Abstract
The central role of HER2 as the disease driver and HER3 as its essential partner has made them rational targets for the treatment of HER2-amplifed breast cancers, and there is considerable interest in developing highly effective treatment regimens for this disease that consist of targeted therapies alone. Much of these efforts are focused on dual targeting approaches, particularly dual targeting of the HER2-HER3 tumor driver complex itself, or vertical combinations that target downstream PI3K or Akt in addition to HER2. There is also potential in lateral combinations based on evidence implicating cross-talk with other membrane receptor systems, particularly integrins, and such lateral combinations can potentially involve either HER2 or HER3. We established a preclinical model of targeting HER3 using doxycycline-inducible shRNA and determined the efficacy of a β1 integrin inhibitor in combination with targeting HER3. We report that targeting HER3 and β1 integrin provides a particularly effective combination therapy approach for HER2-amplified cancers, surpassing the combination of HER2 and β1 integrin targeting, and evading some of the safety concerns associated with direct HER2-targeting. This further validates HER3 as a major hub mediating the tumorigenic functions of HER2 and identifies it as a high value target for lateral combination therapy strategies.
Keywords: HER2, HER3, β1 integrin, AIIB2, OS2966, Combination therapy
Introduction
Abnormalities in human epidermal growth factor (HER) family signaling are a common attribute of many human cancers. This is most directly evident in cancers wherein these receptors are found to be constitutively activated through genomic alterations such as is seen in subsets of breast cancers with amplification and overexpression of HER2 [1, 2]. These receptors function to regulate cell behavior in response to the extracellular environment. The principal extracellular determinant of their activities is their specific ligands, and the HER family of receptors is activated by a large family of ligands [2]. However, when mutationally activated or genomically amplified and overexpressed, these receptors can signal constitutively, in defiance of their requisite extracellular signals, leading to uncontrolled cellular behavior typical of the malignant state [2]. As such, the inhibition of overactive signaling using selective inhibitors provides a rational basis for the treatment of these cancers. However, these receptors must coordinate their signaling activities with other receptor systems, and cross-talk between receptor families is increasingly being recognized as a means for signal integration. Although the aberrant activation of growth factor receptor signaling through mutation or overexpression in cancers may relieve them of their requirement for extracellular ligand stimulation, it does not necessarily liberate them from their cross-talk obligations, and this dependency on other signaling systems provides yet additional opportunities for more effective cancer therapies through co-targeting strategies. One important such parallel signaling pathway is integrin signaling.
Integrins are involved in the regulation of many cellular processes including adhesion to matrix, migration, proliferation, and survival. These processes are of paramount importance to the phenotype of cancer cells and an extensive body of evidence links a variety of integrin complexes with the biology of specific types of cancers [3,4]. Although integrins are not themselves oncogenes, their control over processes critical for tumor initiation and progression makes them attractive targets for cancer therapies and a number of integrin-targeting agents are in development for the treatment of cancer [5]. The ligation of integrin receptors to their extracellular ligands affects growth and proliferation through the regulation of a number of intracellular signaling pathways including the Ras-MAPK and PI3K-Akt signaling pathways [6]. These pathways are also regulated by growth factor receptors, and there is mounting evidence that the proliferative phenotype of many cancers is dependent on the coordinate regulation of these pathways by integrins as well as growth factor receptors [7, 8]. However, the mechanism of cross-talk between growth factor receptors and integrins remains poorly defined. The importance of this cross-talk is most evident in cancers driven by growth factor receptor oncogenes, with particularly compelling evidence in HER2-driven models of cancer. Mouse genetic models reveal that β4 integrin is essential for initiation of tumorigenesis induced by overexpression of HER2, and β1 integrin is essential for the progression of these tumors [9, 10]. The important role of these integrins in the biology of HER2-driven breast cancers makes a compelling case for combined targeting of HER2 and integrins in the treatment of HER2-amplified breast cancers.
Of considerable interest in the biology of HER2-amplified breast cancers is the role of its signaling partner HER3. Although HER3 itself is kinase-inactive, it plays a critical role in HER2 signaling by recognizing the presence of ligands and initiating ligand-induced dimerization with HER2, by functioning as an allosteric activator of the HER2 kinase domain and by mediating the downstream activation of PI3K [11–13]. The functions of HER3 are essential for HER2-driven tumorigenesis and HER2-driven tumors will not develop or will not grow in the absence of HER3 expression [14–16]. Furthermore, the dynamic nature of HER3 signaling underlies resistance to HER2 inhibitors in these cancers. The inhibition of HER2 in HER2-amplified cancer cells results in a compensatory upregulation of HER3 expression and signaling that substantially increases HER2-HER3 signaling throughput, undermining the efficacy of HER2 inhibitors [17–19]. In effect, HER3 functions as the dynamic regulator of HER2-HER3 signaling output in HER2-amplified cancers. Considering the critical regulatory role of HER3 in HER2-amplified cancers, it is plausible that HER3 mediates the cross-talk with integrin signaling in this subtype of cancers.
