Abstract
Purpose
We examined the clinical/pathologic features of ipsilateral second breast cancers (IP-SBCs) following breast-conserving surgery (BCS) for DCIS among community-treated patients, and ascertained the degree of correlation between the features of index DCIS and IP-SBC events.
Methods
From a Cancer Research Network cohort of DCIS patients diagnosed 1990–2001 and treated with BCS, we identified women who subsequently developed an ipsilateral DCIS or invasive breast cancer. All index DCIS tumors underwent expert pathology review. Pathologic characteristics of IP-SBCs were abstracted from available medical records. Logistic regression was used to examine associations between pathologic characteristics and identify factors associated with invasive versus non-invasive IP-SBC.
Results
Of 1969 DCIS patients, 182 developed an IP-SBC within a median of 38 months (range 6–160). IP-SBCs were slightly more commonly non-invasive (53%) vs. invasive (47%). Of invasive IP-SBCs, 31% were high grade, 67% were <20 mm, 74% were estrogen-receptor (ER) positive, 7% were HER2 positive, and 16% were node-positive. Of non-invasive IP-SBCs, 33% were high grade. Comparing index DCIS and IP-SBC specimens, there was moderate-high correlation for HR status and grade. Among patients with IP-SBCs, those who were younger and whose index DCIS tumors were HR negative had shorter intervals (within 3 years) between index and IP-SBC diagnoses. No index DCIS feature was statistically significantly associated with an IP-SBC that was invasive versus non-invasive.
Conclusions
Understanding the characteristics of SBCs and identifying correlations between these and index DCIS events could influence treatment choices for DCIS, and may help patients and providers develop treatment paradigms for SBCs.
Keywords: Breast cancer, DCIS, recurrence, second breast cancer, hormone-receptor, grade
INTRODUCTION
The incidence of ductal carcinoma in situ (DCIS) has increased steadily over the past 25 years, from less than 6/100,000 in the 1970’s to greater than 30/100,000 after 2000.[1–5] This change is due in large part to the adoption of screening mammography. Currently DCIS accounts for approximately one-quarter of all newly diagnosed breast cancers in the United States (US)[2, 3, 6–8], and one-half of all those identified by mammography.[9–11] The majority of women who are diagnosed with DCIS will be treated with breast conserving surgery (BCS)[6]; some will also receive radiation therapy and/or tamoxifen/aromatase inhibitors. Relative survival following DCIS is high (>97%).[12, 13] These factors, together with the aging of the US population and the increased use of screening mammography mean that the number of women living with a history of DCIS will increase substantially in the years to come.
Women who have completed treatment for DCIS are at risk of developing a second breast cancer (SBC) that could either be DCIS or invasive disease. Among those treated with BCS, the risk of developing a SBC is greatest in the ipsilateral breast, where the SBC event could represent re-growth of DCIS, progression to invasive breast cancer, or the development of a new primary. After BCS alone (i.e., without post-lumpectomy radiation therapy), the 5-year risk of developing an ipsilateral SBC (IP-SBC) may be as high as 20%.[14] This may be 3–5 fold-greater than the risk of developing de novo breast cancer experience by the general population.[12, 15–18] As the number of women with a history of DCIS increases and overall life expectancy improves the number of women who develop SBCs will climb. Unfortunately, little is known about the pathologic characteristics of, optimal treatments for, and outcomes experienced by women who develop SBCs.
Studies suggest that approximately half of the IP-SBC events that follow a DCIS diagnosis are invasive,[12, 14, 15, 18–21] and that there may be some degree of correlation between the pathologic features of the index DCIS and IP-SBC events.[22] However, significant questions regarding the characteristics of SBCs remain. Understanding the pathologic characteristics and timing of IP-SBCs could help inform prognostic estimates for patients with DCIS and for those who develop IP-SBCs. More importantly, understanding the extent to which index DCIS and IP-SBC diagnoses are similar and identifying factors associated with having a higher (versus lower) risk IP-SBC could inform treatment options for these patients. Notably, there is ongoing debate regarding how aggressive therapy for primary DCIS should be. If there were a high degree of correlation between the pathologic features of the index DCIS and the IP-SBC this could potentially influence initial decision making. DCIS patients who could expect a lower risk of having an invasive IP-SBC could feel more comfortable with conservative treatment for their initial DCIS diagnosis.
Most epidemiologic studies and clinical trials focus on women with primary breast cancer. While tumor registries, such as the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, identify new primary cancers, they do not record information on cancers considered to be recurrences (although the definition of recurrence has changed over time). Consequently, using registries alone to gather information regarding IP-SBCs could lead to underestimates of risk or biased samples. Because of the resources needed to capture complete information on recurrence status, few population-based data for patients with IP-SBC are available. Even though the number of women who develop SBC will continue to grow, conducting randomized controlled trials in patients with SBC will remain challenging. A National Cancer Institute-funded Cancer Research Network (CRN) cohort of DCIS patients was established to examine the patient, clinical and tumor factors associated with breast cancer recurrence.[23, 24] Using this cohort of patients treated in a real-world community setting, we investigated the clinical and pathologic characteristics of ipsilateral SBCs, investigated whether any of these features was associated with shorter time-to-recurrence, and assessed the degree of correlation between the pathology characteristics of index DCIS and SBC events.
