Abstract
Many clinicians who provide mental health treatment find developmental neuroscience discoveries to be exciting. However, the utility of these findings often seem far removed from everyday clinical care. Thus, the goal of this article is to offer a bridge to connect the fields of applied adolescent treatment and developmental neuroscience investigation. An overview of the relevance of developmental neuroscience in adolescent direct practice and a rationale for how and why this integration could benefit adolescent treatment outcomes is provided. Finally, a series of practical suggestions is generated for enhancing collaborative, interdisciplinary work that ultimately advances treatment response for this important clinical population.
Keywords: Clinical, Adolescents, Developmental neuroscience
From the onset of puberty through age 25, the adolescent brain undergoes profound changes in structure and function (Wetherill and Tapert, 2013). While neuroscience research in developmental cognition and psychopathology is scientifically compelling to many clinicians, few practitioners expect that neuroscience discoveries will contribute to and/or modify their day-to-day clinical practice. This science to practice gap is particularly palpable in adolescent mental health, where most established behavioral treatments have less robust outcomes as compared to adults. While increasing levels of attention are being paid to integrative approaches to cross the clinical–neuroimaging divide (Potenza et al., 2011; Goldstein et al., 2009; Naqvi and Morgenstern, 2015), particularly with adolescents (Merikangas et al., 2015; Giedd, 2015; Spear, 2013), few concrete, working collaboratives have been advanced. Thus, at this time, more publications, research presentations, and communication around this topic must occur in order for this conversation to effectively move forward into the arena of active and productive cooperation and dissemination. This commentary offers one step in this direction by articulating the relevance of this message, which represents a priority area at the National Institutes of Health, and by offering concrete steps to begin engaging in this type of clinical–neuroimaging partnership. Ultimately, this commentary offers suggestions to enhance cross-discipline collaboration, with the goal of improving adolescent treatment outcome.
1. Adolescence as a time of neural responsiveness and risk
Research is steadily unraveling the relation between the developing brain and emerging psychopathology in adolescence. Described as a “sensitive period”, (Blakemore and Mills, 2014) this time of catalytic neuronal change contributes to increased opportunity and risk. In terms of benefit, the adolescent brain has an elevated capacity to encode and synthesize new information (Giedd, 2012). For example, experimentation with new behaviors (e.g., driving) combines with plasticity to yield more efficient brain function (e.g., synaptic pruning affected by experience). In this respect, the adolescent brain is relatively more adaptive than during other developmental periods, as it actively receives and synthesizes novel information to improve individual functioning.
Yet, the same evolutionary drive that steers adolescents toward experimentation also enhances the potential for risk. Supporting their predisposition toward reward seeking, the activated dopaminergic (DA) pathways of the brain encompass regions including the orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and ventral striatum (VS). Largely modulated by the ventral tegmental area (VTA), which has DA projections to these and other salient regions (e.g., amygdala), these networks underlie adolescents’ estimation of rewards, along with salient social information. It is argued that the greater activation of these regions during adolescence reflects the redistribution of DA receptor density in these regions during these years. Relative to other age periods, adolescents subsequently experience a greater release of DA in response to rewarding events. Practically, this means that risk behaviors, often inherently exciting, frightening, and fun, may in fact feel much more so during adolescence. Importantly, many of these same developing mesocorticolimbic systems are also key substrates in the later manifestation of psychopathology, including addiction, during adulthood (Volkow and Baler, 2014). Related, the animal and human literatures suggest that one potential modulator of psychopathology onset and course is exposure to common, neurotoxic substances (including alcohol, tobacco, cannabis) during adolescence.
2. The gap between neurodevelopment and direct clinical practice
Despite strong empirical support for these neurobiological models, many clinicians, including those in addiction, report that these discoveries feel disconnected from their day-to-day practice. At the same time, advances in adolescent psychotherapy research have slowed (Feldstein Ewing and Chung, 2013). Clinical researchers are largely at a loss to explain modest expectations for long-term improvement from treatment [e.g., less robust effect sizes for adolescents (ages 12–23; d = 0.17) (Jensen et al., 2011) as compared with adults (ages 16–62; d = 0.77) (Hettema et al., 2005) 12 months following treatment]. Leading treatment researchers have called for change, “moderate effectiveness calls for improvement and scarce resources call for efficiency…more nuanced analytic methods are…needed” (p. 883) (Magill and Longabaugh, 2013).
Despite the commonly held belief that the brain is at the source of human change, few clinical research teams have looked to the adolescent brain to identify new treatment targets or metrics of outcomes. Understanding how and why the adolescent brain does (or does not) change in the context of treatment might lead to improvements in current treatment approaches such as promoting positive brain response (e.g., greater neural control; activation of contemplation networks). Adolescent brain data offers one promising route to enhance current evidence-based treatments for this high-need, and often underserved, age group.