We have been studying the interplay of HER2 and integrins in HER2-amplified breast cancers. We have shown that dual treatment of SKBR3 and BT474 cells with HER2-inhibitory antibodies in combination with a β1 integrin inhibitory antibody lead to enhanced cytostasis [20]. Furthermore, we reported that lapatinib resistance in these cancer cells is associated with an upregulation of β1 integrin and co-treatment with an inhibitor of β1 integrin can overcome lapatinib resistance [21]. Considering that HER3 is a key regulator of the HER2–HER3 tumor driver in HER2-amplified cancers, we sought to determine whether HER3 can be as good, or perhaps even a better co-target for β1 integrin. In the current body of work, we report the effects of targeting both β1 integrin and HER3 in HER2-amplified cancers and find a significant benefit to this co-targeting approach.
Methods
Cell culture
SKBR3 and HCC1569 cells were obtained from the American Type Tissue Collection (ATCC). HCC1569 M1 cells are a subclone of HCC1569 that were obtained after passage of HCC1569 cells in the mammary fat pad of nude mice and subsequent transfer and re-establishment of tumor cell growth in tissue culture dishes. Both SKBR3 and HCC1569 M1 cell lines were maintained at 37 °C and 5 % CO2. SKBR3 cells were grown in DMEM:Ham’s F-12 media, HCC1569 M1 cells were grown in RPMI 1640 with 10 mM Hepes, and both were supplemented with 10 % tetracycline-free heat inactivated fetal bovine serum (Gemini Bio Products), 1X Penicillin and streptomycin, and 2 mmol/l l-glutamine (Invitrogen).
Modified 3D laminin-rich extracellular matrix (IrECM) cell culture
We used a modified three-dimensional IrECM culture technique that more closely resembles the in vivo tumor environment compared to 2D cell culture on plastic dishes [22]. Briefly, dishes were pre-coated with 100 % growth factor-reduced basement membrane extract (matrigel; Trevigen, MD, #3433-005-01). Cells were maintained in media conditioned with 5 % matrigel and layered on top of a base layer of prepared matrigel. Using this method, cells are not completely embedded in matrigel but float on top of it allowing spheroid structures to form.
Western blotting
Cells were lysed in RIPA buffer supplemented with leupeptin, aprotinin, phenylmethylsulfonyl fluoride, sodium vanadate, and a phosphatase inhibitor cocktail (Roche 04906845001). Lysates were quantified using the Pierce BCA assay (ThermoFisherScientific). 50ug of protein lysate was separated on 4–12 % Bis-Tri gels (Life Technology, Noverx) and samples were transferred to nitrocellulose membranes and immunoblotted with the following antibodies. HER3 (Santa Cruz sc81455), phospho-HER3 (Cell Signaling 4791), AKT (BD 610837), phospho-AKT (Cell Signaling 9271), β1 Integrin (BD 610467), and β-Actin (Sigma A5441).
Inducible HER3 shRNA
A two vector system was used to create both the SKBR3 and HCC1569 M1 inducible HER3 knockdown stable cell lines. In the first step, SKBR3 and HCC1569 M1 lines stably expressing the tet operon repressor protein (TetR) were generated. The pcDNA6/TR Tet repressor plasmid (Invitrogen #V1025-20) containing the blasticidin resistance gene and a gene encoding the TetR protein was transfected into SKBR3 cells using Lipofectamine 2000 (Invitrogen). The pLenti6/TR Tet repressor construct containing the Blasticidin resistance gene and the TetR gene (Invitrogen #K4965-00) was used to make lentivirus and infect HCC1569 M1 cells. Briefly, a 10-cm tissue culture (TC) plate was coated with 0.1 % gelatin and allowed to dry overnight. Lentiviral producing 293FT cells (Invitrogen #R700-07) were transfected with a total of 39 ug DNA and 40 ul of Lipofectamine 2000 using the rapid reverse transfection protocol (15 ug, TetR construct and 3rd generation lentiviral packaging plasmids (15 ug pLP1, 6 ug pLP2, 3 ug pLP/pVSV-G)). Briefly, 5 ml of media was added to the TC dish followed by the Lipofectamine 2000:DNA complexes, and lastly 5.5 × 106 293FT cells suspended in 5 ml of media were added in a drop wise fashion. The TC plate was placed at 37°C overnight and media was changed at 24 h intervals and virus was harvested at 48 and 72 h pooled and filtered through a 0.45-um filter (Millipore). For lentiviral infection, HCC1569 M1 cells were seeded into 6-well plates and infected the following day at ~70 % confluence. 2 ml of fresh lentivirus was mixed with 8 ug/ml final concentration of hexadimethrine bromide (Sequabrene Sigma S 2667) and added to one well of the 6-well plate. Cells were spin inoculated for 45 min at 1800 rpm in an Eppendorf 5800 centrifuge and then returned to the incubator. Spin inoculation was repeated 2 more times over 48 h and cells were allowed to recover in regular media overnight. The next day cells were split into 10-cm dishes and selection for blasticidin resistance was started 72–96-h post-inoculation (5 ug/ml). Both SKBR3 and HCC1569 M1 cells were selected for blasticidin resistance and subclones were tested for TetR expression with Western blotting (MoBiTec (TET)). The clone with the highest TetR protein expression for each cell line was used. In the second step, an inducible HER3 knockdown shRNA cell line was created. HER3 targeting shRNA complementary sequences shown below were cloned into the pSuperior.neo vector (Oligoengine #VEC-IND-0012) using BglII and HindIII sites.