METHODS
Data Source
The CRN is a consortium of integrated health care delivery systems with more than 12 million enrollees. The overall mission of the CRN is “to increase the effectiveness of preventive, curative, and supportive interventions for major cancers through a program of collaborative research, and to determine the effectiveness of cancer control interventions that span the natural history of major cancers among diverse populations and health systems.”[25] Because CRN data are derived from integrated health delivery systems, the capture of events and procedures for covered patients is generally robust.
Previously, three CRN sites developed a cohort of patients with newly diagnosed DCIS: Kaiser Permanente Northern California (KPNC), Kaiser Permanente Southern California (KPSC) and Harvard Pilgrim Health Care (HPHC).[25] The study design and data collection methods for the CRN DCIS cohort study have been described in detail elsewhere and are briefly summarized below.[26] Two of the three sites that participated in the DCIS cohort study collected additional data on SBC events (KPNC and HPHC). Our analysis only included patients from these two sites. The institutional review boards at each participating site approved the protocol as well as the data collection and transmission procedures.
Cohort Development
The original DCIS cohort included approximately 3000 patients who were diagnosed with unilateral non-invasive breast cancer between 1990 and 2001 and treated with BCS. Patients with LCIS were excluded, unless they also had DCIS. Patients were considered eligible if their medical records indicated that they received healthcare services from their primary network site; and were excluded if they had a prior history of breast or other cancer (except non-melanoma skin cancer), a breast cancer in the contralateral breast at the time of the index DCIS diagnosis, or a mastectomy within six months of the DCIS diagnosis. Censoring of follow-up occurred at end of study follow-up, or if a patient disenrolled from the health plan (i.e., network site) or died.
The study described herein focused on a subset of patients from the original DCIS cohort – those who developed a second breast cancer (whether DCIS or invasive disease) in the ipsilateral breast. Surveillance for SBC events started six months after the index DCIS diagnosis, and continued until ipsilateral breast cancer recurrence, ipsilateral prophylactic mastectomy, contralateral breast cancer diagnosis, non-breast invasive cancer diagnosis, death, or last medical record contact, whichever occurred first. For patients with more than one breast cancer event after their index DCIS diagnosis, only the first event was included in this analysis.
Cohort Data Abstraction & Pathology Review
For the original DCIS cohort, data abstracted from the medical record included age at diagnosis, race/ethnicity, menopausal status, body mass index, method of DCIS detection, and pathologic characteristics of the index DCIS (from pathology reports). Therapies administered for the index DCIS within one year of diagnosis were also collected, including the use of radiation therapy and tamoxifen. Medical record abstraction was used to identify subsequent cancer diagnoses. SBC events were classified as either DCIS or invasive. If both types of disease were present in the same specimen, then the event was recorded as invasive.
For all patients who developed an IP-SBC, the diagnostic slides of the index DCIS specimens were obtained and sent to the study breast cancer pathologists for a standardized review. A number of features were assessed during the expert pathology review. Nuclear grade was categorized as low (grade 1/well-differentiated); intermediate (grade 2/moderately differentiated); or high (grade 3/poorly differentiated). DCIS size was based on the number of involved high power-fields. Invasive tumor size was based on the extent of involvement of the pathologic specimen measured in centimeters. Surgical margins were classified as tumor at the margin, tumor <1mm from the margin, tumor 1–3 mm from the margin, or tumor >3 mm from the margin. Sections from archived tumor blocks underwent immuno-staining for the estrogen receptor (ER), progesterone receptor (PR), HER2 receptor, Ki67, and other markers.
Additional medical record abstraction was performed for the current study to collect data regarding the pathologic characteristics of the IP-SBCs, including grade, ER status, PR status and HER2 status.
Statistical Analyses
We analyzed correlations between the pathologic characteristics of index DCIS and SBC events separately for patients who had invasive vs. non-invasive IP-SBCs. For patients with invasive IP-SBCs we looked at ER, PR, and grade. For those with non-invasive IP-SBCs we only evaluated grade, because hormone-receptor status was not available for most recurrent DCIS events. To assess the impact of time from diagnosis, the sample was stratified into two groups based on whether the second event occurred before or after the median time from the index DCIS diagnosis. Correlations between pathologic features were assessed using the C-statistic, which was generated by fitting a simple logistic regression model with the SBC-pathologic characteristic as the dependent variable and the index DCIS-pathologic characteristic as the predictor variable. If the pathologic data were missing, an event was excluded from the analysis (i.e., comparisons only included patients who had pathologic data for both events).
