth inhibition than docetaxel alone, such that tumor volume at the end of study (Day 41) in mice treated with the combination was only 35% (P<0.05) of that achieved with docetaxel monotherapy (Fig.1).
Fig. 1.
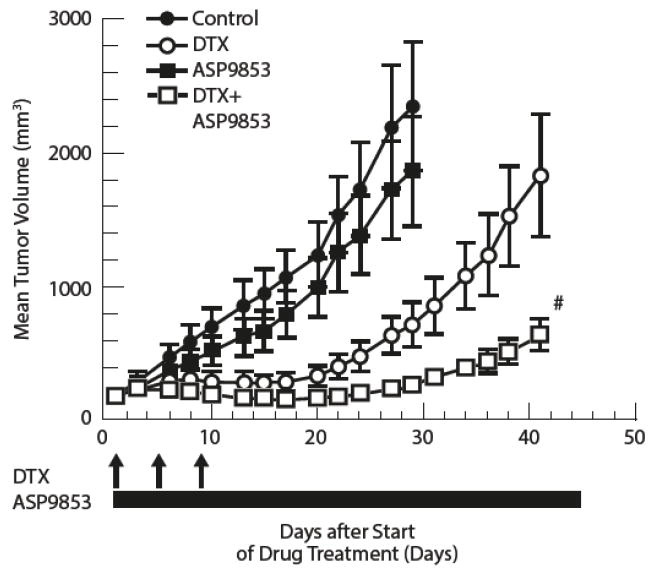
Preclinical effects of ASP9853: Time-course changes of tumor volume in mice xenografted with Calu-6 cells receiving a single cycle of ASP9853 alone, docetaxel alone and ASP9853 combination with docetaxel
The plot represents the mean (SE) of 6 animals. #P<0.05 versus docetaxel group (Student’s t-test). DTX, docetaxel. Control is untreated.
ASP9853 in combination with another taxane, paclitaxel, was also confirmed to produce significantly greater tumor growth inhibition compared with paclitaxel alone (data not shown). ASP9853 in combination with paclitaxel also produced greater tumor growth inhibition in mice in other xenografts including breast cancer (MDA-MB-231), ovarian cancer (MCUAS) and prostate cancer (PC-3) (data not shown).
Phase I clinical study
Baseline patient characteristics and treatment
Between August 2012 and June 2014, twenty-two patients were enrolled in the study. However, one patient discontinued before receiving any study drug, and hence the demographics are reported for 21 patients in Table 1. One patient discontinued after receiving ASP9853 monotherapy so did not continue to receive taxane combination therapy, so that the safety and PK analyses sets are comprised of 20 patients. Most patient demographics and baseline disease characteristics were comparable between cohorts (Table 1). The mean age of the patients was 59.4 years and approximately 24% and 76% had ECOG performance status 0 and 1, respectively, at screening. The most prevalent tumor types were lung (30%), prostate (15%), esophagus and bladder (10% each). All patients received chemotherapy prior to study entry (50% had received a prior taxane, 85% had prior surgery/procedures and 85% had prior radiation treatments. Weight and body mass index (BMI) were slightly lower in Cohort 2 than the other cohorts.
Table 1.
