SUMMARY
Special AT-rich sequence-binding protein-1 (Satb1) governs genome-wide transcriptional programs. Using a conditional knockout mouse, we find that Satb1 is required for normal differentiation of conventional dendritic cells (DCs). Furthermore, Satb1 governs the differentiation of inflammatory DCs by regulating MHC-II expression through Notch1 signaling. Mechanistically, Satb1 binds to the Notch1 promoter, activating Notch expression and driving RBPJ occupancy of the H2-Ab1 promoter, which activates MHC-II transcription. However, tumor-driven, unremitting expression of Satb1 in activated Zbtb46+ inflammatory DCs that infiltrate ovarian tumors results in an immunosuppressive phenotype characterized by increased secretion of tumor-promoting Galectin-1 and IL-6. In vivo silencing of Satb1 in tumor-associated DCs reverses their tumorigenic activity and boosts protective immunity. Therefore, dynamic fluctuations in Satb1 expression govern the generation and immunostimulatory activity of steady-state and inflammatory DCs, but continuous Satb1 overexpression in differentiated DCs converts them into tolerogenic/pro-inflammatory cells that contribute to malignant progression.
INTRODUCTION
Special AT-rich binding protein 1 (Satb1) is a master genomic organizer that coordinates gene expression by forming loops in transcriptionally active chromatin (Cai et al., 2006; Yasui et al., 2002). Such loops bring enhancers and repressors separated by long sequences into close proximity with transcriptional start sites. Because both Satb1 and the DNA-binding zinc finger protein CTCF associates with the nuclear matrix, it is likely that they interact to construct the appropriate higher order chromatin structure (Lee and Iyer, 2012). Satb1 has been shown to regulate transcriptional programs by recruiting writers and erasers of histone modification (Cai et al., 2006; Pavan Kumar et al., 2006) and DNA methylation, as well as crucial transcription factors, including β-catenin (Notani et al., 2010). Satb1 therefore integrates global epigenetic and transcriptional programs that determine cellular phenotypes, differentiation, and activity of leukocyte subsets (Borghesi, 2014).
Recently, we found that Satb1 is a direct target of miR-155 in ovarian-associated DCs (Cubillos-Ruiz et al., 2012), which infiltrate solid ovarian tumors (Cubillos-Ruiz et al., 2009; Huarte et al., 2008; Scarlett et al., 2009; Scarlett et al., 2012). Although heterogeneity and phenotypic overlap complicate the categorization of myeloid subsets under inflammatory conditions (Segura and Amigorena, 2013), we demonstrated that when these leukocytes receive activating signals, they can effectively process and present antigens to T cells (Cubillos-Ruiz et al., 2009; Scarlett et al., 2009). However, in the absence of immunostimulatory interventions, tumor-associated DCs are spontaneously immunosuppressive (Scarlett et al., 2012). One recently identified mechanism driving the immunosuppressive phenotype of tumor-associated DCs is the constitutive activation of XBP1 (Cubillos-Ruiz et al., 2015). Regulatory ovarian cancer DCs express significant levels of CD86 and MHC-II and produce inflammatory pro-tumorigenic cytokines such as IL-6, CCL4, CXCL8 and CCL3 (Cubillos-Ruiz et al., 2009; Nesbeth et al., 2009; Scarlett et al., 2009). This phenotype matches the features of DCs indirectly activated by inflammatory cytokines in the absence of pattern recognition receptor stimulation (Joffre et al., 2009; Vega-Ramos et al., 2014).
To define the role of Satb1 in the differentiation of DCs in the steady-state and cancer, we generated a conditional Satb1-deficient mice. Our results demonstrate that a dynamic pattern of Satb1 expression regulates the generation of conventional DCs as well as the acquisition of MHC-II-dependent immunocompetence by inflammatory DCs through regulation of Notch signaling. However, unremitting expression in already differentiated DCs drives an inflammatory, immunosuppressive, and tumor-promoting phenotype.
RESULTS
Activated inflammatory DCs universally infiltrate solid ovarian tumors in mice and humans
We have previously demonstrated that supplementation of miR-155 to CD11c+DEC205+MHC-II+ leukocytes in the ovarian cancer microenvironment transforms them from an immunosuppressive to an immunostimulatory cell type through a mechanism involving silencing of Satb1 (Cubillos-Ruiz et al., 2009). To further define the phenotype of these cells in established, orthotopic ID8-Defb29/Vegf-a ovarian tumor-bearing mice, we used markers specifically expressed by bona fide DCs. As shown in Figure 1A, most CD11c+MHC-II+ leukocytes in tumor ascites expressed the DC-specific lineage marker Dngr1 (Schraml et al., 2013). Tumor DCs also expressed the DC-specific transcription factor Zbtb46 (Meredith et al., 2012; Satpathy et al., 2012), at levels comparable than CD11c+MHC-II+ bone marrow-derived DCs (BMDCs) and much higher than F4/80+MHC-II+ macrophages in tumor-free mice (Figure 1B). Tumor-associated CD11c+MHC-II+Dngr1+Zbtb46+ cells co-exhibited FcεRI, CD11b and CCR7, phenotypic markers of inflammatory DCs (Segura and Amigorena, 2013). However, the expression of the DC lineage marker Dngr1 suggests that they are ontogenetically true DCs, rather than DC-like monocyte-derived cells (Figure 1A&C). An identical (Dngr1+) phenotype was identified in tumor-associated DCs in orthotopic K-ras-driven solid ovarian carcinomas (Scarlett et al., 2012) (Figure 1D). Unexpectedly, DCs in tumor ascites also co-expressed the activation marker CD83 (Figure 1A). Therefore, although we have previously demonstrated that they primarily exert immunosuppressive activities (Cubillos-Ruiz et al., 2012; Cubillos-Ruiz et al., 2009; Huarte et al., 2008; Scarlett et al., 2009; Scarlett et al., 2012), bona fide DCs at ovarian cancer locations represent an activated cell type.
Figure 1. Activated inflammatory DCs infiltrate syngeneic ovarian tumors in mice.
(A) CD45+CD11c+MHC-II+ cells in tumor ascites express Dngr1 and the activation marker CD83. (B) Zbtb46 transcript levels relative to Gapdh expression in CD11c+MHC-II+ DCs in tumor ascites, total BMDCs at different stages of differentiation and MHC-II+F4/80+ splenic macrophages from tumor-free mice. (C) Tumor-associated DCs express phenotypic markers of inflammatory DCs. (D) Dngr1+CD11c+MHC-II+ DCs are also universally found in freshly dissociated orthotopic p53-driven ovarian tumors at days 62 after adenovirus-Cre-mediated activation of p53 and K-ras mutations. Representative of 4 different tumors analyzed. (E) Western-blot analysis of the thymus of wild-type and Satb1constitutive KO mice define the specificity of only the lower band. (F) Western-blot comparison of Satb1 expression in tumor-derived DCs vs. BMDCs at different stages of differentiation. (G) MHC-II expression in tumor-derived (CD45.2+) CD11c-Cre/Satb1flx/flx vs. (CD45.1+) wild-type CD11c+MHC-II+ DCs in mixed BM chimeras challenged with ID8-Defb29/Vegf-a orthotopic tumors. Representative of three independent experiments.