(5′-CCCAAGAGGATGTCAACGGTTATTCAAGAGA TAACCGTTGACATCCTCTTTTTTTAAGAGGG-3′).
(5′-CCCTCTTAAAAAAAGAGGATGTCAACGGTTA TCTCTTGAATAACCGTTGACATCCTCTTGGG -3′).
Retrovirus was made using phoenix amphotropic cells (ATCC CRL-3213). Briefly, we used the rapid reverse transfection protocol to transfect 15 ug of the pSuperior HER3 shRNA construct or scramble shRNA with 50 ul Lipofectamine 2000 into the phoenix cells and harvested retrovirus at 48 and 72-h post-transfection. 2 ml of filtered retrovirus plus 8 ug/ml sequabrene was used to spin infect both the SKBR3/TR and HCC1569M1/TR cells. G418 selection (400 ug/ml) was started 48–72-h post-infection, cells were subcloned, and clones were tested for induction of HER3 knockdown after treatment with doxycycline (dox) for 72 h (HCC1569 M1 cells) or 168 h (SKBR3 cells).
β1 Integrin inhibitory antibodies
AIIB2 (Aragen) is a rat monoclonal IgG1 antibody that has been shown to functionally inhibit integrin β1 (CD29) [23–25]. AIIB2 was added to IrECM SKBR3 cell culture at 150ug/ml or injected into the intraperitoneal cavity of mice according to the timeline described below.
Apoptosis and proliferation assays
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL assay) and Ki67 nuclear antigen immunostaining were used to measure levels of apoptosis and proliferation in cell culture, respectively. For the TUNEL assay, a commercially available kit (In Situ Cell Death Detection kit, fluorescein; Roche, NJ) was used according to the manufacture instructions. After fixation in 4 % paraformaldehyde, cells were permeabilized in cold 0.1 % Triton X-100 in 0.1 % sodium citrate. Cells were then washed in PBS and incubated in TUNEL reaction mixture at 37 °C for 1 h before mounting the slides. Ki67 immunofluorescence (IF) staining of 3D lrECM was done following previously described protocol [22]. Briefly, samples were fixed in 1:1 methanol/acetone solution for 10 min at −20 °C and air dried. Cells were then washed in PBS and blocked with 10 % gold serum in IF buffer for 1 h at room temperature (RT). After samples were treated with 1:250 dilution of goat anti-mouse IgG Fab fragments (Life Technology, #24526), Ki67 primary antibody (1:500; VPK451) was applied over night at 4 °C. The following day, cells were washed with IF buffer and FITC-conjugated anti-rabbit secondary antibody (1:500, Life Technology) was applied for an additional 1 h at RT. Cells were washed and stained with DAPI (Sigma, #D1388) for 5 min before mounting the slides. Number of nuclei staining positive for Ki67 was determined using Zeiss microscopy with Axio-Cam HRm.
In vivo tumor studies
7–8-week-old female athymic nude mice (Taconic Farms Inc.) were used for xenograph mouse experiments. Mice were cared for under a protocol approved by the Institutional Animal Care and Use Committee at UCSF. Lapatinib was purchased from (GlaxoSmithKline) and pulverized tablets were administered as a suspension in 0.5 % hydroxypropylmethylcellulose and 0.2 % Tween 80 by oral gavage in two daily doses. AIIB2 was purchased from Aragen. Doxycycline was purchased from Sigma. Mice were inoculated s.c. with 4 × 106 HCC1569TR/shHER3 cells in 50 % phenol-free matrigel. When average tumor size of the entire cohort reached ~150 mm3 mice were randomized and treated according the one of several experimental arms or vehicle control. In the HER3-targeting arm, mice were treated with 200 ug/ml doxycycline in drinking water. In the β1 integrin targeting arm, mice were treated with AIIB2 at 1 mg/kg given intraperitoneally twice per week. In the HER2-targeting arm, mice were treated with lapatinib at 50 mg/kg twice daily by oral gavage. Mice were monitored for signs of toxicity and tumor length, width, and diameter was measured twice weekly and tumor volumes were calculated.