Multivariable logistic regression models were used to examine whether patient or index DCIS tumor characteristics were associated with invasive versus non-invasive IP-SBC. The characteristics included demographic factors (age, race/ethnicity), clinical factors (method of index cancer detection, body mass index, menopausal status, year of index DCIS diagnosis), pathologic factors of the index DCIS (size, margin, grade, architectural pattern, associated LCIS, ER, and PR), and use of radiation therapy for the initial DCIS. For each covariate, a bi-variable logistic regression model with invasive IP-SBC as the dependent variable was generated, with the intention that any statistically significant predictors (P<0.05) would be included in the multi-variable model.
We hypothesized that the pathologic features of the index DCIS, such as hormone-receptor status, could influence the characteristics or timing of IP-SBC. We stratified the sample by index DCIS hormone-receptor status, and plotted the proportion of all IP-SBCs that were DCIS, invasive HR positive, or invasive HR negative for each year after the index diagnosis. After dichotomizing the sample based on median time to SBC event, we tested whether ER-status, PR-status, grade, age, or other features of the index DCIS were associated with a shorter time to IP-SBC. All P-values were two-sided, and values <0.05 were considered statistically significant.
RESULTS
From the original CRN DCIS cohort study of 2995 DCIS cases, 10.9% (n=325) experienced an IP-SBC at a median follow-up of 58 months.[25] Among the 1969 patients who were treated at the two sites that contributed to this sub-cohort, 9.2% (n= 182) were diagnosed with an IP-SBC at a median follow-up of 38 months (minimum 6, maximum 160). Among women who had an IP-SBC, the median age at diagnosis of the index DCIS was 55 years (minimum 35, maximum 84). Seventy-two percent of patients were white, 57% were postmenopausal, and 50% were overweight or obese (Table 1). BCS without radiation or tamoxifen was the primary therapy for most (67%) patients.
Table 1.
Baseline characteristics of patients who had DCIS treated with breast conserving surgery and experienced an ipsilateral second breast cancer*
| Characteristic | No. (%) |
|---|---|
| Age (years) | |
| < 40 | 14 (7.7) |
| 40 to < 50 | 48 (26.4) |
| 50 to < 60 | 52 (28.6) |
| 60 to < 70 | 47 (25.8) |
| ≥ 70 | 21 (11.5) |
| Median | 55 |
| Race / ethnicity | |
| White non-Hispanic | 131 (72.0) |
| African-American non-Hispanic | 17 (9.3) |
| Hispanic | 13 (7.1) |
| Asian non-Hispanic | 20 (11.0) |
| Unknown | 1 (0.6) |
| Menopausal status | |
| Premenopausal | 71 (39.0) |
| Postmenopausal | 104 (57.1) |
| Unknown | 7 (3.9) |
| Body mass index | |
| < 20 | 9 (5.0) |
| 20 to < 25 | 74 (40.7) |
| 25 to < 30 | 55 (30.2) |
| ≥ 30 | 36 (19.8) |
| Unknown | 8 (4.4) |
| Presentation | |
| Mammography | 148 (81.3) |
| Symptomatic (e.g., palpation) | 33 (18.1) |
| Unknown | 1 (0.6) |
| Year of diagnosis | |
| 1990–1993 | 57 (31.3) |
| 1994–1997 | 94 (51.7) |
| 1998–2001 | 31 (17.0) |
| Index DCIS management | |
| Lumpectomy alone | 122 (67.0) |
| Lumpectomy + radiation | 56 (30.8) |
| Lumpectomy + radiation + tamoxifen | 4 (2.2) |
Abbreviation: DCIS = ductal carcinoma in situ
Approximately 14% of index DCIS diagnoses had a positive margin (Table 2). In addition 37% were high grade, 74% were ER positive, and 63% were comedo pattern. Ipsilateral SBCs were slightly more likely to be non-invasive (53%) vs. invasive (47%). Of the invasive IP-SBCs, 31% were high grade, 67% had a tumor size <20 mm, 74% were ER positive, 7% were HER2 positive, and 16% were node-positive (Table 3). Of the non-invasive IP-SBC’s, 33% were high grade. Comparisons between the pathologic features of index DCIS and IP-SBC cases appear in Table 4 (a detailed correlation table appears in the supplemental materials). For patients who developed an invasive IP-SBC, the C-statistics, comparing characteristics of the index and IP-SBC tumors, were 0.92 for ER status, 0.73 for PR status, and 0.66 for grade, suggesting strong to moderate correlation. For patients who developed non-invasive IP-SBCs, the C-statistic for grade was 0.74, consistent with a moderate correlation. We tested each covariate listed in Table 1 as a potential predictor invasive versus non-invasive IP-SBC, but none was statistically significant (not shown).
Table 2.
