Pre-exposure prophylaxis (PrEP) with daily tenofovir disoproxil fumarate (TDF), alone or in fixed-dose combination with emtricitabine (FTC/TDF), decreases the risk of sexual transmission of HIV.1-5 In HIV-infected individuals, there is a small risk of acute kidney injury and proximal tubulopathy,6-8 and cumulative TDF exposure has been associated with decreased glomerular filtration rate (eGFR).9,10 In clinical trials of healthy HIV-uninfected adults receiving TDF-containing PrEP, there was no statistically significant increase in renal adverse events; however, the trials were not powered to detect rare safety events.2-5,11-16 Secondary analyses of three large PrEP trials demonstrated small but statistically significant declines in eGFR or calculated creatinine clearance (CrCl) in participants assigned to active PrEP.17-19 We performed a systematic review and meta-analysis of randomized, placebo-controlled trials to assess the risk of renal adverse events in healthy subjects receiving TDF-containing PrEP.
This meta-analysis was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.20 Detailed methods are provided in the Supplementary Appendix. We included randomized clinical trials evaluating daily TDF-based PrEP, alone or in combination with FTC. All studies used a graded elevation in serum creatinine as the primary measure of renal adverse events. Most studies used the National Institutes of Health Division of AIDS (DAIDS) toxicity table;21 several studies modified the DAIDS definition (Supplementary Table 1). For the pooled analysis, we defined the endpoint as any Grade 1 or higher elevation in serum creatinine as reported by the study investigators. Analyses were based on the number of participants with at least one graded creatinine elevation, divided by the number at risk as defined by the study investigators. We also considered a modified intention to treat analysis including only HIV-negative participants who were dispensed at least one dose of study drug.
Data were analyzed using Stata 11 (Stata Corp LP) and RevMan 5 (The Cochrane Collaboration). We chose a conservative analytic approach using random effects due to differences in patient population, duration of PrEP exposure, loss-to-follow up and adherence, and frequency of creatinine assessment across studies. Results are expressed as the pooled odds ratio (OR); we re-expressed this result as number needed to harm to illustrate the magnitude of risk. Potential heterogeneity in the estimated effect of PrEP versus placebo was explored via random-effects meta-regression.
We identified 2657 potential articles, and 10 studies were eligible for inclusion (Supplementary Table 1 and Supplementary Figure 1). Analysis of publication quality is summarized in Supplementary Table 2. The included studies enrolled a total of 19,507 HIV-positive participants, including 17,220 participants randomized to daily oral PrEP (n=9913) versus placebo (n=7307). Eight studies followed participants for 12 months or longer, while two small studies followed participants for only 4 months. No individual study demonstrated a statistically significant difference in the odds of graded creatinine elevation between participants assigned to active PrEP versus placebo (Figure 1).
Figure 1.
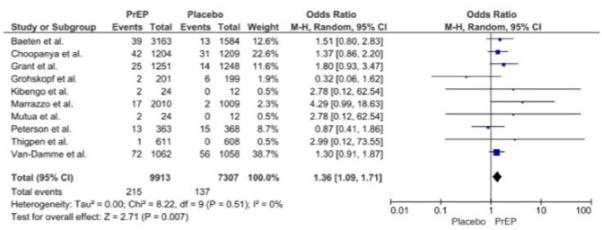
Daily oral PrEP and graded creatinine events: forest plot
The majority of elevations were Grade 1 (1.1-1.3 x upper limit of normal, ULN) regardless of treatment assignment. Of the 352 participants assigned to daily active PrEP or placebo who experienced a graded creatinine elevation, only 23 experienced Grade 2 elevations (1.4-1.8 x ULN), including 16 assigned to PrEP and 7 to placebo. Eight participants experienced Grade 3 or 4 elevations (≥1.9 x ULN), 4 in each group; six of those participants were enrolled in a single study evaluating persons who inject drugs.2
Although we did not detect significant statistical heterogeneity across trials (I2=0%, P= 0.51), we chose a conservative approach using random effects due to relevant differences between the studies. In meta-analysis, participants assigned to daily TDF-based PrEP versus placebo had a modestly increased risk of Grade 1 or higher creatinine events (pooled OR=1.36, 95% CI 1.09-1.71; Figure 1). The absolute risk increase was small (pooled risk increase 0.6%; 95% CI 0.1-1.2%), with a number needed to harm=167. In sensitivity analysis, excluding the study with the highest weight and highest number of events14 reduced our statistical power, but resulted in a similar pooled estimate (pooled OR=1.40, 95% CI 1.05-1.88). Standardizing the number at risk across all studies to include only participants who were HIV-negative at baseline and who were dispensed at least one dose of study drug also resulted in similar results (pooled OR=1.37, 95% CI 1.09-1.71). Finally, the results were similar using a fixed effects model (pooled OR=1.38, 95% CI 1.121-1.72).