Patient demographics and baseline disease characteristics
| Parameter/statistic | Totala (n=21) |
|---|---|
|
| |
| Sex | |
| Male, n (%) | 15 (71.4) |
| Female, n (%) | 6 (28.6) |
|
| |
| Race | |
| White | 19 (90.5) |
| Black or African American | 2 (9.5) |
| Asian | 0 |
| American Indian/Alaska Native | 0 |
| Native Hawaiian/other Pacific Islander | 0 |
| Other | 0 |
|
| |
| Age (years) | |
| Mean (SD) | 59.4 (11.25) |
| Median (range) | 60.0 (36–87) |
|
| |
| Weight (kg), mean (SD) | 76.6 (18.06) |
|
| |
| Height (cm), mean (SD) | 171.7 (8.27) |
|
| |
| BMI (kg/m2)b, mean (SD) | 26.0 (5.77) |
|
| |
| ECOG status, n (%)c | |
| Grade 0 | 5 (23.8) |
| Grade 1 | 16 (76.2) |
|
| |
| Primary tumor type, n (SAF) | n=20 |
|
| |
| Lung | 6 |
| Prostate | 3 |
| Bladder | 2 |
| Esophagus | 2 |
| Other | 2 |
| Bile duct | 1 |
| Gastroesophageal junction | 1 |
| Kidney | 1 |
| Pancreas | 1 |
| Skin | 1 |
|
| |
| Prior chemotherapy, n (%) (SAF) | 20 (100.0) |
| Prior taxane, n (%) | 10 (50.0) |
|
| |
| Prior surgery/procedures, n (%) | 17 (85.0) |
|
| |
| Prior radiation treatment, n (%) | 17 (85.0) |
From FAS (all enrolled patients), unless specified. SAF, safety analysis set (all patients all patients who received at least one dose of study drug);
Total refers to ‘Total ASP9853 combination with docetaxel;
Body mass index (BMI) = weight (kg)/height (m2);
At screening
Dose escalation and dose-limiting toxicities
Five patients experienced AEs that were considered to be potential DLTs. Two of these patients experienced AEs that were treated as DLTs but after review did not meet the formal protocol-defined DLT criteria. The three patients who met the protocol-defined DLT criteria were in Cohorts 1 and 2.
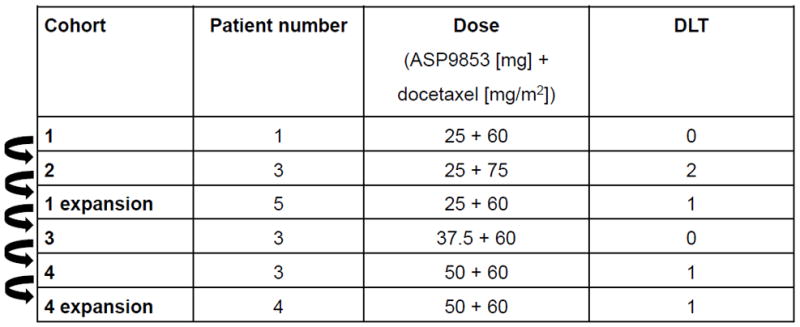
Cohort 1 was initiated as a single-patient cohort at 25 mg ASP9853 with 60 mg/m2 docetaxel based on accelerated escalation rules (Table 2). Although it did not meet the DLT criteria, a Grade 3 neutropenia was noted in Cohort 1, triggering the transition from single patient cohorts to the more conservative dose-escalation schema of 3+3 dose design. Cohort 2 was opened at 25 mg ASP9853 with 75 mg/m2 docetaxel. Cohort 2 exceeded the MTD with two of the three patients experiencing neutropenic fever DLTs. Based upon review of the Cohort 2 data and in agreement with the Investigators, expanded safety testing of 25 mg ASP9853 + 60 mg/m2 docetaxel (expansion of Cohort 1) was undertaken. One patient experienced febrile neutropenia meeting the DLT criteria in the Cohort 1 expansion. Docetaxel dose was reduced to below standard of care (from 75 to 60 mg/m2) due to fever and neutropenia requiring growth factor support.
Table 2.
Dose escalation and DLTs
|
Patients in Cohort 3 received 37.5 mg ASP9853 with 60 mg/m2 docetaxel; three patients were included and no DLTs were observed. Three patients in Cohort 4 received 50 mg ASP9853 with 60 mg/m2 docetaxel. One DLT occurred (leukopenia and neutropenia) and the cohort was expanded to include four more patients. One additional DLT (leukopenia and neutropenia) occurred in the Cohort 4 expansion. Both of these DLT events in Cohort 4 and Cohort 4 expansion did not meet the formal protocol-defined DLT criteria (the Grade 4 neutropenia and leukopenia were consistent with data observed in previous cohorts; dose escalation was not allowed due to pending internal review of aggregate data resulting in program termination).