Certain mice were sacrificed and tumors harvested for analysis by flash-freezing and by fixation and paraffin embedding. Flash frozen tumor samples were processed into powder while still frozen using a BioPulverizer (BioSpec #59012MS), and the tissue powder was immediately lysed in RIPA buffer supplemented with leupeptin, aprotinin, phenylmethylsulfonyl fluoride, sodium vanadate, and a phosphatase inhibitor cocktail (Roche 04906845001) and used for gel separation and immunoblotting. For histology, tumor samples were fixed in 10 % neutral buffered formalin and embedded in paraffin. 5-μm tissue sections were dewaxed and stained with hematoxylin and eosin (H&E) according to standard methods. Microscopy and imaging were performed with a Zeiss microscope using an AxioCam HRm camera.
Results
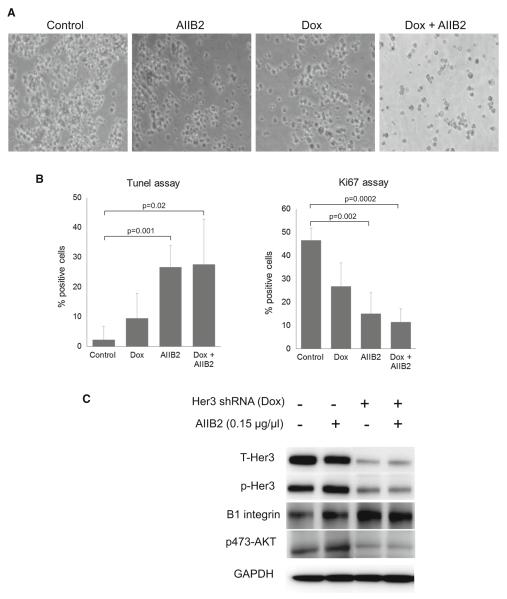
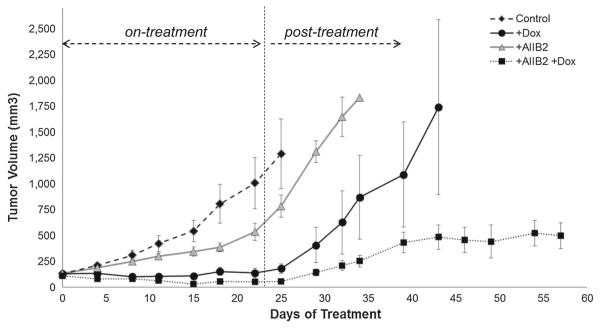
The HER2-amplified SKBR3 breast cancer cells expressing doxycycline-inducible HER3 shRNA were used in 3D culture to study the effects of HER3 inhibition. Although HER3 shRNA is highly effective at eliminating HER3 expression, its effects on proliferation and apoptosis are modest, with a 10 % induction of apoptosis and a 45 % reduction in proliferative activity (Fig. 1a, b). Interestingly, knockdown of HER3 results in a compensatory and significant upregulation of integrin β1 (Fig. 1c). This suggests a linkage in the signaling pathways driven by these receptors and led us to investigate the effects of targeting integrin β1 inhibitor alone or concomitant with knockdown of HER3 in these tumor cells. Treatment of SKBR3 cells with the β1 integrin blocking antibody AIIB2 by itself produces a 66 % reduction of proliferative activity and a 25 % induction of apoptotic activity (Fig. 1a, b). The combination of HER3 knockdown and integrin β1 inhibition does not further increase apoptotic activity over integrin β1 inhibition alone, and additive anti-proliferative activity does not reach statistical significance in these in vitro assays (Fig. 1a, b). Similar results were seen when wildtype HCC1569 cells were compared with HCC1569 cells wherein HER3 expression has been eliminated by CRISPR/Cas targeting of the HER3 gene (Supplementary figure S1). These data suggested to us that co-targeting HER3 and integrin β1 may be of particular interest and have specific benefit in the treatment of HER2-amplified cancer cells. To test this, we studied this combination approach on tumor growth in vivo. Since SKBR3 cells are not tumorigenic in mouse models, we used HCC1569 HER2-amplified breast cancer cells as the model. These cells were similarly engineered to express a doxycyclineinducible HER3 shRNA leading to elimination of HER3 expression upon exposure to doxycycline (Supplementary Figure S2). Mice were inoculated with HCC1569TR-shHER3 cells and upon the development of tumors, they were randomly assigned to treatment arms including control, oral doxycycline in drinking water, AIIB2 administered intraperitoneally, or both. Treatments were continued for a duration of 3 weeks. During this time, AIIB2 treatment had only a modest effect on tumor growth, whereas HER3 knockdown had a more profound effect with near complete inhibition of tumor growth (Fig. 2). However, the combined treatment produced the most profound anti-tumor effects with actual tumor regression, which was not observed with either treatment alone. After cessation of drug therapies