3. Bridging adolescent neuroscience and treatment
Cutting-edge brain imaging methodologies are a highly sensitive set of tools to empirically explore neural substrates underlying successes and failures of current clinical treatments. Beginning with more fundamental association studies of brain structure and function (Volkow and Li, 2005), many treatment teams are now evaluating how adult and adolescent brains respond to treatment. For example, in the context of addiction, initial explorations with adults have evaluated brain response to pharmacotherapies.
Arguably, these explorations may be even more salient to the advancement of behavioral treatment. Neuroimaging data are critical in clinical research so that clinicians and scientists can fully understand the mechanisms underlying treatment successes and failures. Specifically, at this time, our behavioral metrics of adolescent treatment response (e.g., reward response) are not sufficiently sensitive to guide clinical decision making. Thus, with brain data in hand, we might learn that a particular behavioral treatment (e.g., contingency management) dampens adolescents’ neural reward response to drug cues. This information could directly inform clinical decision making, such as determining whether to enhance this behavioral treatment (e.g., contingency management) with medication and/or to include another adjunctive behavioral treatment that has gained empirical support in dampening adolescent neural reward response. Further, through this approach, one might learn that one element of reward neurocircuitry is more plastic and responsive to behavioral treatment than another. Moreover, this approach might uncover that different treatment elements (e.g., motivationally focused vs. reward-focused behavioral treatments) have different neural targets. Ultimately, learning how clients’ brains do or do not respond to these treatment elements could guide us to the selection of one treatment target over another. Finally, querying the response of the adolescent brain to different treatment approaches might uncover which treatments (e.g., behavioral approaches vs. medication vs. their combination) have the most enduring effects, and in which neural regions. Together, structural and functional neuroimaging will generate neural targets that can concretely help clinical researchers strengthen existing treatment options. Understanding the biological mechanisms of behavioral change is fundamental to advance growth and make substantive advances in the field of adolescent addiction treatment.
In terms of the clinical–neuroscience divide, novel examinations have begun to evaluate the neural substrates of in-session clinical exchanges (client change talk; therapist statements) by examining functional brain response (Feldstein Ewing et al., 2013, 2011a). By replaying in-session clinical excerpts back to individuals within the scanner, Feldstein Ewing and colleagues found that human brains respond differently to the clients’ own in-session statements in favor of change (e.g., “I need to cut back on smoking weed”) when contrasted with their own in-session statements in favor of staying the same (e.g., “I like smoking weed—it’s fun!”). Notably, the location of functional brain response varied by developmental period: mesocorticolimbic reward regions for adults (ages 35–52) (Feldstein Ewing et al., 2011a) and introspection/contemplation areas for adolescents (ages 14–17) (Feldstein Ewing et al., 2013). Further, adolescents who showed greater brain response in the precuneus, cingulate gyrus, posterior cingulate, and globus pallidus showed greater behavior change, as measured by significantly less cannabis use post-treatment (Feldstein Ewing et al., 2013). Collectively, these studies illuminate the neural processes behind spontaneous and self-generated client thought, both in-session and after leaving a practitioner’s office (Feldstein Ewing et al., 2014). Other cutting-edge studies are exploring the role of self-generated thought (Andrews-Hanna et al., 2014), the relationship between brain response and relapse (Vollstadt-Klein et al., 2011), and neurofeedback (Canterberry et al., 2013). Together, these studies reveal that neuroimaging can unlock a previously unavailable empirical window, allowing investigators to access, observe, and understand critical brain processes underlying within- and post-treatment behavior change.
4. Neuroimaging in the context of psychotherapy for adolescent psychopathology
Although a handful of functional neuroimaging modalities exist, concerns about radioactive material (e.g., PET) and accurate localization (e.g., EEG, MEG) can limit their application to treatment contexts. Two modalities, magnetic resonance imaging (MRI) and functional MRI (fMRI) (Buxton et al., 2014), are excellent for use with youth, as they are non-invasive and offer high-resolution localization.
Despite the advantages of MRI and fMRI, they also have limitations (for a review, see Logothetis, 2008). As examples, fMRI sessions have a restricted duration, hampering psychosocial treatment sessions in the scanner. fMRI requires multiple trials of behavior (i.e., repetitions of the event of interest) to produce observable and reliable effects; a condition of interest needs to occur a number of times. Thus, active collaboration between clinicians and neuroimagers is requisite to synchronize protocols to yield translational projects that have ecological clinical validity, and that yield usable, empirical data regarding treatment processes in the brain.