Safety and tolerability
The most commonly reported AEs (in ≥30% of patients) receiving ASP9853 in combination with docetaxel, regardless of relationship to study drug, were neutropenia (50.0%), fatigue (45.0%), nausea (35.0%), decreased white blood cells (35.0%), alopecia (30.0%), and decreased appetite (30.0%). Similarly, fatigue, decreased white blood cells, diarrhea, neutropenia, nausea and vomiting were the most commonly reported AEs possibly related to ASP9853 in combination with docetaxel across the cohorts (Table 3). The incidence of AEs possibly related to ASP9853 in the lead-in phase was low (overall 7 [35%]; leukopenia, gastrooesophageal reflux disease, nausea, vomiting, asthenia, QT prolonged electrocardiogram, arthralgia, pollakiuria, all n=1).
Table 3.
Adverse events potentially related to ASP9853 occurring in ≥10% of patients’
| Adverse event, n (%) | Grade | ASP9853 Lead-in (n=20) | Cohort 1 (n=6) | Cohort 2 (n=4) | Cohort 3 (n=3) | Cohort 4 (n=7) | Totala (n=20) |
|---|---|---|---|---|---|---|---|
| Overall | All | 7 (35) | 5 (83) | 3 (75) | 3 (100) | 7 (100) | 18 (90) |
| 3/4 | 0 | 2 (33) | 3 (75) | 2 (67) | 4 (57) | 11 (55) | |
| Fatigue | All | 0 | 2 (33) | 3 (75) | 2 (67) | 2 (29) | 9 (45) |
| 3/4b | 0 | 0 | 1 (25) | 0 | 1 (14) | 2 (10) | |
| White blood cell count decreased | All | 0 | 1 (17) | 0 | 2 (67) | 2 (29) | 5 (25) |
| 3/4b | 0 | 1 (17) | 0 | 2 (67) | 1 (14) | 4 (20) | |
| Neutropenia | All | 0 | 1 (17) | 0 | 1 (33) | 2 (29) | 4 (20) |
| 3/4 | 0 | 1 (17) | 0 | 1 (33) | 2 (29) | 4 (20) | |
| Neutrophil count decreased | All | 0 | 0 | 0 | 1 (33) | 1 (14) | 2 (10) |
| 3/4 | 0 | 0 | 0 | 1 (33) | 1 (14) | 2 (10) | |
| Febrile neutropenia | All | 0 | 0 | 2 (50) | 0 | 0 | 2 (10) |
| 3/4 | 0 | 0 | 2 (50) | 0 | 0 | 2 (10) | |
| Diarrhea | All | 0 | 1 (17) | 2 (50) | 1 (33) | 0 | 4 (20) |
| 3/4b | 0 | 0 | 1 (25) | 0 | 0 | 1 (5) | |
| Nausea | All | 1(5) | 0 | 2 (50) | 1 (33) | 1 (14) | 4 (20) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vomiting | All | 1 (5) | 0 | 3 (75) | 0 | 1 (14) | 4 (20) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anemia | All | 0 | 1 (17) | 1 (25) | 0 | 1 (14) | 3 (15) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Constipation | All | 0 | 1 (17) | 0 | 1 (33) | 1 (14) | 3 (15) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dizziness | All | 0 | 0 | 0 | 1 (33) | 2 (29) | 3 (15) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Asthenia | All | 1 (5) | 0 | 2 (50) | 0 | 0 | 2 (10) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Decreased appetite | All | 0 | 0 | 1 (25) | 1 (33) | 0 | 2 (10) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 |
Total refers to ‘Total ASP9853 combination with docetaxel.’
Only Grade 3. SAF.
There were two deaths during the study: one patient died due to progressive disease between Day -7 and -2 during the ASP9853 lead-in period prior to the first dose of docetaxel. This patient had esophagogastric junction cancer with metastases to the bone, lung, liver, node and left suboccipital lobe. A second patient in Cohort 2 died as a result of sepsis, aspiration of water, respiratory failure, and hypotension 12 days after ASP9853 discontinuation. Neither of these deaths were considered to be related to either ASP9853 or docetaxel.