at 3 weeks, the mice were continually observed and the re-growth of their tumors was continuously measured. The mice treated with either HER3 knockdown or integrin β1 inhibition experienced rapid tumor re-growth, which was similar in rate to untreated controls. However, a similar recovery of tumor growth was not seen in mice that had been treated with the combination therapy approach (Fig. 2). Tumor growth in these mice continued to be substantially impaired compared with controls, despite the absence of continued treatment, indicating an anti-tumor effect of combination therapy that persists beyond cessation of therapy. While the control and monotherapy arms had to be terminated at days 25, 33, and 44, in compliance with our institutional guidelines for maximum tumor size, the combination therapy arm was terminated at day 58 as there was no evidence of continued tumor progression, having been off therapy for more than 3 weeks, and with tumors not growing any further and their sizes well beneath the regulatory ceiling of 1000 mm3. Looking at the efficacy of AIIB2 treatment on these tumors, it is apparent that while this β1 integrin targeted therapy by itself has minimal anti-tumor efficacy, it enhances the efficacy of HER3-targeting in a profound way.
Fig. 1.
Effect of targeting HER3 or β1 integrin on growth of SKBR3 cells in 3D lrECM culture. a SKBR3 cells were grown in 3D lrECM culture according to the indicated treatment arms for 7 days. Phase contrast microscopy images were taken at 10× magnification. b Apoptotic and proliferative activity of the cells were determined on day 7 using the Tunnel (left panel) and Ki67 (right panel) assays. Results shown are the average of three experiments with the indicated standard error of the mean. Significance values were calculated using the unpaired T test. c SKBR3 cells grown in 3D lrECM culture were harvested on day 2 of treatment and cell lysates used for immunoblotting as indicated. This figure depicts the fusion of image sections from the same film with removal of extraneous lanes from the center
Fig. 2.
Effect of targeting HER3 or β1 integrin on in vivo growth of HCC1569 tumors. Nude mice bearing HCC1569TR-shHER3 tumor xenografts were randomly assigned to one of four treatment arms consisting of control (n = 11), oral doxycycline (200 ug/ml) in drinking water (n = 12), AIIB2 administered twice weekly IP (1 mg/kg) (n = 12), or both (n = 12). Treatments were terminated after three weeks and the mice were continuously monitored thereafter in the post-treatment phase. Error bars indicated the standard error of the mean
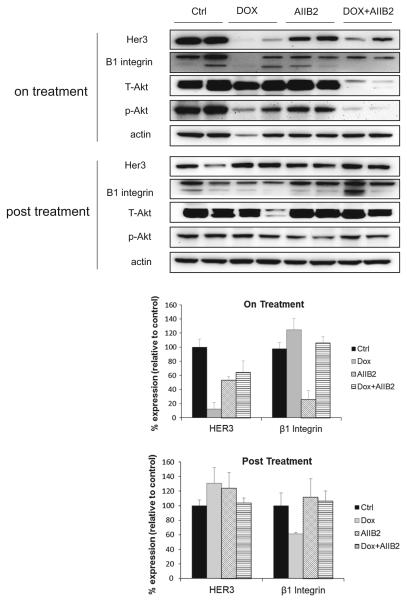
Representative mice from each group were sacrificed while on treatment and their tumors resected for immunoblotting and sectioning. This analysis confirms that the knockdown of HER3 induced by doxycycline treatment (Fig. 3). There is also a reduction in HER3 expression seen with AIIB2 treatment that was not seen in in vitro studies and may be unique to the in vivo growth environment. There is an apparent more effective suppression of Akt signaling with combined HER3 and β1 integrin targeting when compared with either one alone, although this could be due to reduced tumor cellularity in these tumors. On H&E sections, there is reduced tumor cellularity evident during either of the treatment arms and increased cellularity in the post-treatment period (Supplementary Figure S3).
Fig. 3.