To define the role of Satb1 in tumor-associated DCs, we generated conditional Satb1 knockout mice on a B6 background. We found that Abcam antibody#EPR3895 produced two clear bands. However, only the lower band at ~103kDa disappeared in thymocytes from constitutive Satb1 KO mice (Figure 1E) and therefore truly represented Satb1. More importantly, Satb1 expression levels in CD11c+MHC-II+ DCs sorted from tumor ascites were significantly higher than in differentiated BMDCs, in which Satb1 expression starts decreasing coinciding with the transition to CD11c+MHC-II+ from CD11c+MHC-II− precursors (day 5–6; Figure 1F). Supporting the role of Satb1 overexpression in mature tumor-associated DCs, congenic Satb1+ DCs in tumor ascites exhibited higher levels of MHC-II than their CD11c-Cre/Satb1flx/flx DCs in mixed BM chimeras (Figure 1G).
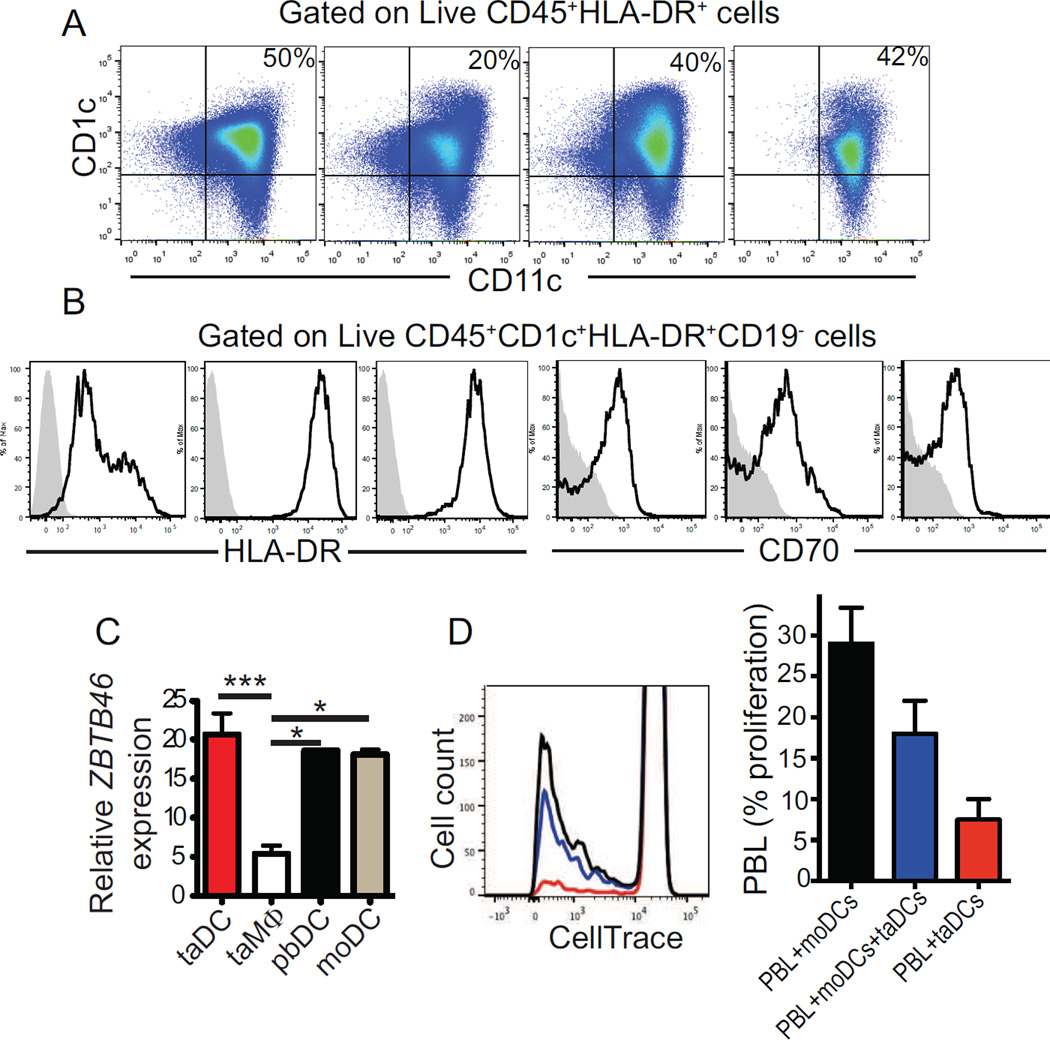
Notably, the accumulation of bona fide DCs in ovarian mouse tumors recapitulates the immunoenvironment of human ovarian carcinomas because CD1c+CD11c+MHC-II+CD19−CD70high leukocytes (attributes of the human counterpart of inflammatory DCs (Segura and Amigorena, 2013; Segura et al., 2013)) were found in all freshly dissociated solid advanced tumors samples analyzed (n=12), at proportions ranging from 19% to 67% of total CD45+MHCII+ cells (Figure 2A&B). Tumor-infiltrating human DCs expressed levels of ZBTB46 similar to their counterparts in matching peripheral blood or autologous monocyte-derived DCs (Figure 2C). As with their murine counterparts, despite showing relatively high expression of costimulatory molecules and MHC-II, ovarian cancer-infiltrating DCs from 3 randomly selected patients did not elicit allogeneic responses, while autologous peripheral blood monocyte-derived DCs induced measurable proliferations (Figure 2D). Taken together, these results indicate that bona fide DCs universally accumulate in the ovarian cancer microenvironment. Although Satb1 overexpression contributes to their activated phenotype, DC immunostimulatory function is severely abrogated at tumor locations.
Figure 2. Inflammatory DCs universally infiltrate solid human ovarian carcinomas.
(A) CD45+CD1c+CD11c+HLA-DR+ DCs in four different dissociated human ovarian carcinomas (representative of 12 tumors analyzed). (B) Representative histograms of activation markers CD70 and HLA-DR in DCs infiltrating 3 different human ovarian carcinomas. Grey, isotype control used in each experiment. Representative of 12 different specimens analyzed. (C) Zbtb46 mRNA expression relative to GAPDH expression in tumor-derived DCs (CD45+HLA-DR+CD11c+CD1c+), tumor-derived Macrophages (CD45+HLA-DR+CD11b+CD1c−), peripheral-blood DCs (CD45+HLA-DR+CD11c+CD1c+) and monocyte-derived DCs from 3 patients. (D) Representative mixed Lymphocyte Reaction assay performed by co-culturing matched-monocyte or tumor-derived DC and allogeneic T cells (left). Proportions of allogeneic T cells proliferating in response to DCs from 3 different patients (right). All data represent mean ± SEM. *p<0.05, ***p<0.001.