Pathologic characteristics of index DCIS*
| Characteristics | No. (%) |
|---|---|
| Tumor size (# of LPF) | |
| 1–5 | 65 (35.7) |
| 6–9 | 29 (15.9) |
| 10–19 | 41 (22.5) |
| ≥20 | 47 (25.8) |
| Margin (mm from tumor) | |
| Positive | 25 (13.7) |
| <1 | 64 (35.2) |
| 1–3 | 20 (11) |
| >3 | 49 (26.9) |
| Unknown | 24 (13.2) |
| Nuclear grade | |
| High | 67 (36.8) |
| Intermediate | 103 (56.6) |
| Low | 12 (6.6) |
| Estrogen receptor† | |
| Positive | 134 (73.6) |
| Negative | 25 (13.7) |
| Unknown | 23 (12.6) |
| Progesterone receptor† | |
| Positive | 113 (62.1) |
| Negative | 36 (19.8) |
| Unknown | 33 (18.1) |
| Histology Subtype | |
| Comedo | 114 (62.6) |
| Non-comedo | 68 (37.4) |
| HER2† | |
| 0–1+ | 92 (50.5) |
| 2+ | 32 (17.6) |
| 3+ | 26 (14.3) |
| Unknown | 32 (17.6) |
| LCIS† | |
| Present | 17 (9.3) |
| Absent | 165 (90.7) |
Abbreviations: DCIS = ductal carcinoma in situ; # LPF = number of involved low power fields; HER2 = human epidermal growth factor receptor 2; LCIS = lobular carcinoma in situ
Immuno-histochemical analysis obtained via central pathology review
Table 3.
Pathologic characteristics of ipsilateral second breast cancers*
| DCIS only (N=97) | Invasive (N=85) | |
|---|---|---|
| Characteristic | No. (%) | No. (%) |
| Tumor size (mm) | ||
| 1 to 5 | 19 (19.6) | 16 (18.8) |
| >5 to 10 | 18 (18.6) | 22 (25.9) |
| >10 to 20 | 7 (7.2) | 19 (22.4) |
| >20 to 50 | 4 (4.1) | 8 (9.4) |
| >50 | 1 (1.0) | 1 (1.2) |
| Unknown | 48 (49.5) | 19 (22.4) |
| Grade | ||
| Low | 9 (9.3) | 11 (12.9) |
| Intermediate | 28 (28.9) | 29 (34.1) |
| High | 32 (33.0) | 26 (30.6) |
| Unknown | 28 (28.9) | 19 (22.4) |
| Histology subtype† | - | |
| Ductal | - | 66 (77.7) |
| Lobular | - | 2 (2.4) |
| Ductal and lobular | - | 4 (4.7) |
| Tubular/colloid/papillary | - | 5 (5.9) |
| Other/Unknown | - | 8 (9.4) |
| Estrogen receptor‡ | ||
| Negative | 2 (2.1) | 9 (10.6) |
| Positive | 4 (4.1) | 63 (74.1) |
| Unknown | 91 (93.8) | 13 (15.3) |
| Progesterone receptor‡ | ||
| Negative | 3 (3.1) | 22 (25.9) |
| Positive | 2 (2.1) | 49 (57.6) |
| Unknown | 92 (94.8) | 14 (16.5) |
| HER2†,‡ | ||
| Negative (degree of staining unknown) | - | 33 (38.8) |
| 0–1+ | - | 9 (10.6) |
| 2+ | - | 8 (9.4) |
| 3+ | - | 5 (5.9) |
| Positive (degree of staining unknown) | - | 1 (1.2) |
| Unknown | - | 29 (34.1) |
| Lympho-vascular Invasion† | ||
| Positive / present | - | 7 (8.2) |
| Negative / absent | - | 28 (32.9) |
| Unknown | - | 50 (58.8) |
| Lymph nodes (No. positive)† | ||
| Clinical negative | - | 20 (23.5) |
| Pathologic negative | - | 51 (60.0) |
| Pathologic positive (1–3) | - | 10 (11.8) |
| Pathologic positive (4+) | - | 4 (4.7) |
| Distant recurrence† | ||
| Present | - | 4 (4.7) |
| Absent | - | 81 (95.3) |
Abbreviations: DCIS = ductal carcinoma in situ; HER2 = human epidermal growth factor receptor 2.
Characteristics only available for invasive recurrences
Immuno-histochemical data obtain from medical record abstraction of primary data
Table 4.
Concordance between index DCIS and ipsilateral SBC hormone-receptor expression and tumor grade stratified by the type of SBC
| Characteristic (Index / Second Event) |
No. Identical Cases^ / No. Eligible |
% Concordant | C-statistic** |
|---|---|---|---|
| DCIS / Invasive | |||
| ER | 63 / 65 | 96.9 | 0.92 |
| PR | 48 / 59 | 81.4 | 0.73 |
| Grade | 31 / 66 | 47.0 | 0.64 |
| DCIS / DCIS | |||
| Grade | 42 / 69 | 60.9 | 0.74 |
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; PR, progesterone receptor.
Indicates number of cases with the same hormone receptor status or grade between index DCIS and second breast cancer.