Effect of targeting HER3 or β1 integrin on tumor signaling in vivo. Two representative mice from each treatment arm of Fig. 2 were sacrificed and tumor lysates were assayed by Western blotting as indicated. The relative intensities of the HER3 and β1 integrin bands are quantitatively shown below as an average of the two samples in each arm
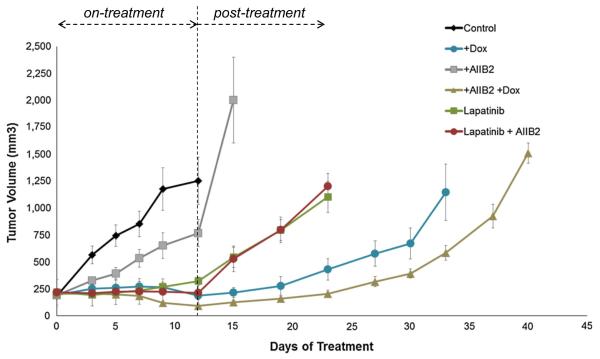
As discussed above, mouse genetic models reveal an important role for β1 integrin in the growth and progression of HER2-driven tumors and suggests cross-talk between β1 integrin and HER2 signaling. This suggests that targeting HER2 and β1 integrin together may be particularly effective. Since the HER2-HER3 complex is the functionally relevant driver of HER2-driven cancers and HER3 is the dynamic regulator of this complex, it is possible that the functionally important cross-talk of β1 integrin is with HER3 rather than HER2. As such, it is possible that targeting HER3 and β1 integrin may be even more beneficial than targeting HER2 and β1 integrin. We directly compared these modes of co-targeting in mice bearing doxycycline-inducible HCC1569TR-HER3shRNA tumor xenografts. After tumors were established, mice were randomly assigned to 5 treatment arms and a control arm. These included three monotherapy arms targeting HER2 (lapatinib), HER3 (doxycycline), or β1 integrin (AIIB2) and 2 dual therapy arms targeting β1 integrin with either HER2 or HER3. From these experiments, it is evident that dual targeting of β1 integrin and HER3 is superior to dual targeting of β1 integrin and HER2 (Fig. 4). This is consistent with the notion that HER3 is the link that ties β1 integrin function to HER2-driven tumorigenic growth and suggests that dual targeting of these two receptors would be particularly advantageous in the treatment of HER2-amplified cancers, even in the absence of direct HER2-targeting.
Fig. 4.
Effect of targeting β1 integrin in combination with HER2 or HER3. Nude mice bearing HCC1569TR-shHER3 tumor xenografts were randomly assigned to control (n = 7) or one of five treatment arms consisting of oral doxycycline (200 ug/ml) in drinking water (n = 8), AIIB2 administered twice weekly IP (1 mg/kg) (n = 8), daily lapatinib by oral gavage (50 mg/kg) (n = 8), or the indicated combinations (n = 8, n = 8). Treatments were terminated after 12 days and the mice were continuously monitored thereafter in the post-treatment phase. Error bars indicated the standard error of the mean. The increased efficacy of HER3 and β1 integrin targeting compared with HER2 and β1 integrin targeting is highly significant (AIIB2 + dox vs AIIB2 + lapatinib; comparison at day 24, two-tailed t test p < 0.0001)
Discussion
There is considerable interest and a track record of success in the treatment of HER2-amplified breast cancers using targeted therapies. A number of HER2-targeting therapies including HER2-targeting antibodies and tyrosine kinase inhibitors have already become part of the clinical standard of care for the management of this disease. The efficacies of targeted therapies available thus far remain too low for use alone, and they are predominantly used in combination with cytotoxic chemotherapies. As such, there continues to be much interest in developing combinations of targeted therapies with enhanced efficacies such as to obviate the requirement for chemotherapeutics, or further enhance the efficacies of combination therapies in the management of HER2-amplified cancers. Here we report a highly favorable co-targeting strategy that involves targeting HER3 and β1 integrin. This is based on considerable evidence that links HER family signaling with integrin signaling and that links the signaling and tumorigenic functions of HER2 with HER3. The mouse genetic models that reveal the important role of β1 integrin in the progression of HER2-driven tumors [9] already suggest an interdependency between these two receptor systems and a suggestion that co-targeting β1 integrin with HER2 may be more effective than targeting HER2 alone. A relationship between β1 integrin and HER2 is also apparent in clinical studies that show elevated expression of β1 integrin in HER2-amplified breast cancers compared with non-amplified cases [26]. We have been interested in the role of β1 integrin in HER2-amplified breast cancers and previously reported that HER2-amplified cancer cells made resistant to the HER2 inhibitor lapatinib exhibit elevated expression of β1 integrin and increased FAK signaling, also consistent with cross-talk between these two receptor systems [21]. As such, co-targeting HER2 and β1 integrin is a rational strategy for combination therapy. The therapeutic index of such a combination therapy remains to be defined by clinical studies; however, additional combinations with greater efficacy or wider therapeutic index would be of interest, as there are at least theoretical concerns with co-targeting HER2 and β1 integrin. HER2-targeted therapies have a risk for inducing cardiac damage, a finding that is consistent with the role of HER2 in myocardial protection [27, 28]. β1 integrin is also expressed in the mycocardium where it plays an important function [29], and conditional mouse knockout models reveal its critical role in maintaining cardiac health [30]. Importantly, however, the functions of HER2 in mediating neuregulin signaling and promoting cardiac development and preserving cardiac health are mediated through its co-receptor HER4 [31–34]. The fact that the tumorigenic function of HER2 involves its partner HER3, yet the cardiac functions of HER2 involve its partner HER4, provides an opportunity in HER3 as a more suitable co-target for β1 integrin in the treatment of HER2-driven cancers. The data presented in this paper identify HER3-β1 integrin co-targeting as an effective and alternative approach to HER2-β1 integrin targeting and yet the abundance of evidence suggests that it would be expected to have less cardiac toxicity, providing for a highly suitable combination therapy approach.