Satb1 governs the generation and maturation of multiple subsets of conventional DCs
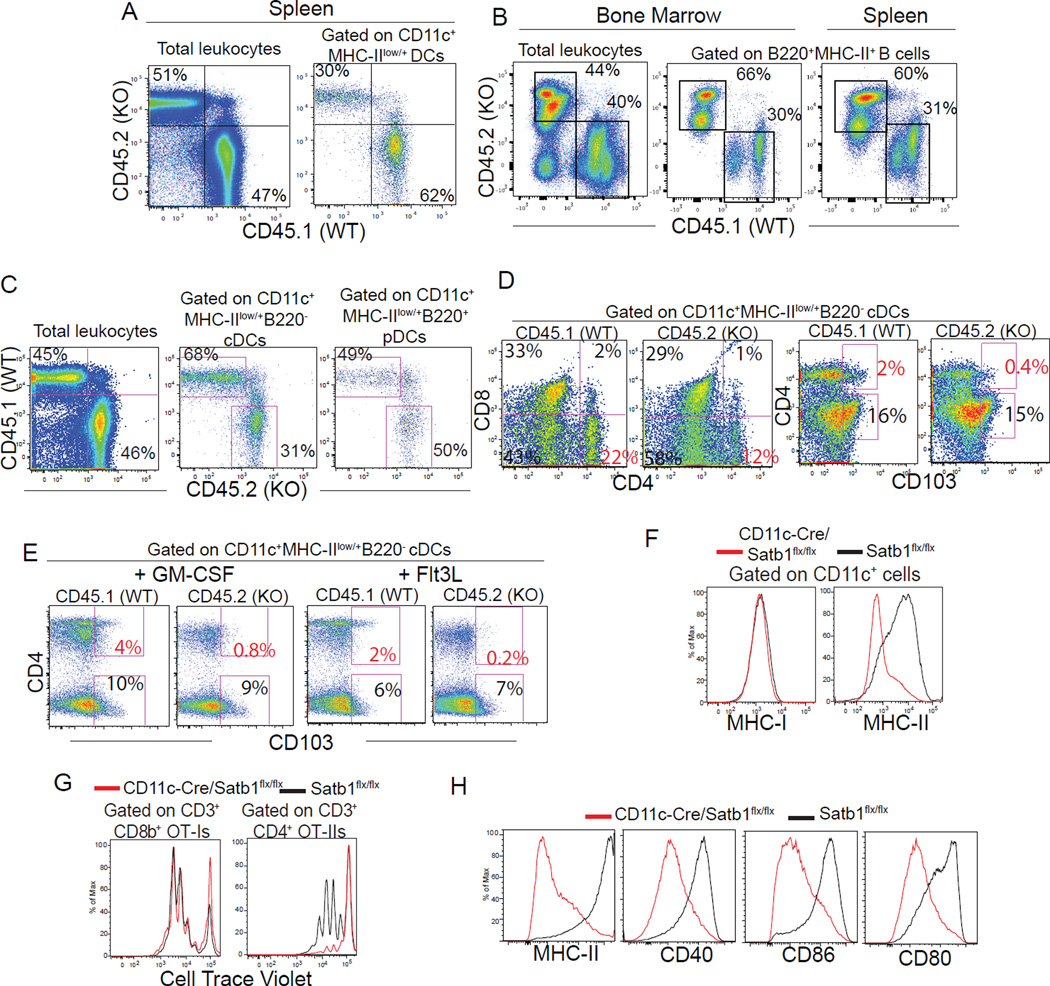
To understand the role of Satb1 in DC differentiation, we next performed competitive BM reconstitution experiments using BM from both constitutive and Vav1-Cre-driven Satb1-deficient mice. As shown in Figure 3A age-matched congenic wild-type and Satb1 constitutive KO BM showed similar capacity to repopulate total leukocytes in the spleen, but also a defect in the reconstitution of total DCs. Supporting previous reports (Alvarez et al., 2000), we observed a severe defect in T cell reconstitution in Satb1-deficient leukocytes (not shown), compensated by the expansion of B cells (Figure 3B). We again found a reproducible decrease in the reconstitution of total splenic DCs arising from Vav1-Cre-Satb1flx/flx BM, compared to congenic wild-type progenitors, but this reduction selectively affected conventional (but not plasmacytoid) DCs (Figure 3C). Satb1 deficiency also had divergent effects on different subsets of conventional DCs, causing a significant reduction of overall CD4+ DCs in multiple experiments, while promoting the relative expansion of CD11c+CD4−CD8−B220− DCs (Figure 3D). Interestingly, Satb1 ablation resulted in the elimination of a CD103+CD4+ subset, both in the steady-state and upon DC mobilization induced by different cytokines (Figure 3D&E).
Figure 3. Satb1 influences the generation and maturation of conventional DCs.
(A) Mice reconstituted with equal proportions of age-matched wild-type (CD45.1) and constitutive Satb1-deficient BM (CD45.2) analyzed ~2 months after reconstitution. (B-C) Analysis of spleens from mixed BM chimeras ~2 months after competitive reconstitution with equal ratios of adult age-matched wild-type (CD45.1) and Vav1-Cre/Satb1flx/flx (CD45.2) BM. Representative of ≥3 independent experiments with ≥7 mice/group. (D) Reduction of Satb1-deficient total CD4+ and CD4+CD103+ conventional DCs with concomitant expansion of CD4−CD8− subsets in the spleen of mixed wild-type/Vav1-Cre/Satb1flx/flx BM chimeras. (E) Ablation of Satb1-deficient CD4+CD103+ conventional DCs in response to intraperitoneal administration of Flt3L (10 µg/mouse) and GM-CSF (5 µg/mouse) for 5 consecutive days to induce DC mobilization. Both representative of ≥2 independent experiments and readouts at day 6. (F) MHC-I and MHC-II expression in (CD45.1+) wild-type and (CD45.2+) CD11c-Cre/Satb1flx/flx BMDCs co-cultured for 8 days in the presence of GM-CSF. Representative of 3 independent experiments. (G) Wild-type and CD11c-Cre/Satb1flx/flx BMDCs differentiated for 8 days were pulsed with full-length Ovalbumin and incubated with Cell Trace Violet-labeled OT-I or OT-II T cells. Representative of 3 independent experiments. (H) Expression of co-stimulatory markers in (day 9) wild-type and CD11c-Cre/Satb1flx/flx BMDCs after 18 hours incubation with 1 µg/ml LPS.
To dissect the role of Satb1 in the differentiation of Antigen-Presenting Cells (APCs) generated under inflammatory conditions (Segura et al., 2013), we treated wild-type and CD11c-Cre/Satb1flx/flx BM with GM-CSF in vitro. As shown in Figure 3F, left, the ablation of Satb1 in lineage-committed BMDCs triggered by CD11c expression played no significant role in the acquisition of MHC-I, or cross-presentation of SIINFEKL to OT-1 CD8 T cells when differentiated CD11c+ cells were pulsed with full-length OVA (Figure 3G, left). In contrast, only a small proportion of Satb1-deficient CD11c+ cells acquired surface expression of MHC-II (Figure 3F, right). Correspondingly, the same Satb1-deficient APCs that effectively presented antigen through class I, failed to stimulate OT-II CD4 T cells (Figure 3G, right). In addition, Satb1-deficient APCs were unresponsive to TLR-mediated activation in terms of up-regulation of MHC-II and multiple co-stimulatory molecules (Figure 3H). Together, these experiments indicate that Satb1 plays divergent roles in the differentiation of conventional DC subsets and contributes to expression of MHC-II during inflammatory APC differentiation and TLR-dependent increase of co-stimulatory molecules.
Satb1 drives the expression of MHC-II on inflammatory DCs through a transient Notch signaling-dependent transcriptional mechanism
Supporting a transcriptional defect, we found significantly lower H2-Ab1 mRNA levels in APCs generated in the absence of Satb1 (Figure 4A). Similar transcriptional differences were obtained with sorted CD11c+CD135+CD115− cells, specifically derived from Dngr1+ DC precursors (Helft et al., 2015) (Figure 4B). Surprisingly, we found no Satb1-dependent differences in the expression levels of Ciita, which controls MHC-II transcription by associating with the basal transcription machinery (Lochamy et al., 2007) (Figure 4C).
Figure 4. Satb1 drives the expression of MHC-II on the surface of differentiated DCs by regulating the Notch1 signaling.