We found no evidence of publication bias (Supplementary Figures 3 and 4). In meta-regression, there was no statistically significant heterogeneity across the studies due to variability in age, sex, follow-up time, or self-reported adherence (Supplementary Table 3).
In this meta-analysis of randomized trials, assignment to TDF-based PrEP was associated with a modest increase in the risk of graded creatinine events in HIV-negative individuals. Nonetheless, the absolute risk increase was small (number needed to harm 167), and the majority of events in these carefully monitored clinical trial participants were mild. Our findings are consistent with the results of secondary analyses reported by three trials of TDF-based PrEP, which demonstrated small but statistically significant declines in eGFR or CrCl in participants assigned to active PrEP.17-19 The clinical significance of the declines in kidney function is unclear, as most were mild and self-limited or resolved after stopping PrEP.17 Our findings expand on those studies by confirming that the small but significant effects observed in those studies are representative of the available renal safety data for daily oral PrEP.
In clinical practice, PrEP recipients may not be monitored as closely, and PrEP may be given to individuals with other risk factors for kidney disease, who were excluded from the clinical trials. Data on the risk of renal adverse events during implementation of PrEP will be important. In the interim, the decision to prescribe PrEP should weigh the small risk of kidney injury against the risk of HIV infection in a given individual. In a meta-analysis of PrEP efficacy including 6 of the 10 trials included in our analysis, the absolute risk reduction for HIV infection was 2.04% overall (number needed to treat 49).22 The number needed to treat is even smaller among those at highest risk for HIV acquisition,23,24 supporting a favorable risk: benefit ratio when PrEP is used by healthy individuals at substantial risk of acquiring HIV infection.
An important limitation of this analysis is the reliance on serum creatinine as the only indicator of kidney function. We were unable to evaluate the effect of PrEP on proximal tubulopathy, as comprehensive evaluation of proximal tubular function has been performed in only a small subset of PrEP trial participants to date.17 Although serum phosphorus was routinely measured as a surrogate marker of proximal tubular function, the clinical relevance of mild hypophosphatemia is unclear because of the influence of dietary intake, intra-individual variability in results, and the overlap of DAIDS toxicity criteria with normal reference values for serum phosphorus. There are also differences in the serum creatinine assays used across studies, but these differences should have minimal impact on intra-individual variation in creatinine.25
All meta-analyses are subject to limitations of the original studies. Although not statistically significant, there was a high degree of heterogeneity between the studies with respect to patient population, duration of PrEP exposure, and frequency of creatinine assessment. These differences may explain some of the variability in event rates. Although self-reported study drug adherence was high in all studies, pharmacological evidence of adherence ranged from 30% to 80% across studies.3,5,13,14,16 Low adherence in some studies may have attenuated the risk of renal adverse events. Because objective measures of adherence were only available for a subgroup of participants, the current analysis is based on intention to treat. Finally, studies varied in defining the population at risk; pooled results were similar in a modified intention to treat analysis including only HIV-negative participants dispensed at least one dose of study drug.