Three patients had AEs that led to treatment discontinuation. In Cohort 2 one patient discontinued ASP9853 on Day 14. They experienced febrile neutropenia concurrent with diarrhea; both febrile neutropenic fever and diarrhea were considered probably related to both ASP9853 and docetaxel; an episode of neutropenia in this patient was deemed not related to ASP9853, but probably related to docetaxel. As described above, another patient in Cohort 2 died. One patient in Cohort 4 permanently discontinued study drug due to disease progression and worsening chronic right shoulder pain (not treatment related).
Serious adverse events
A total of 7 patients (35%) experienced 18 serious adverse events (SAEs). Febrile neutropenia was observed in three patients (Cohort 1, n=1; Cohort 2, n=2); neutropenia and leukopenia were both reported in two patients in Cohort 4. Other SAEs, which were reported in only one patient each, were observed during the lead-in phase (atrial fibrillation), during treatment in Cohort 1 (upper abdominal pain, neck pain, metastases to central nervous system, leucocytosis), and Cohort 2 (diarrhea, fatigue, sepsis, pneumothorax, respiratory failure, aspiration, hypotension).
The SAEs considered related to ASP9853 were leukopenia (Cohort 4, n=1), febrile neutropenia (Cohort 2, n=2), neutropenia (Cohort 4, n=1), and diarrhea (Cohort 2, n=1).
Laboratory and vital evaluations
All patients experienced neutropenia during treatment. At baseline, 17 patients had normal neutrophil counts and three patients had Grade 1 neutropenia. Post-baseline, three patients had a maximum Grade 1 neutropenia; two Grade 2; six Grade 3; and eight Grade 4.
No clinically significant changes from baseline were observed in liver function tests, vital signs, or electrocardiogram (ECGs).
Drug exposure and disposition
The median (range) duration of ASP9853 exposure during the study was 49.0 days (6–203). Fifteen percent of patients had exposure of less than or equal to 28 days, whereas 60.0% of patients had exposure of 28–56 days. One and four patients had exposures of 56–78 and more than 78 days, respectively.
The four patients receiving ASP9853 for over 78 days had the following tumor types: NSCLC [last dose day: 93]; renal [last dose day: 119], prostate [last dose day: 203] and SCLC [last dose date: 132). For docetaxel, the median (range) number of cycles was 2.0 (1–9).
Treatment was interrupted in eight patients (reasons given for interruptions included febrile neutropenia, leukopenia, neutropenia and non-serious fatigue) and ASP9853 dose was reduced during the study in one patient. The primary reason for discontinuation was progressive disease (81.0%).
ASP9853 and docetaxel pharmacokinetics
For Cycle 1/Day 1 the median AUC24 for ASP9853 in cohorts 1, 2, 3 and 4 was 3769, 8839, 7075 and 6922 hr*ng/mL, respectively (Fig. 2a) and the median ASP9853 Cmax in Cohorts 1, 2, 3, and 4 were 747, 1019, 2450, and 1490 ng/mL, respectively. ASP9853 was absorbed with a Tmax within 0.5–4.2 hours. Median plasma ASP9853 concentrations 24 hours after administration were approximately 11% or less of the peak concentration. The median half-life for ASP9853 in the four cohorts ranged between 3.5 and 6.8 hours and more than 95% of ASP9853 was cleared from plasma within 24 hours after administration.
Fig. 2.