(A) Quantification of H2-Ab1 transcripts relative to Gapdh expression at days 6 and 10 of GM-CSF-induced DC differentiation from Satb1flx/flx and CD11c-Cre/Satb1flx/flx BM. Representative of 3 experiments in triplicate. (B) Q-PCR quantification of H2-Ab1 mRNA in CD11c+CD135+CD115− cells sorted after 10 days of GM-CSF-induced DC differentiation as in (A). Representative of 2 experiments in triplicate. (C) Ciita expression in Satb1flx/flx and CD11c-Cre/Satb1flx/flx BMDCs at different times of GM-CSF-induced differentiation. Representative of 3 experiments. (D) Expression of full-length and Intracellular Notch1 during GM-CSF-induced DC differentiation of BM from Satb1flx/flx and CD11c-Cre/Satb1flx/flx. Representative of 3 independent experiments. (E) Dynamics of GM-CSF-induced differentiation of wild-type CD11c+MHC-II+ BM cells in these cultures. (F) Satb1 binding to the Notch1 promoter. Chromatin was immunoprecipitated with anti-Satb1 Abs or control IgG from CD11c+APCs differentiated for 7 days with GM-CSF, derived from the BM of wild-type (WT) vs. CD11c-Cre/ Satb1flx/flx (KO; control) mice. Enrichment of the Notch1 promoter sequence in chromatin IPed with anti-Satb1 Abs vs. irrelevant IgG was quantified by Real-Time Q-PCR. Representative of two independent experiments in triplicate, with similar results. (G) MHC-II expression in CD45+CD11c+ BM cells from either Satb1flx/flx, CD11c-Cre/Satb1flx/flx or RosaNotch mice differentiated with GM-CSF. Representative of 3 independent experiments. (H) Quantification of H2-Ab1 transcripts relative to Gapdh expression at days 6 and 10 of GM-CSF-induced DC differentiation from CD11c-Cre/Satb1flx/flx and RosaNotch BM. Representative of 3 experiments. (I) Q-PCR of CD11c+ APCs as described above for H2-ab1 promoter sequences in chromatin IPed with anti-Rbpj Abs vs. irrelevant IgG. Representative of two independent experiments in triplicate, with comparable results. All data represent mean ± SEM.*p<0.05, **p<0.01, ***p<0.01.
To identify Satb1-dependent regulators of MHC-II expression, we focused on Notch1. Although Notch1 signaling is typically associated with lymphocytic differentiation, recent studies indicate that Delta-like1-signaling drives cell surface expression of MHC-II in mast cells (Nakano et al., 2009). Accordingly, we found that at day 6–7 of GM-CSF-differentiation of wild-type BM, precisely coinciding with the up-regulation of MHC-II in CD11c+ cells, Notch expression is elevated in immature myeloid cells and is progressively down-regulated upon terminal differentiation of APCs (Figure 4D&E). This occurs in a Satb1-dependent manner, because a significant decrease in both full-length and intracellular Notch1 was identified in nascent APCs upon CD11c-induced ablation of Satb1.
To determine whether Satb1 physically associates with the Notch1 promoter, we performed chromatin immunoprecipitation (ChIP) with specific Abs, using Satb1−/− APCs as a control. As shown in Figure 4F, we observed significant enrichment of the Notch1 promoter in Satb1-DNA precipitates from day 7 BM-derived CD11c+MHC-II+ APCs, compared to control pull-downs with an irrelevant IgG. Supporting the specificity of the binding to the Notch1 promoter, no significant enrichment was found in identically differentiated APCs from CD11c-Cre/Satb1flx/flx mice (Figure 4F).
We next generated triple (CD11c-Cre/Satb1flx/flx/RosaNotch) transgenic mice and brought them to a B6 background. In this system CD11c expression triggers Satb1 ablation while simultaneously driving the expression of intracellular (constitutively activated) Notch1 (Murtaugh et al., 2003) during GM-CSF-induced differentiation of BM cells into APCs. Supporting the role of Notch signaling in MHC-II transcription, Cre-mediated Notch activation restored cell surface expression of MHC-II (Figure 4G) by up-regulating H2-Ab1 mRNA levels (Figure 4H). Finally, we observed significant enrichment of H2-ab1 promoter sequences in Rbpj-DNA precipitates (but not Hes1-DNA precipitates; not shown), indicating that Notch signaling turns-on MHC-II through transcriptional activation (Figure 4I). As expected, Rbpj occupancy of the H2-ab1 promoter decreased significantly in APCs (Figure 4I). Together, our results unveil a mechanism whereby Satb1 drives the expression of Notch in APC precursors (including bona fide pre-DCs) by directly activating the promoter. By governing MHC-II transcription, Notch signaling drives cell surface expression of MHC-II during APC differentiation, licensing them for antigen presentation.
Silencing Satb1 in tumor-associated DCs boosts anti-tumor immunity and delays malignant progression
The aforementioned results indicate that Satb1 is required for full DC differentiation and MHC-II-dependent T cell stimulatory activity. Correspondingly, CD11c-Cre/Satb1flx/flx BM-reconstituted mice succumbed to tumor challenge significantly faster than wild-type BM-reconstituted controls, suggesting that DCs were incapable of orchestrating protective immunity (Figure 5A).
Figure 5. Silencing Satb1 in tumor-associated DCs boosts anti-tumor immunity and delays malignant progression.
(A) Survival of mice reconstituted with wild-type vs. CD11c-Cre/Satb1flx/flx BM after ID8-Defb29/Vegf-a tumor challenge. Two independent experiments pooled (9–10 mice/group). (B) Mice bearing ID8-Defb29/Vegf-a tumor ascites received S100A9 (25 µg) or β-Estradiol (0.01 mg) i.p. CD11c+MHC-II+ DCs were FACS-sorted and analyzed for Satb1 expression 34–48 hours later. Representative of 3 independent experiments. (C) Rhodamine-labeled Satb1-targeting siRNA-PEI nanoparticles were i.p. injected into 3 week ID8-Defb29/Vegf-a tumor-bearing mice. Rhodamine+ DCs were identified ~36 hours later. (D) Satb1 and Notch1 expression in CD11c+MHC-II+ DCs sorted from tumor ascites ~36–48 hours after i.p. administration of non-targeting (NT) or Satb1-targeting siRNA-PEI. (F.L = Full length, I.C.=Intracellular). (E-F) Mice received indicated treatments at days 8, 13, 18, 23 and 28 after tumor challenge. T cells from ascites were used for IFN-γ- and Granzyme B-ELISPOT analysis (E) or expression of CD44 and CD69 levels (F). Representative of two independent experiments (4–6 mice/group). (G) ID8-Defb29/Vegf-a tumor-bearing mice treated for three weeks with siRNA-carrying nanocomplexes or PBS received 0.6 mg full length endotoxin-free ovalbumin i.p. 3 hours after the last treatment, followed 18 hours later by i.p. transfer of 2×106 CFSE-labeled SIINFEKL-specific OT-1 T cells. Peritoneal wash was collected after 48 hours and CFSE dilution was quantified. Representative of 3 independent experiments (6 mice/group). (H) Division Index of proliferating cells in (f). (I) ID8-Defb29/Vegf-a tumor-bearing mice received 5 treatments every 5 days and were monitored for survival. Data represent two independent experiments with 10 mice total per group. All data represent the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
To reconcile the overexpression of Satb1 in tumor DCs with their immunosuppressive phenotype, we focused on the fact that, unlike tumor-associated DCs, steady-state DCs down-regulate Satb1 after maturation (Figure 1F). We found that inflammatory factors such as S100A8/A9 were sufficient to further up-regulate Satb1 in vivo in DCs in the TME, while estrogen signaling had the opposite effect (Figure 5B). To define the effect of inflammation-induced Satb1 overexpression in already differentiated DCs (as opposed to DC precursors), we silenced Satb1 specifically in tumor-associated DCs using siRNA-carrying nanoplexes. Polyethylenimine (PEI)-based nanoparticles carrying fluorescently labeled siRNA were selectively engulfed by CD11c+ leukocytes at peritoneal (ID8-Defb29/Vegf-a tumor) locations, as we reported (Cubillos-Ruiz et al., 2012; Cubillos-Ruiz et al., 2009) (Figure 5C). Satb1-targeting, but not control (non-targeting), nanoparticles effectively silenced Satb1 expression in vivo in ovarian cancer DCs (Figure 5D) and, accordingly, induced Notch1 down-regulation. Most importantly, silencing Satb1 throughout the course of malignant progression significantly enhanced anti-tumor immunity, as determined by both Granzyme B and IFN-γ ELISPOT analysis (Figure 5E). Accordingly, the accumulation of antigen-experienced (CD44+) cytotoxic T cells exhibiting markers of recent activation was also significantly increased upon Satb1 silencing (Figure 5F). Furthermore, OT-I T cells responded significantly more strongly to cognate antigen in situ in the ovarian cancer microenvironment when Satb1-silenced tumor DCs were pulsed in vivo with full-length ovalbumin, compared to DCs identically pulsed in mice treated with irrelevant nanoparticles (Figure 5G&H). Supporting the relevance of enhanced protective immunity, repeated Satb1 silencing specifically in DCs resulted in significantly longer survival rates, compared to control irrelevant siRNA nanocomplexes (Figure 5I). Therefore, inflammatory cytokines in the TME up-regulate Satb1, resulting in an immunosuppressive, tumor-promoting phenotype.