One more critical consideration is that neuroimaging studies are, by and large, correlational in nature (Naqvi and Morgenstern, 2015; Gabrieli et al., 2015). Much of the neuroimaging literature has hinged on examining comparisons of regions and relationships of interest within or between small samples. Ultimately, to generate maximal benefit to advancing our understanding treatment mechanisms, the field must move toward causation, by generating empirical data that identify which neuropsychological systems are necessary to successful treatment response (behavior change) (Naqvi and Morgenstern, 2015). Given attention to issues of replicability and reproducibility in the field of psychological science at this time (Collaboration, 2015), many laboratories are currently working on methods, including setting aside a small set of subjects for validation purposes (e.g., “training” vs. “test” sets), to address and minimize prediction errors that stem from the use of small datasets in these evaluations (e.g., issue of “optimism”) (Gabrieli et al., 2015; Whelan and Garavan, 2014). It is also important to note that some have found that neuroimaging data has yielded greater predictive power than behavioral metrics alone (Gabrieli et al., 2015; Magnan et al., 2013). One caveat here is that group differences in neural response could represent either neural systems impacted by the disorder itself (e.g., the characterizing features of the disorder) or the mechanisms by which treatment exerts its response (e.g., the areas of the brain impacted by the treatment, which subserve behavior change) (Gabrieli et al., 2015). In some cases, these areas may be regions related to the etiology and progression of the disease, rather than treatment response. Moreover, these may also be neural regions that have no intuitive or theoretical link to the disorder, its treatment response and/or its resolution. Ultimately, these issues represent the crux of the problem wherein “data has been disconnected from practice”. These conversations and considerations are precisely the types of discussions that neuroscientists must have with clinicians in order to advance the field.
Another common problem is that typical and widely occurring individual and environmental differences can interfere with interpretation of scan data (e.g., left-handedness, past-hour tobacco use). These variables may extend from the most fundamental of variables (e.g., time of day, degree of sleepiness, whether or not a child has eaten, brought their corrective lenses, consumed caffeine, refined sugar, and/or exercised prior to the scan session) to more intransigent variables that could impact fMRI and structural data, including pre-existing genetic, neurological, and/or developmental differences in brain function and structure (e.g., pubertal development status and menstrual cycle, natural differences in age and growth and related cognitive development). Further, there are inherent risks in using functional, task-dependent metrics, where approaches to measures of attention, for example, can vary widely by laboratory (e.g., anti-saccade vs. GoNoGo; various versions of GoNoGo); in this respect, structural and resting state measures offer some more technical consistency that can improve generalizability of scientific approach and subsequent empirical findings (Gabrieli et al., 2015).
Limiting these confounding factors is often considered necessary to isolate the true neural signal within the therapeutic effect of interest. Yet, this can inadvertently homogenize samples and their generalizability to broader clinical and non-clinical populations. While many developmental neuroscience teams exclude youth with co-occurring Axis I disorders (outside the target disorder) and common psychotropic medicines (e.g., antidepressants, anxiolytics), the recommendation here is to include these samples, and empirically compare brain response and treatment outcomes [e.g., between youth who have comorbid substance use disorder (SUD) and attention deficit hyperactivity disorder (ADHD) vs. youth who only have SUD]. Collaborating with clinical investigators expert on which disorders or patient populations may be integrated (vs. those that would render the data un-interpretable) is key.
Currently, it is straightforward to evaluate brain change in relation to behavior, for example, relative levels of BOLD signal pre- vs. post-treatment to pictures of spiders in the treatment of specific phobia (M age = 24.15) (Lipka et al., 2014). However, in some contexts, it might be more important to evaluate a more elusive aspect or mechanism of the behavior disorder [such as the nature of substance use – e.g., craving vs. self-regulation – within adolescent addiction, as evaluated with samples of 14–17-year-olds (Feldstein Ewing et al., 2013)]. The cognitive neuroscience literature is replete with sophisticated tasks to measure the clinical target of interest (e.g., social processing, theory of mind, attention, distraction, behavioral activation, self-thought).