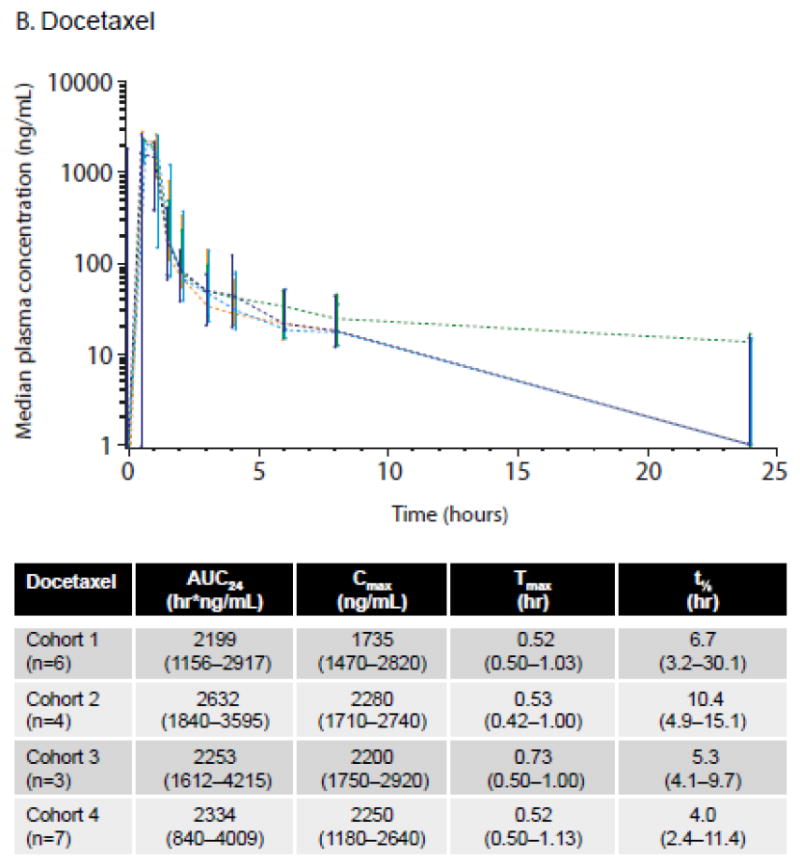
ASP9853 and docetaxel PK profiles by cohort on Cycle 1/Day 1. a. Median plasma concentration of ASP9853 (graph) and median ASP9853 PK parameters (table); b. Median plasma concentration of docetaxel (graph) and median docetaxel PK parameters (table)
Values are median (range). Data from the PKAS
The median AUC24 for docetaxel by cohort and summary of docetaxel PK parameters for Cycle 1/Day 1 are shown in Fig. 2b. The effect of docetaxel on ASP9853 PK parameters was assessed, showing that the ratio of Cmax Day -1 (no docetaxel) to Cmax Day 1 (docetaxel) ranged from 70.1 to 107.9%. Overall when administered in combination with docetaxel, ASP9853 plasma exposure, as measured by AUC24, appeared lower; however, the cohort sample size was too small to determine statistical significance (data not shown).
Pharmacokinetics and neutrophil kinetics for patients experiencing neutropenic DLTs
ASP9853 plasma concentrations in the patients who experienced neutropenic DLTs (<500 cells/mm3 for more than 7 consecutive days), their ASP9853 plasma concentrations were compared with those in other patients within the cohort and with the overall median concentration achieved in the cohort (Fig. 3). There was high interpatient variability with no clear difference in ASP9853 PK profiles observed between patients with and without neutropenic DLTs.
Fig. 3.