Association between histopathology factor of the index DCIS and that of the second breast cancer assessed by the C-statistic, generated by fitting a simple logistic regression with recurrence status as a dependent variable and index DCIS status as a predictor
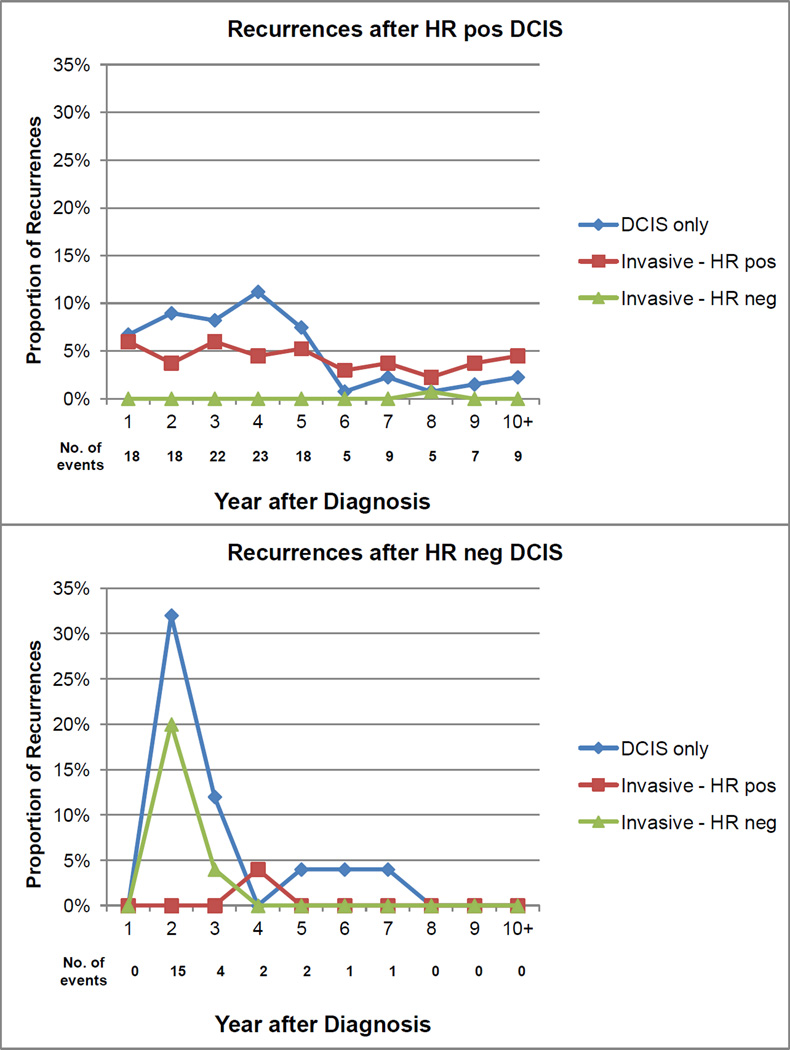
The sample was stratified into two groups based on whether the IP-SBC occurred before or after the median time from index DCIS diagnosis (38 months). Factors associated with an early IP-SBC included ER negative index DCIS (P=0.03), PR negative index DCIS (P<0.01), and young age (P<0.01). To describe this finding in more detail, we displayed the proportion of all IP-SBC events that were non-invasive, invasive-ER positive, and invasive-ER negative for each year after diagnosis stratified by index DCIS hormone-receptor status (Figure 1). For HR-positive index DCIS cases, a relatively smaller proportion of recurrences occurred within 4 years, and many were attributable to non-invasive or HR-positive invasive disease. For HR-negative index DCIS cases, a relatively larger proportion of recurrences occurred within 4 years and many were attributable to non-invasive or HR-negative invasive disease. While interesting, these findings should be viewed cautiously, because relatively few patients had 6–10 years of follow-up and the number of IP-SBC events among patients originally diagnosed with HR-negative DCIS was relatively small. Note that there was no association between margin and time to recurrence (i.e., patients with close/positive margins were no more likely to experience an earlier recurrence; P=0.45).
Figure 1.
Proportions of SBCs that were DCIS only, invasive HR-positive, or invasive HR negative stratified by the HR status of the index DCIS diagnosis. Proportions are presented for each year after the index DCIS through a median follow-up of 58 months. There were 134 recurrences after HR-positive DCIS, and 25 recurrences after HR-negative DCIS. Abbreviations: DCIS=ductal carcinoma in situ; HR=hormone receptor.
DISCUSSION
Among 182 patients who developed an ipsilateral SBC after BCS for DCIS we identified moderate-to-high degrees of correlation in hormone-receptor status and grade between the two breast cancer events. Neither patient factors nor pathologic features of the index DCIS were associated with having an invasive versus a non-invasive IP-SBC. Patients who developed an IP-SBC sooner tended to be younger and to have had a hormone-receptor negative index DCIS.