In this study, we targeted HER3 through an shRNA approach rather than through the use of one of several HER3-targeting agents being developed in industry. This provides definitive inactivation of HER3 in order to obtain the proof-of-principle evidence that we were seeking regarding the functional interaction of HER3 and β1 integrin in these cancers. Future studies will determine whether HER3-targeting agents currently in clinical or preclinical studies can recapitulate these findings in combination with this or other β1 integrin targeting agents. But the pharmacologic inactivation of HER3 has thus far been a challenging endeavor. There are numerous HER3-targeting agents in the developmental pipelines, but the ability of any of these agents to induce a loss-of-function of HER3 in HER2-amplified cancers remains contentious. Monoclonal antibodies targeting its extracellular region can interfere with its ligand-binding or conformational activation [35–39]. These may be of particular benefit in disease states driven by ligand stimulation but they show decreased efficacy in cancers with HER2 amplification where HER3 signaling is engaged through ligand-independent mechanisms. Small molecule ATP-analog classes of inhibitors targeting its kinase domain are ineffective at inhibiting the signaling functions of HER3 [40]. This is because unlike most other receptor tyrosine kinases, its kinase domain is catalytically inactive and functions predominantly as an allosteric activator in dimeric complexes [13]. Many other approaches are underway to develop effective inhibitors of HER3 function.
β1 integrin inhibitors are currently being evaluated in preclinical and clinical studies. Among these is OS2966, the humanized version of AIIB2, which is currently in preclinical development. Our study provides preclinical rationale for combining this agent with HER3 inhibitors for the treatment of patients with HER2-amplified cancer.
Supplementary Material
Acknowledgments
MRC was supported by a AACR-Genentech Postdoctoral Fellowship and a Susan G. Komen Postdoctoral fellowship. CP was supported by National Institutes of Health R01 CA174929. MMM was supported by the National Institutes of Health R01 CA122216 and The California Breast Cancer Research Program 18IB-0030 and 16OB-0150. The project employed the services of the Helen Diller Family Comprehensive Cancer Center core facilities including the Preclinical Therapeutics Core and the mouse pathology core.
Footnotes
Compliance with ethical standards
Conflicts of Interest CP is co-founder of Oncosynergy, Ltd.
Electronic supplementary material The online version of this article (doi:10.1007/s10549-016-3698-y) contains supplementary material, which is available to authorized users.
References
- 1.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19(56):6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 2.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65(10):1566–1584. doi: 10.1007/s00018-008-7440-8. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer metastasis reviews. 2010;29(1):223–237. doi: 10.1007/s10555-010-9211-x. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 5.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9(10):804–820. doi: 10.1038/nrd3266. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 6.Cabodi S, Di Stefano P, Leal Mdel P, Tinnirello A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D, Tornillo G, Defilippi P. Integrins and signal transduction. Adv Exp Med Biol. 2010;674:43–54. doi: 10.1007/978-1-4419-6066-5_5. [DOI] [PubMed] [Google Scholar]
- 7.Beattie J, McIntosh L, van der Walle CF. Cross-talk between the insulin-like growth factor (IGF) axis and membrane integrins to regulate cell physiology. J Cell Physiol. 2010;224(3):605–611. doi: 10.1002/jcp.22183. doi: 10.1002/jcp.22183. [DOI] [PubMed] [Google Scholar]
- 8.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418(3):491–506. doi: 10.1042/BJ20081948. doi: 10.1042/bj20081948. [DOI] [PubMed] [Google Scholar]
- 9.Huck L, Pontier SM, Zuo DM, Muller WJ. β1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc Natl Acad Sci. 2010;107(35):15559–15564. doi: 10.1073/pnas.1003034107. doi: 10.1073/pnas.1003034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126(3):489–502. doi: 10.1016/j.cell.2006.05.047. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 11.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res. 2010;16(5):1373–1383. doi: 10.1158/1078-0432.CCR-09-1218. doi: 10.1158/1078-0432.ccr-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltoff SP, Carraway KL, 3rd, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14(6):3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human HER3 receptor. Proc Nat Acad Sci USA. 2009;106:21608–21613. doi: 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100(15):8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68(14):5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 16.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, Sanchez V, Koland J, Muller WJ, Arteaga CL, Cook RS. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72(10):2672–2682. doi: 10.1158/0008-5472.CAN-11-3594. doi: 10.1158/0008-5472.can-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445(7126):437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin DN, Sergina N, Ahuja D, McMahon M, Blair JA, Wang D, Hann B, Koch KM, Shokat KM, Moasser MM. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2010;2(16):16ra17. doi: 10.1126/scitranslmed.3000389. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, Manning HC, Chang J, Arteaga CL. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA. 2011;108(12):5021–5026. doi: 10.1073/pnas.1016140108. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigelt B, Lo A, Park C, Gray J, Bissell M. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122(1):35–43. doi: 10.1007/s10549-009-0502-2. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Park CC, Hilsenbeck SG, Ward R, Rimawi MF, Wang YC, Shou J, Bissell MJ, Osborne CK, Schiff R. beta1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res. 2011;13(4):R84. doi: 10.1186/bcr2936. doi: 10.1186/bcr2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. β1 Integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66(3):1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. doi: 10.1158/0008-5472.can-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takada Y, Puzon W. Identification of a regulatory region of integrin beta 1 subunit using activating and inhibiting antibodies. J Biol Chem. 1993;268(23):17597–17601. [PubMed] [Google Scholar]
- 24.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95(25):14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137(1):231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.dos Santos P, Zanetti J, Ribeiro-Silva A, Beltrao E. Beta 1 integrin predicts survival in breast cancer: a clinicopathological and immunohistochemical study. Diagnostic Pathology. 2012;7(1):104. doi: 10.1186/1746-1596-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewer MS, O’Shaughnessy JA. Cardiac toxicity of trastuzumab-related regimens in HER2-overexpressing breast cancer. Clin Breast Cancer. 2007;7(8):600–607. [PubMed] [Google Scholar]
- 28.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–465. doi: 10.1038/nm0502-459. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 29.Hornberger LK, Singhroy S, Cavalle-Garrido T, Tsang W, Keeley F, Rabinovitch M. Synthesis of extracellular matrix and adhesion through beta(1) integrins are critical for fetal ventricular myocyte proliferation. Circ Res. 2000;87(6):508–515. doi: 10.1161/01.res.87.6.508. [DOI] [PubMed] [Google Scholar]
- 30.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90(4):458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 31.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 32.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273(17):10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 33.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–394. doi: 10.1038/378390a0. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 34.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17(5):627–638. doi: 10.1038/ncb3149. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- 35.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, Fulgham A, Song Y, Nielsen UB, Engelman JA, Wong KK. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70(6):2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. doi: 10.1158/0008-5472.can-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackburn E, Zona S, Murphy ML, Brown IR, Chan SK, Gullick WJ. A monoclonal antibody to the human HER3 receptor inhibits Neuregulin 1-beta binding and co-operates with Herceptin in inhibiting the growth of breast cancer derived cell lines. Breast Cancer Res Treat. 2012;134(1):53–59. doi: 10.1007/s10549-011-1908-1. doi: 10.1007/s10549-011-1908-1. [DOI] [PubMed] [Google Scholar]
- 37.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, Gu J, Kohli N, Wallace M, Feldhaus MJ, Kudla AJ, Schoeberl B, Nielsen UB. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11(3):582–593. doi: 10.1158/1535-7163.MCT-11-0820. doi: 10.1158/1535-7163.mct-11-0820. [DOI] [PubMed] [Google Scholar]
- 38.Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li S, Sineshchekova O, Saxena P, Sutton CR, Chen D, Chen Y, Wang H, Liang J, Das R, Mosher R, Gu J, Huang A, Haubst N, Zehetmeier C, Haberl M, Elis W, Kunz C, Heidt AB, Herlihy K, Murtie J, Schuller A, Arteaga CL, Sellers WR, Ettenberg SA. An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res. 2013;73(19):6024–6035. doi: 10.1158/0008-5472.CAN-13-1198. doi: 10.1158/0008-5472.can-13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kugel CH, 3rd, Hartsough EJ, Davies MA, Setiady YY, Aplin AE. Function-blocking ERBB3 antibody inhibits the adaptive response to RAF inhibitor. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-14-0464. doi: 10.1158/0008-5472.can-14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Littlefield P, Moasser MM, Jura N. An ATP-competitive inhibitor modulates the allosteric function of the HER3 pseudokinase. Chem Biol. 2014;21(4):453–458. doi: 10.1016/j.chembiol.2014.02.011. doi: 10.1016/j.chembiol.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.