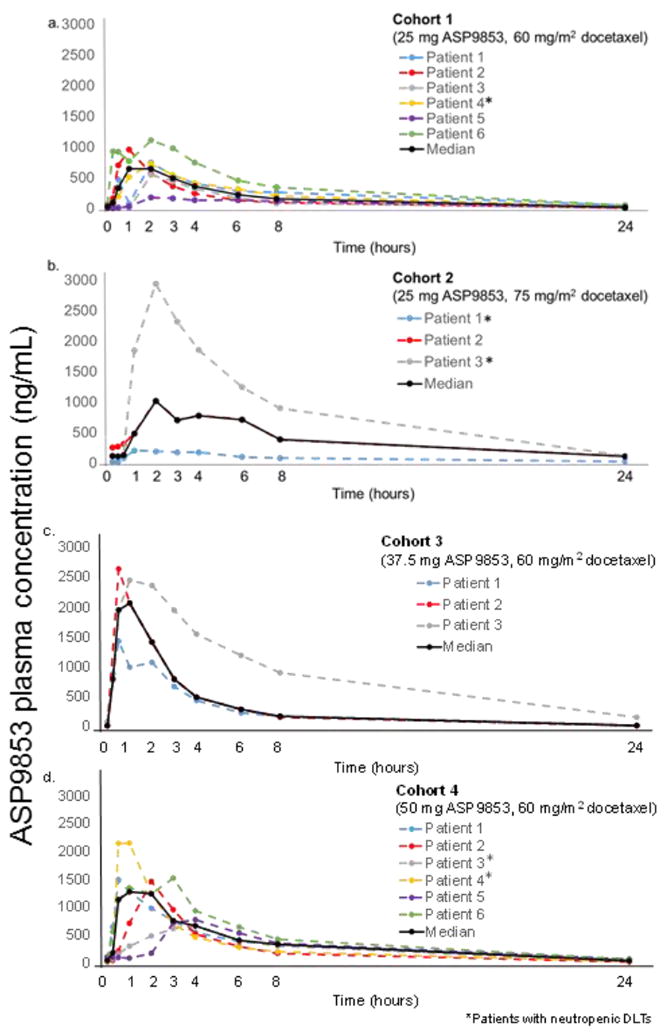
By-patient and median ASP9853 plasma concentration (Cycle 1, Day 1)
Similarly, ANCs for patients experiencing neutropenic DLTs were compared with the median for the cohort (Supplemental Fig. 2).
Efficacy
Eighteen patients remained on study long enough to receive restaging scans hence were evaluable for response, though no objective radiographic responses were observed. Eight patients (38.1%) had a best overall response of stable disease, which was maintained for a mean (SD) of 49.4 (47.2) days (median [range]: 78 [1–133]) days).
Study termination
Based on the observed toxicities, inability to escalate to full dose docetaxel and lack of objective responses, the study was terminated by the sponsor without determination of MTD and RP2D.
Discussion
This was a Phase I study investigating the iNOS inhibitor ASP9853 in combination with docetaxel for the treatment of solid tumors, in which toxicity limited both dose escalation and further clinical development. This clinical study followed preclinical investigations finding a greater degree of tumor growth inhibition with ASP9853 plus docetaxel versus docetaxel alone. This first-in-human Phase I study investigated whether ASP9853 plus docetaxel showed any activity against advanced solid tumors in vivo and was well tolerated.
Pharmacokinetic parameters in patients with advanced solid tumors showed that ASP9853 plasma concentrations peaked quickly (within 1–2 hours after administration) with a half-life between 3 and 6 hours. When ASP9853 was administered in combination with docetaxel, ASP9853 plasma exposure appeared lower; however, the statistical significance of this finding was difficult to determine due to the small number of subjects in each cohort. The docetaxel PK, when in combination with ASP9853, appeared similar to that reported for docetaxel alone [29].
Although fever and neutropenia are common with taxane treatment, and the overall kinetics of neutropenia appeared similar to that of docetaxel alone, ASP9853 appeared to potentiate the neutropenia and leukopenia associated with docetaxel, as the severity and duration of such events were marked in some patients and more than would be expected from docetaxel alone [30]. Despite no changes in docetaxel exposure, a mechanism is not immediately clear, hence this toxicity suggests that iNOS inhibition may have direct effects on the myeloid compartment and potentiate taxane-induced neutropenia.
The combination of ASP9853 and docetaxel did not produce objective responses, and stable disease achieved was of modest duration. The ASP9853 concentrations predicted for efficacy based on the preclinical data were not achieved in this clinical study as dose escalation was limited by the increase in docetaxel-related toxicity above what would be expected from docetaxel alone. In addition, to mitigate toxicity, the docetaxel dose was reduced to 60 mg/m2, which is below the standard of care (75 mg/m2). It is also important to note, however, that some of the included patients had tumor types where taxanes are known to be of limited benefit (eg. melanoma) or who had previously progressed on a taxane-based regimen (50% in this study).