To our knowledge, only two prior studies have compared the pathologic characteristics of SBC events with those of index DCIS cases.[22, 27] Both found concordant histology in a majority of cases, but each had limitations. One study was based on patients enrolled in a single clinical trial[27], and the other study included patients who were treated within a network of comprehensive cancer centers[22]. These cohorts may not represent DCIS patients in the general population. For example, the study of patients treated at comprehensive cancer centers demonstrated a lower than expected rate of SBCs. The patient characteristics and patterns of care observed in our cohort of DCIS patients diagnosed 1990–2001 are likely more representative of the population of DCIS patients in the US, because our cohort included a significant proportion of patients who were non-white, had close/positive margins, and did not receive post-lumpectomy radiation therapy. Notably, the 9.2% risk of developing an IP-SBC at 5 years seen in our cohort is closer to what was seen in previous clinical trials and a population-based sample from Sweden.[28–30] Also, it is important to note that the rate of recurrence has decreased over time, as mammography has increased and surgery, radiation therapy, and hormonal therapy practices have changed.[17, 24]
One possible explanation for the high degree of pathologic correlation of HR status and grade could be that the IP-SBC events represented re-growth/progression of occult cells from the index DCIS. Perhaps the association in our analysis was particularly strong, because all SBC events were ipsilateral, most occurred within 5 years of the index diagnosis, our cohort had a relatively high rate of close/positive margins, or many patients did not receive adjuvant radiation therapy. Regardless, this hypothesis is supported by a study that used comparative genomic hybridization techniques to analyze index DCIS cases and second ipsilateral DCIS events.[31] In a sample of 18 patients, there was a high degree of concordance in chromosomal alterations (median 81%) that was independent of the time from index DCIS diagnosis to IP-SBC. However, this analysis did not include invasive or contralateral recurrences. A study of women with DCIS who developed either an ipsilateral or contralateral SBC found that the degree of hormone-receptor status concordance was greater for patients who 1) developed an ipsilateral versus contralateral event; 2) did not versus did receive radiation therapy; and 3) had an early versus late SBC.[22] Altogether, these and other findings suggest that a substantial proportion of SBC cases are recurrent events, rather than new primary cancers.[24]
An alternative explanation for the high degree of pathologic concordance could be that patients are predisposed to developing specific types of breast cancer. Interestingly, a previous analysis identified a significant association between the grade of an index DCIS and the grade of a contralateral SBC.[22] Perhaps patient-specific factors are contributing to the association. Molecular genomic profiling could help clarify the relative importance of patient- versus cancer-specific factors on the development of low versus high risk SBC.
A number of studies have demonstrated that hormone-receptor positive invasive breast cancer can recur early or late, whereas hormone-receptor negative invasive breast cancer usually recurs within 5–6 years of diagnosis.[32, 33] We are not aware of previous studies describing the patterns of recurrence after treatment for non-invasive breast cancer. It is notable to have demonstrated that the hormone-receptor status of an index DCIS may be associated with the time at which an IP-SBC develops. Our findings should not be interpreted as an indication that hormone-receptor negative DCIS has a substantially higher risk of recurrence. Rather, SBCs that occur after a diagnosis of hormone-receptor negative DCIS may be more likely to develop early compared with SBCs that occur after a diagnosis of hormone-receptor positive DCIS.
The ability of the CRN cohort study, which obtained the patient, treatment, and outcomes information used in this analysis, to identify SBCs is high, because only those patients who received health care from one delivery system were included in the cohort. That having been said, some patients could have been lost to follow-up, because they transferred their care to a different network. There was no evidence to suggest that patients’ DCIS characteristics were associated with their likelihood of following-up at the same CRN site. Our analysis was strengthened by its focus on patients who received care from community-based practices, rather than clinical trials or comprehensive cancer centers.
This study has some limitations. An expert, comprehensive pathology review was conducted for the index DCIS samples, but we had to rely on a different methodology, medical record abstraction, to identify the pathologic features of the IP-SBC samples. Consequently, some IP-SBC pathologic characteristics were missing for a substantial proportion of patients. This was a particular problem when analyzing the hormone-receptor status of non-invasive IP-SBCs. Hormone-receptor testing was not done routinely until 2000, when tamoxifen was approved for treatment of DCIS. Among patients who developed an IP-SBC, we looked for but were not able to identify factors statistically significantly associated with an invasive (i.e., higher risk) versus a non-invasive (i.e., lower risk) SBC event. Even with 182 patients, the power to identify correlations between the features of initial DCIS and IP-SBC events was relatively modest. A previous analysis of patients treated for DCIS, including those who did and did not go on to develop an IP-SBC, found that having a symptomatic presentation for the index DCIS was a significant risk factor for the development of an invasive SBC; while African-American race, younger age and larger tumor size were more strongly associated with the development of a non-invasive SBC.[24] Further, larger tumor size and close-involved surgical margins were associated with developing any type of SBC. Two other DCIS studies have reported that higher nuclear grade, close/positive surgical margins, and necrosis were associated with the development of any IP-SBC.[23, 34] Future studies should assess the impact of primary DCIS treatments on the pathologic features of SBCs. While our study followed patients through a median of 58 months, breast cancer recurrences can occur 10 or more years after the original diagnosis; we were not able to assess correlations with late events. Additional efforts to identify and integrate patient, pathologic, and genomic [35] determinants of recurrence after BCS for DCIS are warranted.