Satb1 governs genome-wide transcriptional modifications
To define the spectrum of transcriptional changes induced by Satb1 overexpression, we treated mice with ovarian cancer-induced ascites with fluorescently-labeled Satb1-specific vs. non-targeting PEI-based nanocomplexes. After 20–24 hours, CD11c+MHC-II+ DCs targeted by functional Satb1-specific or identically delivered control siRNA were collected and subjected to RNA deep-sequencing. In the first experiment, mRNA of Satb1 was reduced 4.8 fold and overall, 1003 genes changed their expression by at least 50% (Supplemental file 1). In the second experiment, when Satb1 reduced expression only by 20%, we found 521 genes that significantly changed expression with any fold (Supplemental file 1). Significant overlap between the two sets of 72 genes (Figure 6A, p=3x10−11 by hypergeometric test) indicates that there is a subset of genes sensitive to Satb1 expression even when the expression changes are very small. The full list of 72 genes significantly up- and down-regulated with any level of Satb1 changes in both experiments, which includes an array of inflammatory cytokines and chemokines, is shown in Figure 6B.
Figure 6. Transcriptional and regulatory changes caused by Satb1 overexpression.
(A) Numbers of significantly changed genes and their overlap between two independent experiments, that changed expression of Satb1 4.8 fold and 1.2 fold. The overlap was 2.7 more than expected by random chance alone (p=3x10−11 by hypergeometric test). (B) Expression heatmap and fold changes for 72 genes affected by high and low level of Satb1 change. N or NT indicate NT siRNA treated samples, S or Satb1 indicate Satb1-siRNA treated samples. (C) Canonical pathways significantly affected by Satb1 overexpression. Il6-mediated pathways and pathways for Granulocyte and Agranulocyte Adhesion and Diapedesis were among the most significantly affected and were highlighted to demonstrate expression changes of individual genes in the pathway. (D) Predicted upstream regulators whose regulatory function was significantly affected by Satb1 as evidenced by mRNA expression changes of their known target genes.
In addition, Ingenuity Pathway Analysis of the 1003 genes that underwent expression change of at least 1.5 fold upon Satb1 silencing of 4.8 fold in tumor-associated DCs revealed many signaling pathways affected in Satb1-overexpressing cells (Figure 6C). Inflammatory networks driven by Satb1 overexpression included Il6-mediated pathways and pathways for Granulocyte and Agranulocyte Adhesion and Diapedesis. Upstream regulator analysis revealed multiple regulators affected by Satb1 (Figure 6D) as estimated from mRNA changes in their known targets. For example, Ifnb1 and Tnf, in which mRNA levels were most significantly altered, were indeed functionally more active with Satb1 overexpression, as 38 targets for Ifnb1 and 139 targets for Tnf displayed significant mRNA changes, indicative of the regulators’ activation.
In accordance with results of pathway analysis, Il6 target genes demonstrated that Il6 was more active when Satb1 was present. Supporting our in vitro results, we also observed similar effects on Notch1 signature and, interestingly, an Lgals1 signature. Galectin-1, encoded by the Lgals1 gene, is a pleiotropic protein that binds to surface glycoconjugates containing N-acetyllactosamine sequences, cross-linking these saccharide structures on immune cells and driving potent immunosuppression (Dalotto-Moreno et al., 2013; Rubinstein et al., 2004; Rutkowski et al., 2015; Toscano et al., 2007). Together, these data indicate that unremitting overexpression of Satb1 in already differentiated inflammatory DCs in the TME drives genome-wide transcriptional changes that globally affect the transcriptome and promotes an immunosuppressive phenotype.
Satb1-dependent secretion of galectin-1 is sufficient to accelerate tumor progression
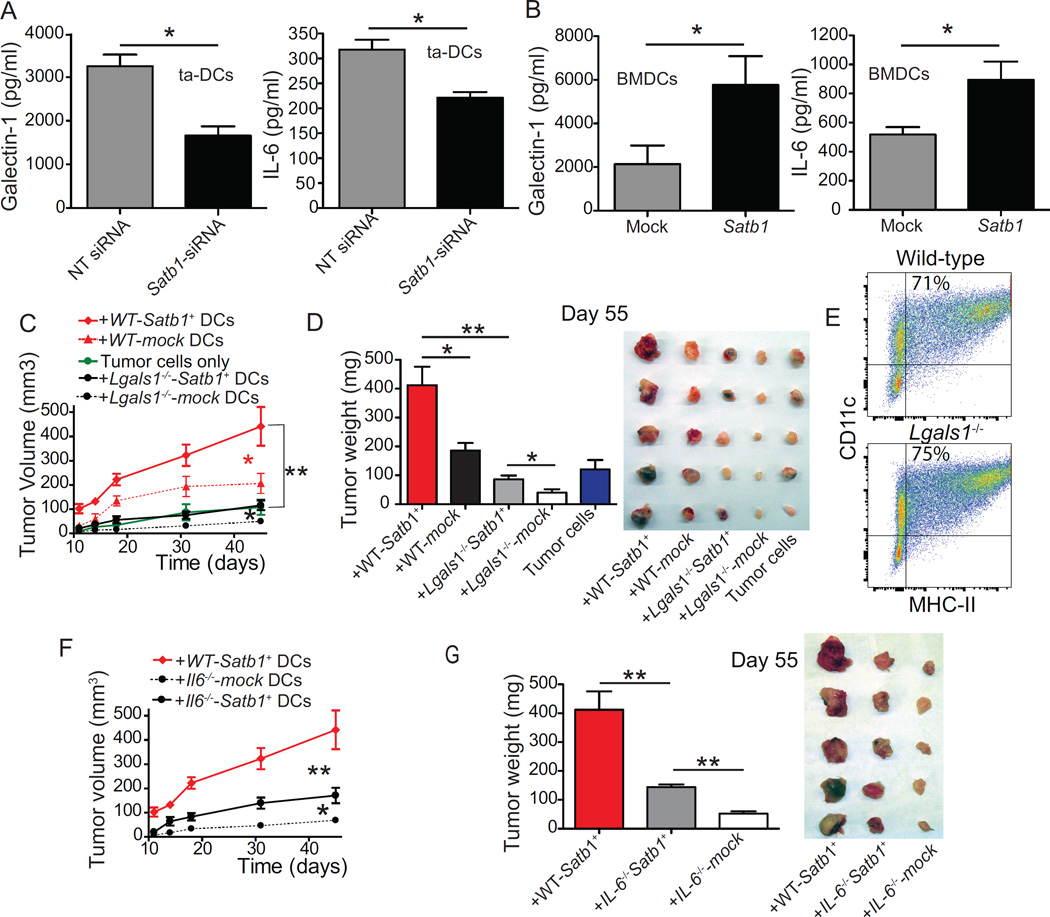
Substantiating the relevance of our genomic analysis, FACS-sorted DCs engulfing Satb1-silencing nanocomplexes secreted significantly lower levels of both IL-6 and galectin-1, compared to non-targeted siRNA-untreated controls (Figure 7A). Correspondingly, enforced retroviral-mediated expression of Satb1 augmented their production of both galectin-1 and tumor-promoting IL-6 (Figure 7B).