These likely impacted the antitumor activity observed and emphasize that modulation of the iNOS pathway may limit dosing of taxane chemotherapy. Despite the preclinical evidence for the attenuation of tumor cytotoxicity of anticancer drugs by NO [18-21], the attenuation of docetaxel by ASP9853 was not substantiated in this clinical study due to toxicity and lack of efficacy. These findings may impact other such combinations of iNOS inhibitors and chemotherapeutic agents investigated in a clinical setting.
Based on the experience in this trial, iNOS inhibitors may be difficult to administer in combination with standard chemotherapy. A more productive approach may involve combinations with immune checkpoint blockade. iNOS plays a role in the activation and recruitment of myeloid-derived suppressor cells (MDSCs); therefore, iNOS inhibition may reverse MDSC accumulation and consequent immunosuppression [31-34]. Currently, another iNOS inhibitor, RTA408, is being studied in combination with ipilimumab in patients with advanced melanoma [https://clinicaltrials.gov/ct2/show/NCT02259231]).
In conclusion, inhibition of iNOS in combination with taxane chemotherapy was not found to be feasible in patients with advanced solid tumors. The therapeutic value of such an approach could not be fully investigated and alternative approaches for combining chemotherapy with modulation of NO biology will be necessary, in addition, further insight into the mechanisms of the neutropenia observed will be required. Manipulation of the iNOS pathway, with or without chemotherapy, appears to be more challenging than initially anticipated.
Supplementary Material
Acknowledgments
We would like to thank Michelle Utton-Mishra, Choice Healthcare Solutions, for medical writing assistance, funded by Astellas Pharma Inc.
Conflict of interest
JL has no COIs; PLR has received consultancy/advisory fees from Astellas, Pfizer, Genetech and Astex; GIS reports funding to the Dana-Farber Cancer Institute Early Drug Development Center for conduct of the study; JI has not COIs; AK, RS, TY, AF and CD are employees of Astellas Pharma Global Development, Inc.
References
- 1.Sessa WC. Molecular control of blood flow and angiogenesis: Role of nitric oxide. J Thromb Haemost. 2009;1(7 Suppl):35–37. doi: 10.1111/j.1538-7836.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4:3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DD, Miranda KM, Colton CA, Citrin D, Espey MG, Wink DA. Heme proteins and nitric oxide (NO): The neglected, eloquent chemistry in no redox signaling and regulation. Antioxid Redox Signal. 2003;5:307–317. doi: 10.1089/152308603322110887. [DOI] [PubMed] [Google Scholar]
- 5.Landar A, Darley-Usmar VM. Nitric oxide and cell signaling: Modulation of redox tone and protein modification. Amino Acids. 2003;25:313–321. doi: 10.1007/s00726-003-0019-7. [DOI] [PubMed] [Google Scholar]
- 6.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 7.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 8.Predonzani A, Cali B, Agnellini AH, Molon B. Spotlights on immunological effects of reactive nitrogen species: When inflammation says nitric oxide. World J Exp Med. 2015;5:64–76. doi: 10.5493/wjem.v5.i2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janakiram NB, Rao CV. Inos-selective inhibitors for cancer prevention: Promise and progress. Future Med Chem. 2012;4:2193–2204. doi: 10.4155/fmc.12.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–373. [PMC free article] [PubMed] [Google Scholar]
- 11.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin Cancer Biol. 2005;15:277–289. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Hibbs J, Taintor R, Vavrin Z, Granger D, Drapier J-C, Amber I, Lancaster J. Synthesis of nitric oxide from a terminal guanidino nitrogen atom of l-arginine: A molecular mechanism regulating cellular proliferation that targets in intracellular iron. In: Moncada s, Higgs E., editors. Nitric oxide from l-arginine: A bioregulatory system. Elsevier; Amsterdam: 1990. pp. 189–223. [Google Scholar]