By shedding light on the characteristics of the cancers that develop after an initial DCIS diagnosis, the findings from this study could influence the treatment plans devised for both index DCIS and SBC events. Knowing what type of IP-SBC would be likely to develop if a woman with excised DCIS were to have a recurrence could influence her approach to treating the initial DCIS diagnosis. For example, a woman who had surgery for a low grade, ER positive DCIS may feel less anxious about a close margin or less inclined to consider radiation or tamoxifen therapy if she thought any IP-SBC that could develop would probably be low grade/ER positive. Additionally, knowing that the characteristics of an IP-SBC tend to mimic those of the initial breast cancer could help patients and providers feel more comfortable applying treatment paradigms developed for an initial diagnosis to patients who develop an IP-SBC. Newer, gene-expression profile-based analyses may also help identify DCIS patients at greater risk of recurrence.[35] Regardless, uncertainty surrounding the impact of treatments on the outcomes of women who develop IP-SBCs persists, arguing strongly in favor of clinical trials to evaluate the efficacy and tolerability of treatments for this growing patient population.
Supplementary Material
Acknowledgments
Funding
This work was supported by contract # HHSA290200500161 from the Agency for Healthcare Research and Quality and by the National Institutes of Health (R01 CA81302 to L.A.H.; U19 CA079689 to Ed Wagner).
Dr. Hassett received salary support from a Susan G. Komen for the Cure Career Catalyst Award.
The views expressed in this article are those of the authors, and no official endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services is intended or should be inferred.
Footnotes
Disclosures and Potential Conflicts of Interests
Conflicts of Interests: The authors declare that they have no conflicts of interest.
Contributor Information
Michael J. Hassett, Email: michael_hassett@dfci.harvard.edu.
Wei Jiang, Email: wjiang3@partners.org.
Laurel A. Habel, Email: laurel.habel@kp.org.
Larissa Nekhlyudov, Email: larissa_nekhlyudov@harvardpilgrim.org.
Ninah Achacoso, Email: ninah.s.achacoso@nsmtp.kp.org.
Luana Acton, Email: luana.acton@kp.org.
Stuart J Schnitt, Email: sschnitt@caregroup.harvard.edu.
Deb Schrag, Email: deb_schrag@dfci.harvard.edu.
Rinaa S. Punglia, Email: rpunglia@lroc.harvard.edu.
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. [ http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf] [Google Scholar]
- 2.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. Journal of the National Cancer Institute. 2010;102(3):170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 3.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, Yankaskas BC, Rosenberg R, Carney PA, Kerlikowske K, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. Journal of the National Cancer Institute. 2002;94(20):1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 4.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275(12):913–918. [PubMed] [Google Scholar]
- 5.Sumner WE, 3rd, Koniaris LG, Snell SE, Spector S, Powell J, Avisar E, Moffat F, Livingstone AS, Franceschi D. Results of 23,810 cases of ductal carcinoma-in-situ. Annals of surgical oncology. 2007;14(5):1638–1643. doi: 10.1245/s10434-006-9316-1. [DOI] [PubMed] [Google Scholar]
- 6.Sumner WE, 3rd, Koniaris LG, Snell SE, Spector S, Powell J, Avisar E, Moffat F, Livingstone AS, Franceschi D. Results of 23,810 cases of ductal carcinoma-in-situ. Ann Surg Oncol. 2007;14(5):1638–1643. doi: 10.1245/s10434-006-9316-1. [DOI] [PubMed] [Google Scholar]
- 7.Desantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA: a cancer journal for clinicians. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 8.Fenton JJ, Xing G, Elmore JG, Bang H, Chen SL, Lindfors KK, Baldwin LM. Short-term outcomes of screening mammography using computer-aided detection: a population-based study of medicare enrollees. Annals of internal medicine. 2013;158(8):580–587. doi: 10.7326/0003-4819-158-8-201304160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrow M, Schmidt R, Cregger B, Hassett C, Cox S. Preoperative evaluation of abnormal mammographic findings to avoid unnecessary breast biopsies. Arch Surg. 1994;129(10):1091–1096. doi: 10.1001/archsurg.1994.01420340105021. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm MC, Edge SB, Cole DD, deParedes E, Frierson HF., Jr Nonpalpable invasive breast cancer. Ann Surg. 1991;213(6):600–603. doi: 10.1097/00000658-199106000-00010. discussion 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakovitch E, Pignol JP, Chartier C, Hanna W, Kahn H, Wong J, Mai V, Paszat L. The management of ductal carcinoma in situ of the breast: a screened population-based analysis. Breast cancer research and treatment. 2007;101(3):335–347. doi: 10.1007/s10549-006-9302-0. [DOI] [PubMed] [Google Scholar]