Figure 7. Satb1 overexpression of tumor DCs accelerates malignant progression by promoting the secretion of IL-6 and galectin-1.
(A) ELISA quantification of galectin-1 and IL-6 released by PMA/Ionomycin (500 ng/1 µg/mL)-stimulated CD11c+MHC-II+ DCs sorted from ~day 35 tumor ascites, 20–24 hours after treatment with 100 µg of Rhodamine-labeled NT or Satb1-targeting siRNA-carrying polyplexes. Pooled from two independent experiments. (B) GM-CSF-differentiated wild-type BM cells were transduced at days 2 and 3 with empty or Satb1-encoding retroviruses. Galectin-1 and IL-6 quantified in supernatants of PMA/Ionomycin-stimulated Mock or Satb1-transduced BMDCs. Pooled from two independent experiments in triplicate. (C) Wild-type and Lgals1−/− BMDCs were transduced with Satb1 or the empty retrovirus and admixed (1:10 ratio) with 106 ID8-Defb29/Vegf-a cells for injection into the axillary flank. Tumor growth was monitored. With the exception of tumor cells alone and mock transduced Lgals1−/− DCs, representative of three independent experiments (≥5 mice/group). (D) Tumor weight and size comparison of tumors resected at day 55 in one of the experiments. (E) Comparable proportions of CD11c+MHC-II+ DCs generated from wild-type and Lgals1−/− BM after 9 days of culture with GM-CSF. Representative of three independent experiments. (F) Mice (5/group) were challenged with ID8-Defb29/Vegf-a tumors admixed with Satb1/mock-transduced wild-type vs. Il6−/− BMDCs as in (C) and tumor growth was monitored. (G) Tumor weight and size comparison of tumors resected at day 55. All data represent the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
We have previously identified galectin-1 as a major pathogenic factor in a variety of tumors (Rutkowski et al., 2015). To determine the relevance of Satb1-dependent up-regulation of galectin-1 specifically in tumor microenvironmental DCs, we ectopically over-expressed Satb1 in wild-type and galectin-1-deficient BMDCs, independently admixed them with tumor cells, and used the mixture to challenge different cohorts of naïve mice. As shown in Figure 7C&D, these slowly progressing tumors grew significantly faster when admixed with Satb1-overexpressing wild-type DCs, compared to mock-transduced control DCs. However, Satb1-dependent acceleration of tumor growth (vs. the administration of tumor cells without DCs) only occurred when tumor-associated DCs had the capacity to up-regulate galectin-1 (Figure 7C). Importantly, galectin-1-deficient BMDCs showed the same differentiation kinetics as their wild-type counterparts (Figure 7E). Similarly, when tumor cells were admixed with Satb1-transduced IL-6−/− DCs they still grew significantly faster than tumors containing mock-transduced IL-6−/− DCs, but significantly more slowly than tumors admixed with Satb1-transduced wild-type DCs (Figure 7F&G). Together, these results indicate that Satb1 unrelenting overexpression drives complex tumor-promoting, pro-inflammatory activities in tumor-associated DCs, for which the secretion of immunosuppressive galectin-1 and IL-6 are significant contributors.
DISCUSSION
We find that Satb1 governs a genome-wide transcriptional program required for terminal steady-state DC differentiation and effective antigen presentation through MHC-II. Paradoxically, Satb1 needs to be extinguished after DC maturation for effective immunostimulatory activity. Accordingly, unremitting expression of Satb1 in fully committed inflammatory DCs in solid ovarian tumors drives an immunosuppressive cell type that outnumbers canonical macrophages and contributes to accelerate malignant progression.
Inflammatory TLR signals appear to be necessary to activate DCs for effective T cell priming, but cytokines such as IL-1β, TNF or type I IFNs can activate DCs in an alternative manner. Although these inflammatory cytokines induce DC maturation, they are not sufficient to generate T cell stimulatory DCs. Thus, DCs that were activated by cytokines in the absence of TLR agonists do not efficiently prime effector T cell responses and are regarded as poor stimulators of T cell-mediated immunity (Joffre et al., 2009). Inflammation, therefore, does not substitute for pattern recognition receptor signaling, but it does alter the nature and magnitude of cytokines secreted by DCs. Cytokine-activated DCs, for instance, produce much higher amounts of IL-6, CCL3 or CCL4 in response to TLR activation, compared to their immature counterparts (Vega-Ramos et al., 2014).
Unlike other tumors that accumulate immature myeloid progenitors but few mature DCs, we have previously identified activated bona fide inflammatory DCs as an abundant population in the microenvironment of solid ovarian carcinomas (Cubillos-Ruiz et al., 2009; Huarte et al., 2008; Scarlett et al., 2009; Scarlett et al., 2012). Their DC nature is supported by the expression of Zbtb46 transcripts (Meredith et al., 2012; Satpathy et al., 2012). Additionally, ovarian cancer DCs can be induced to process full-length OVA in vitro (Conejo-Garcia et al., 2004) and in vivo (Cubillos-Ruiz et al., 2009; Scarlett et al., 2009), to present processed SIINFEKL to T cells. Although inflammatory DCs are thought to originate from monocytes (Segura and Amigorena, 2013), murine ovarian cancer DCs express the DC lineage marker Dngr1 (Schraml et al., 2013), suggesting that they may alternatively arise from DC precursors. Their mature phenotype suggests a role for indirect inflammatory activation in the acquisition of a tumor-promoting phenotype by ovarian cancer-associated DCs (Huarte et al., 2008; Scarlett et al., 2012). Supporting this proposition, we found that S100A8/A9 proteins up-regulate Satb1 in DCs in vivo and in vitro, and that Satb1 expression enhances the secretion of inflammatory cytokines and chemokines by DCs. Accordingly, silencing Satb1 expression selectively in tumor-associated DCs in vivo at tumor locations reduced the level of inflammation and immunosuppression in the TME, and boosted T cell-mediated immune protection. Notably, 22% of transcripts underwent ≥2-fold changes in expression levels upon Satb1 knockdown. Therefore, consistent with its role as a master genomic organizer, Satb1 drives major phenotypic changes that are sufficient to accelerate malignant progression and suppress anti-tumor immunity. Although this study cannot segregate the precise contribution of Satb1-dependent loop formation to the entire range of genome-wide effects, it is likely that these dramatic phenotypic changes are the result of both looping and transcriptional activator/repressor activities. Of note, although Satb1 is highly similar to Satb2 and both regulate chromatin remodeling and gene regulation, the latter is not expressed in BMDCs (not shown) and therefore all observed effects can only be attributed to Satb1.