- 13.Albina JE, Reichner JS. Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 1998;17:39–53. doi: 10.1023/a:1005904704618. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. [PubMed] [Google Scholar]
- 16.Takahashi M, Fukuda K, Ohata T, Sugimura T, Wakabayashi K. Increased expression of inducible and endothelial constitutive nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997;57:1233–1237. [PubMed] [Google Scholar]
- 17.Grimm EA, Sikora AG, Ekmekcioglu S. Molecular pathways: Inflammation-associated nitric-oxide production as a cancer-supporting redox mechanism and a potential therapeutic target. Clin Cancer Res. 2013;19:5557–5563. doi: 10.1158/1078-0432.CCR-12-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanvorachote P, Nimmannit U, Stehlik C, Wang L, Jiang BH, Ongpipatanakul B, Rojanasakul Y. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through s-nitrosylation and inhibition of bcl-2 ubiquitination. Cancer Res. 2006;66:6353–6360. doi: 10.1158/0008-5472.CAN-05-4533. [DOI] [PubMed] [Google Scholar]
- 19.Engels K, Knauer SK, Loibl S, Fetz V, Harter P, Schweitzer A, Fisseler-Eckhoff A, Kommoss F, Hanker L, Nekljudova V, Hermanns I, Kleinert H, Mann W, du Bois A, Stauber RH. NO signaling confers cytoprotectivity through the survivin network in ovarian carcinomas. Cancer Res. 2008;68:5159–5166. doi: 10.1158/0008-5472.CAN-08-0406. [DOI] [PubMed] [Google Scholar]
- 20.Fetz V, Bier C, Habtemichael N, Schuon R, Schweitzer A, Kunkel M, Engels K, Kovacs AF, Schneider S, Mann W, Stauber RH, Knauer SK. Inducible NO synthase confers chemoresistance in head and neck cancer by modulating survivin. Int J Cancer. 2009;124:2033–2041. doi: 10.1002/ijc.24182. [DOI] [PubMed] [Google Scholar]
- 21.Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, Hailemichael Y, Jayaraman P, Myers JN, Grimm EA, Overwijk WW. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16:1834–1844. doi: 10.1158/1078-0432.CCR-09-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang HX, Deng C, Liu OS, Liu XL, Wu F, Wang JJ, Feng YQ, Hu CH, Tang ZG. Inducible nitric oxide inhibitor enhances the anti-tumor effect of cisplatin on CNE-2 cells by inducing cell apoptosis. Eur Rev Med Pharmacol Sci. 2014;18:2789–2797. [PubMed] [Google Scholar]
- 23.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the tax 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 25.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 non-small cell lung cancer study group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 26.Chida N, Hirasawa Y, Ohkawa T, Ishii Y, Sudo Y, Tamura K, Mutoh S. Pharmacological profile of FR260330, a novel orally active inducible nitric oxide synthase inhibitor. Eur J Pharmacol. 2005;509:71–76. doi: 10.1016/j.ejphar.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Kenmotsu H, Tanigawara Y. Pharmacokinetics, dynamics and toxicity of docetaxel: Why the japanese dose differs from the western dose. Cancer Sci. 2015;106:497–504. doi: 10.1111/cas.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell PL, Basser R, Chipman M, Grigg A, Cebon J, Davis ID, Zalcberg J, Ng S, Appia F, Green M. A phase I dose-escalation study of docetaxel with granulocyte colony-stimulating factor support in patients with solid tumours. Ann Oncol. 2003;14:788–794. doi: 10.1093/annonc/mdg202. [DOI] [PubMed] [Google Scholar]
- 31.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit t cell responses by an no-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 33.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayaraman P, Parikh F, Lopez-Rivera E, Hailemichael Y, Clark A, Ma G, Cannan D, Ramacher M, Kato M, Overwijk WW, Chen SH, Umansky VY, Sikora AG. Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. J Immunol. 2012;188:5365–5376. doi: 10.4049/jimmunol.1103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