- 12.Bijker N, Meijnen P, Peterse JL, Bogaerts J, Van Hoorebeeck I, Julien JP, Gennaro M, Rouanet P, Avril A, Fentiman IS, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(21):3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 13.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA oncology. 2015 doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 14.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, Land SR, Margolese RG, Swain SM, Costantino JP, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. Journal of the National Cancer Institute. 2011;103(6):478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emdin SO, Granstrand B, Ringberg A, Sandelin K, Arnesson LG, Nordgren H, Anderson H, Garmo H, Holmberg L, Wallgren A. SweDCIS: Radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45(5):536–543. doi: 10.1080/02841860600681569. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 17.Habel LA, Daling JR, Newcomb PA, Self SG, Porter PL, Stanford JL, Seidel K, Weiss NS. Risk of recurrence after ductal carcinoma in situ of the breast. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7(8):689–696. [PubMed] [Google Scholar]
- 18.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 19.Fisher ER, Dignam J, Tan-Chiu E, Costantino J, Fisher B, Paik S, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86(3):429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Price P, Sinnett HD, Gusterson B, Walsh G, A'Hern RP, McKinna JA. Duct carcinoma in situ: predictors of local recurrence and progression in patients treated by surgery alone. Br J Cancer. 1990;61(6):869–872. doi: 10.1038/bjc.1990.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solin LJ, Kurtz J, Fourquet A, Amalric R, Recht A, Bornstein BA, Kuske R, Taylor M, Barrett W, Fowble B, et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(3):754–763. doi: 10.1200/JCO.1996.14.3.754. [DOI] [PubMed] [Google Scholar]
- 22.Arvold ND, Punglia RS, Hughes ME, Jiang W, Edge SB, Javid SH, Laronga C, Niland JC, Theriault RL, Weeks JC, et al. Pathologic characteristics of second breast cancers after breast conservation for ductal carcinoma in situ. Cancer. 2012;118(24):6022–6030. doi: 10.1002/cncr.27691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins LC, Achacoso N, Haque R, Nekhlyudov L, Quesenberry CP, Jr, Schnitt SJ, Habel LA, Fletcher SW. Risk Prediction for Local Breast Cancer Recurrence Among Women with DCIS Treated in a Community Practice: A Nested, Case-Control Study. Annals of surgical oncology. 2015 doi: 10.1245/s10434-015-4641-x. [DOI] [PubMed] [Google Scholar]
- 24.Collins LC, Achacoso N, Haque R, Nekhlyudov L, Fletcher SW, Quesenberry CP, Jr, Schnitt SJ, Habel LA. Risk factors for non-invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast cancer research and treatment. 2013;139(2):453–460. doi: 10.1007/s10549-013-2539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habel LA, Achacoso NS, Haque R, Nekhlyudov L, Fletcher SW, Schnitt SJ, Collins LC, Geiger AM, Puligandla B, Acton L, et al. Declining recurrence among ductal carcinoma in situ patients treated with breast-conserving surgery in the community setting. Breast Cancer Res. 2009;11(6):R85. doi: 10.1186/bcr2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habel LA, Achacoso NS, Haque R, Nekhlyudov L, Fletcher AW, Schnitt SJ, Collins LC, Geiger AM, Puligandla B, Acton L, et al. Declining recurrence among ductal carcinoma in situ patients treated with breast-conserving surgery in the community setting. Breast Cancer Research & Treatment. 2009;11 doi: 10.1186/bcr2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bijker N, Peterse JL, Duchateau L, Robanus-Maandag EC, Bosch CA, Duval C, Pilotti S, van de Vijver MJ. Histological type and marker expression of the primary tumour compared with its local recurrence after breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2001;84(4):539–544. doi: 10.1054/bjoc.2000.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, Fisher ER, Wickerham DL, Deutsch M, Margolese R, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(2):441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 29.Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, Forbes JF, Bishop H, Fentiman IS, George WD. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12(1):21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warnberg F, Bergh J, Holmberg L. Prognosis in women with a carcinoma in situ of the breast: a population-based study in Sweden. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1999;8(9):769–774. [PubMed] [Google Scholar]
- 31.Waldman FM, DeVries S, Chew KL, Moore DH, 2nd, Kerlikowske K, Ljung BM. Chromosomal alterations in ductal carcinomas in situ and their in situ recurrences. Journal of the National Cancer Institute. 2000;92(4):313–320. doi: 10.1093/jnci/92.4.313. [DOI] [PubMed] [Google Scholar]
- 32.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(10):2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 33.Kiba T, Inamoto T, Nishimura T, Ueno M, Yanagihara K, Teramukai S, Kato H, Toi M, Fukushima M. The reversal of recurrence hazard rate between ER positive and negative breast cancer patients with axillary lymph node dissection (pathological stage I-III) 3 years after surgery. BMC Cancer. 2008;8:323. doi: 10.1186/1471-2407-8-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudloff U, Jacks LM, Goldberg JI, Wynveen CA, Brogi E, Patil S, Van Zee KJ. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(23):3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 35.Rakovitch E, Nofech-Mozes S, Hanna W, Baehner FL, Saskin R, Butler SM, Tuck A, Sengupta S, Elavathil L, Jani PA, et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast cancer research and treatment. 2015;152(2):389–398. doi: 10.1007/s10549-015-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.