Among the spectrum of Satb1-dependent mechanisms, we identified the direct activation of the Notch1 promoter as a driver of terminal inflammatory DC differentiation. Notch1 is transiently up-regulated during the switch from CD11c+MHC-II− to CD11c+MHC-II+ APCs. Using genetically engineered systems, our data indicate that ectopic expression of Notch is sufficient to restore MHC-II surface expression in the absence of Satb1, through a transcriptional mechanism dependent of direct binding of Rbpj to the H2-Ab1 promoter. Although seminal studies by the Gabrilovich lab had previously identified a role for Notch in DC differentiation (Zhou et al., 2009), the requirement of Notch signaling for the acquisition of MHC-II immunocompetence in inflammatory DCs is surprising and opens avenues to understanding how transient Notch signaling could contribute to emergency myelopoiesis.
Finally, our study identifies galectin-1, an immunosuppressive lectin, as a major target of Satb1 in tumor-associated DCs. As galectin-1 coordinates tolerogenic programs in human and mouse DCs (Ilarregui et al., 2009), limits survival of Th1 and Th17 cells (Toscano et al., 2007) and promotes the differentiation of tumor Foxp3+ Treg cells (Dalotto-Moreno et al., 2013), our findings suggest that Satb1 may contribute to tumor progression by hierarchically activating galectin-1-driven cancer immune evasive pathways. The dramatic effect that Satb1-dependent, DC-derived galectin-1 production in nascent tumors has on subsequent tumor growth supports the relevance of this mechanism. Overall, our results show an alternative Satb1-driven mechanism that controls DC function in healthy conditions and cancer.
EXPERIMENTAL PROCEDURES
Mice and Cell lines
Satb1 transgenic mice used in this work were generated by Wistar’s Mouse Transgenic Facility with clones of Embryonic Stem cells carrying a targeted/floxed version of Satb1 procured from EUCOMM and injected into blastocysts. The resulting heterozygous colony founders were crossed with C57BL/6 breeders to confirm parental transmission of the targeted version of Satb1, genotyped for LoxP and LacZ and further crossed until homozygosis to obtain a Satb1 full knockout mice. Complete absence of Satb1 expression in Satb1 knock-out mice was verified by western-blot in the thymus, an abundant source of Satb1 expression. To be able to specifically ablate Satb1 expression in certain cell compartments, Satb1floxed/wt mice were crossed with the deleter strain ACTFLPe (stock#003800, Jackson Labs) that express the FLP recombinase under the direction of the ACTB promoter to ubiquitously delete the FRT-flanked sequence. Successively, Satb1floxed/floxed mice were crossed with either CD11c-Cre (Jackson Labs#008068) or Vav1-Cre (Jackson Labs#008610) mice.
Notch1-Rosa26 mice were kindly provided by Dr. B. Z. Stanger (University of Pennsylvania Perelman School of Medicine) and back-crossed for at least 10 generations to a C57BL/6 background. These transgenic mice have a floxed version of Notch1 interrupted by a STOP codon at the permissive locus Rosa26. Notch1-Rosa26 mice were crossed with Satb1floxed/floxed CD11c-Cre-recombinase to obtain conditional triple transgenic mice that simultaneously overexpress Notch1 and ablate Satb1 expression in the CD11c compartment. Galectin-1-deficient (Lgals1−/−) mice were originally generated by F. Poirier (Jacques Monod Institut, Paris). OT-I and OT-II transgenic mice were purchased from Taconic. C57BL/6 and congenic CD45.1+ mice (5–6 weeks old) were purchased from the Frederick Cancer Research Facility at the NCI.
All animals were maintained in pathogen free barrier facilities. All experimental procedures were conducted in agreement with The Institutional Animal Care and Use Committee of the Wistar Institute.
Parental ID8 cells were provided by K. Roby (Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS) (Roby et al., 2000) and retrovirally transduced to express Defb29 and Vegf-a (Conejo-Garcia et al., 2004). Flank tumors with ID8-Defb29-Vegf-a cells were admixed at a 10:1 ratio with BMDCs using factor-reduced Matrigel (BD Biosciences) and injected into the axillary flank. Tumor volume was calculated using the following equation: (L×W2)/2, where L is length and W is width. For retroviral transduction of Satb1, virus-containing media was produced transfecting the ecotropic Phoenix retroviral packaging cell line (Allele Biotech).
Mice bearing ID8-Defb29/Vegf-a tumors for 32–35 days were i.p. injected with S100A9 (25 µg/mouse; Fitzgerald). After 36–48 hours, sorted tumor-associated DCs were analyzed for Satb1 expression as indicated.
Human Specimens
Advanced (stage III-IV) human ovarian carcinoma tissues were freshly procured under a protocol approved by the Institutional Review Boards at Christiana Care Health System (#32214) and The Wistar Institute (#21212263). Informed consent was obtained from all subjects. Tumors were mechanically dissociated and directly analyzed, or cryopreserved as viable single cell suspensions for future analysis.
Generation of Bone Marrow Chimeras
Mononuclear BM cells collected from adult age-matched CD45.1 (congenic) WT or CD45.2 Satb1−/− donor mice (1–2 × 106) were mixed in a 1:1 ratio and retro-orbitally injected into lethally irradiated (~950 rad) adult recipients. Mixed chimeras were analyzed after 7–8 weeks as indicated.
Antigen presentation
For in vitro experiments, CD11c+MHC-II+ sorted BMDCs (day 7) were incubated with 50 µg/mL full-length ovalbumin (Sigma-Aldrich) for 3–15 hours, thoroughly washed and co-cultured with Cell Trace Violet (Life Technologies)-stained OT-I or OT-II isolated T cells in a 1:10 ratio for 65–75 hours. T cell proliferation was analyzed by FACS on the basis of Cell Trace Violet and CD3+CD8b+ or CD3+CD4+, respectively. Division index was calculated by FlowJo.
Retroviral transduction
Murine Satb1 cDNA (Source Bioscience) was subcloned into the retroviral expression vector pBMN-I-GFP (Addgene). BM cells were isolated from wild-type or Lgals1−/− mice and cultured as with GM-CSF for BMDC differentiation. At days 2 and 3 of culture, cells were washed and resuspended in Mock or Satb1 pBMN virus-containing media supplemented with 8 mg/mL of Polybreen (Millipore). Cells were then spin-infected at 2500 RPM for 2 hours at 32° C, and the media was renewed 18 hours later. After 4 days of viral transduction, BMDCs were FACS sorted for GFP+CD11c+ and tested for different readouts as indicated.
In vivo delivery of PEI-siRNA nanoparticles/Satb1 silencing
Selective in vivo targeting of Satb1 in tumor-associated DCs was previously described6,7. In brief, murine on-Targeting (Ambion) or Satb1 (Ambion or Integrated DNA Technologies) siRNA-polyethylenimine nanocomplexes were administered i.p to mice bearing ID8-Defb29/Vegf-a tumors, using endotoxin-free “in vivo-Jet-PEI” FluoR at an N/P ratio of 6 following the recommendations of the manufacturer (Polyplus Transfection).