Our results were similar using random effects and fixed effect models. Regardless of the model used, individual studies are assigned a weight inversely proportional to the within-study variance, such that the study with the most precise estimate of effect size is assigned the highest weight. The assignment of weights does not consider other relevant factors such as duration of exposure or adherence to study drug. Follow-up time and self-reported adherence did not contribute to significant heterogeneity in meta-regression, and removal of the study with the highest weight and lowest adherence14 yielded similar results.
These results demonstrate a small increase in the relative and absolute risk of kidney injury with daily oral PrEP, which is outweighed by the more substantial reduction in risk of HIV infection in situations where PrEP is indicated. Experience with PrEP in individuals with traditional risk factors for kidney disease is limited, as individuals with hypertension, diabetes mellitus, or concomitant nephrotoxic medications were excluded. More frequent monitoring of kidney function is warranted in these groups until more data are available.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the contribution of the investigators who have generously provided or confirmed data from the included studies.
Funding Sources: This work was supported in part by funding from the National Institutes of Health, grants T32DK00775716 (GNN), R01 DK100272 (CMW, KKM, JMB), and P01DK056492 (CMW).
Disclosures: CMW has received honoraria from Bristol-Myers Squibb and her institution has received research funding related to emtricitabine/ tenofovir disoproxil fumarate from the National Institutes of Health and, indirectly through a subcontract from another academic institution, from Gilead Sciences. Gilead Sciences has donated study medication, without other funding, to research studies directed by RMG and JMB.
References
- 1.Centers for Disease C, Prevention Update to Interim Guidance for Preexposure Prophylaxis (PrEP) for the Prevention of HIV Infection: PrEP for injecting drug users. MMWR. Morbidity and mortality weekly report. 2013;62(23):463–465. [PMC free article] [PubMed] [Google Scholar]
- 2.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Touzard Romo F, Smeaton LM, Campbell TB, et al. Renal and metabolic toxicities following initiation of HIV-1 treatment regimen in a diverse, multinational setting: a focused safety analysis of ACTG PEARLS (A5175) HIV clinical trials. 2014;15(6):246–260. doi: 10.1310/hct1506-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitisci L, Demeester R, Legrand JC. Prevalence and European AIDS Clinical Society (EACS) criteria evaluation for proximal renal tubular dysfunction diagnosis in patients under antiretroviral therapy in routine setting. J Int AIDS Soc. 2014;17(4 Suppl 3):19564. doi: 10.7448/IAS.17.4.19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta SK, Anderson AM, Ebrahimi R, et al. Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PLoS One. 2014;9(3):e92717. doi: 10.1371/journal.pone.0092717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24(11):1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 10.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutua G, Sanders E, Mugo P, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103. doi: 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grohskopf LA, Chillag KL, Gvetadze R, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64(1):79–86. doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 13.Kibengo FM, Ruzagira E, Katende D, et al. Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: a randomized, clinical trial. PLoS One. 2013;8(9):e74314. doi: 10.1371/journal.pone.0074314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS clinical trials. 2007;2(5):e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014;28(6):851–859. doi: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175(2):246–254. doi: 10.1001/jamainternmed.2014.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin M, Vanichseni S, Suntharasamai P, et al. Renal function of participants in the Bangkok tenofovir study--Thailand, 2005-2012. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(5):716–724. doi: 10.1093/cid/ciu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 21.Division of AIDS table for grading the severity of adult and pediatric adverse events. 2004 Dec; http://www.niaid.nih.gov/labsandresources/resources/daidsclinrsrch/documents/daidsaegradingtable.pdf
- 22.Jiang J, Yang X, Ye L, et al. Pre-exposure prophylaxis for the prevention of HIV infection in high risk populations: a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e87674. doi: 10.1371/journal.pone.0087674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murnane PM, Celum C, Mugo N, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27(13):2155–2160. doi: 10.1097/QAD.0b013e3283629037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. The Lancet. Infectious diseases. 2014;14(6):468–475. doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peake M, Whiting M. Measurement of serum creatinine--current status and future goals. The Clinical biochemist. Reviews / Australian Association of Clinical Biochemists. 2006;27(4):173–184. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