For survival, ELISPOTs, phenotypic and antigen presentation experiments, ID8-Defb29/Vegf-a-challenged mice were i.p. treated with 50 µg of siRNA-PEI nanocomplexes on days 8, 13, 18, 23 and 28 after tumor challenge. Survival was monitored periodically from this time-point. For phenotypic experiments, total cells were obtained from RBC lysed peritoneal washes of siRNA-PEI treated tumor-bearing mice 24 h after the last treatment and analyzed by flow cytometry. Alternatively, total peritoneal cells were co-cultured for 48 h, in coated and blocked ELISPOT plates with BMDCs (Day 7) in a 10:1 ratio, which were previously pulsed (overnight) with Gamma and UV-irradiated ID8-Defb29/Vegf-a cells (10 dendritic cells:1 tumor cell). Analysis for IFN-γ and Granzyme B spots was performed according to the manufacturer’s protocol (eBioscience).
To test antigen presentation capabilities of tumor-associated-DCs in vivo, mice bearing ID8-Defb29/Vegf-a for 25–30 days were i.p. injected with 0.6 mg of full-length ovalbumin. After 3 hours, mice were either left untreated or injected i.p with Non-targeting or Satb1 siRNA-PEI nanocomplexes. Then, negatively isolated CFSE-labeled CD3+ OT-I splenocytes (2x106/mouse) were injected i.p 18 hours after the siRNA treatment. 48 hours later, CD3+CFSE+OT-I+ cells were harvested from peritoneal cavity and analyzed by FACS for proliferation.
Genomic and Bioinformatical analysis
RNA-seq data are accessible at NCBI's Gene Expression Omnibus (http://ncbi.nlm.nih.gov/geo/; GSE76776). Additional details are available in Supplemental Information Methods.
Supplementary Material
LIST OF CHANGES.
Following your advice, we have revised the title of the manuscript.
As requested, we have replaced the term “epigenetic” in the first sentence of the abstract by the term “transcriptional”, which more accurately convey what Satb1 affects in Dendritic Cells.
We have strived to improve the grammar throughout the manuscript, for which we have requested the advice of other colleagues. We of course remain open to any editorial suggestions.
We have reduced the contrast of Figure 5D, which appeared overcontrasted in the previous version due to the low number of cells recovered from the tumor microenvironment.
We have deposited our RNA deep sequencing data and included the Accession number.
We have double checked all author names and panels.
We have completed the final file checklist and formatted the files according to the guidelines. Please, note that we have checked those boxes that apply to our manuscript, but we believe that we are in compliance with all guidelines.
Acknowledgments
Support for Shared Resources was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute. We are grateful to the Flow Cytometry Facility at Wistar for outstanding technical insight; P. Jiang for the generation of Satb1-targeted mice; and Dr. B. Stanger (U. Pennsylvania) for generously providing the RosaNotch mouse. This study was supported by R01CA157664, R01CA124515, R01CA178687, U54CA151662, P30CA10815, The Jayne Koskinas & Ted Giovanis Breast Cancer Research Consortium at Wistar and Ovarian Cancer Research Fund Program Project Development awards. MJA and NS were supported by T32CA009171. APP was supported by the Ann Schreiber Mentored Investigator Award (OCRF). AJT was a nested Teal Scholar in DoD grant OC100059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTIONS: A.J.T. designed, performed and analyzed most experiments. M.R.R. helped performing and designing flank tumor treatments and mice chimeras and in vivo DC mobilization. E.B. performed MLRs and phenotypic analysis of human samples. T.L.S. designed overexpression experiments and contributed to the generation of the Satb1 KO. M.J.A., N.S. and A.P.P did and analyzed in vitro experiments and contributed to the design of in vivo experiments. J.N. did and analyzed in vitro experiments. M.E.B. and J.T. provided clinical specimens and expertise and helped writing the manuscript. G.R. provided the galectin-1 KO and intellectual support, and helped writing the manuscript. J.W. and A.V.K. performed the bioinformatics analysis and provided intellectual support. J.R.C.G. oversaw and designed the study and experiments, analyzed data and co-wrote the manuscript.
REFERENCES
- Alvarez JD, Yasui DH, Niida H, Joh T, Loh DY, Kohwi-Shigematsu T. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000;14:521–535. [PMC free article] [PubMed] [Google Scholar]
- Borghesi L. Hematopoiesis in steady-state versus stress: self-renewal, lineage fate choice, and the conversion of danger signals into cytokine signals in hematopoietic stem cells. J Immunol. 2014;193:2053–2058. doi: 10.4049/jimmunol.1400936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet. 2006;38:1278–1288. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP, Stupirski JC, Mazal D, Osinaga E, Toscano MA, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73:1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis ESC. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity. 2015;42:1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, Benencia F, Stan RV, Keler T, Sarobe P, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Lee BK, Iyer VR. Genome-wide studies of CCCTC-binding factor (CTCF) and cohesin provide insight into chromatin structure and regulation. J Biol Chem. 2012;287:30906–30913. doi: 10.1074/jbc.R111.324962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochamy J, Rogers EM, Boss JM. CREB and phospho-CREB interact with RFX5 and CIITA to regulate MHC class II genes. Mol Immunol. 2007;44:837–847. doi: 10.1016/j.molimm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N, Nishiyama C, Yagita H, Koyanagi A, Akiba H, Chiba S, Ogawa H, Okumura K. Notch signaling confers antigen-presenting cell functions on mast cells. J Allergy Clin Immunol. 2009;123:74–81. e71. doi: 10.1016/j.jaci.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Nesbeth Y, Scarlett U, Cubillos-Ruiz J, Martinez D, Engle X, Turk MJ, Conejo-Garcia JR. CCL5-mediated endogenous antitumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res. 2009;69:6331–6338. doi: 10.1158/0008-5472.CAN-08-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S. Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol. 2010;8:e1000296. doi: 10.1371/journal.pbio.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan Kumar P, Purbey PK, Sinha CK, Notani D, Limaye A, Jayani RS, Galande S. Phosphorylation of SATB1, a global gene regulator, acts as a molecular switch regulating its transcriptional activity in vivo. Mol Cell. 2006;22:231–243. doi: 10.1016/j.molcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- Rubinstein N, Alvarez M, Zwirner N, Toscano M, Ilarregui J, Bravo A, Mordoh J, Fainboim L, Podhajcer O, Rabinovich G. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG, et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, Rogers NC, Moncaut N, Carvajal JJ, Reis ESC. Genetic Tracing via DNGR-1 Expression History Defines Dendritic Cells as a Hematopoietic Lineage. Cell. 2013;154:843–858. doi: 10.1016/j.cell.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, Dalod M, Soumelis V, Amigorena S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Vega-Ramos J, Roquilly A, Zhan Y, Young LJ, Mintern JD, Villadangos JA. Inflammation conditions mature dendritic cells to retain the capacity to present new antigens but with altered cytokine secretion function. J Immunol. 2014;193:3851–3859. doi: 10.4049/jimmunol.1303215. [DOI] [PubMed] [Google Scholar]
- Yasui D, Miyano M, Cai S, Varga-Weisz P, Kohwi-Shigematsu T. SATB1 targets chromatin remodelling to regulate genes over long distances. Nature. 2002;419:641–645. doi: 10.1038/nature01084. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity. 2009;30:845–859. doi: 10.1016/j.immuni.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.