Further, methodological approaches to evaluate adolescent brain volume and integrity of component tracts at rest, provide critical information regarding the “wiring” of the adolescent brain even when youth are not behaviorally or cognitively engaged. Disaggregation of treatment components to identify active ingredients linked to these types of structural change may provide additional clues about more enduring brain response to treatment. For example, youth who receive a coping skills training module might show strengthened white matter connectivity between the prefrontal cortex and subcortical areas as compared to youth who receive no treatment. Research that examines variation across extent of treatment exposure (e.g., number of sessions) might identify the crucial duration necessary for improved connectivity (and therefore possibility of lasting treatment response and decreased rates of high-risk behavior). Recent studies have also been examining how mindfulness may enhance emotion regulation and dampen affective in-the-moment decision making (ages 18–24) (Murakami et al., 2012); through a synergistic examination, scientists could determine whether this treatment is causally linked to more developmentally mature and/or “healthier” adolescent brain structure. If so, additional exercises that improve brain health may maximize the benefits of this treatment.
5. Practical recommendations regarding how data from neuroimaging studies can enhance current adolescent evidence-based treatments
Improved clinical outcomes informed by neuroscience will ultimately depend on an iterative and integrated series of highly controlled protocols with studies of neural substrates of clinically relevant specific behaviors (e.g., braking when driving fast among adolescents rated consistently as trait impulsive; ages 14–18) (Chein et al., 2011). Researchers may be well advised to identify their clinical constructs of interest, disaggregate them behaviorally and neurally, and/or after identifying structural and functional correlates of isolated contributing behaviors, and test whether the combination relates to the more clinically relevant target. To accomplish this, collaboration between clinicians and neuroscientists will be paramount.
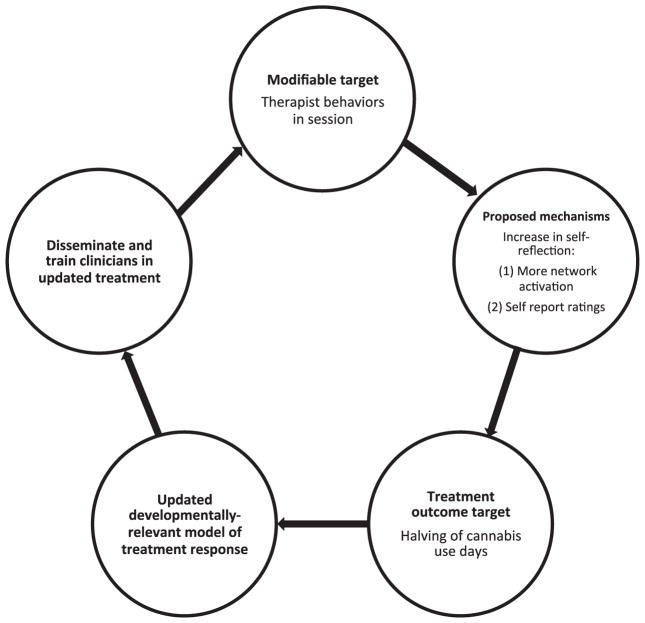
In Fig. 1, five steps are recommended, with data from Dr. Feldstein Ewing’s ongoing line of empirical research as a tangible example of how these efforts could be applied.
Fig. 1.
Example model and overview of steps: Step 1: Identify developmentally relevant modifiable treatment target. Step 2: Identify developmentally relevant treatment mechanism (biology and behavior). Step 3: Identify developmentally relevant treatment outcome. Step 4: Empirically evaluate the model. With resultant data, update empirical model of treatment response. Step 5: Disseminate and train clinicians in updated treatment approach. Example model.
Step 1 is in line with recent recommendations by Allen and Dahl (Allen and Dahl, 2015), wherein the first foundational step in this collaborative is to identify the “modifiable” elements of treatment response that are of interest to the investigative team. Here, the research team must select which factors they believe may be fundamental to an adolescent’s positive treatment response (or lack thereof). For example, both behavioral and neuroimaging work by Feldstein Ewing and colleagues has found that certain in-session therapist behaviors positively predict youth treatment response (ages 13–19) (Feldstein Ewing et al., in press, 2015a). Thus, for this step, one proposed modifiable target could be “in-session therapist behaviors”. It is suggested that this treatment element (therapist behaviors) may be able to directly enhance adolescents’ process of self-examination and potentially self-regulation. Importantly, this is a treatment target that is highly amenable and responsive to modification (e.g., through clinical supervision and didactics).
Step 2 revolves around identifying what the research team believes are the developmentally relevant mechanisms of positive treatment response, both in terms of neurobiology and behavior. Here, Feldstein Ewing and colleagues have found that for substance-using youth, activation of brain networks involved in introspection and contemplation predicted adolescents’ successful treatment response (ages 14–19) (Feldstein Ewing et al., 2013, 2015a). Therefore, for this model, one might expect an increase in self-reflection across both neural metrics of self-reflection (e.g., greater network activation), as well as in behavioral estimates of self-reflection (e.g., self-report ratings of self-reflection and contemplation).
Step 3 includes identifying a concrete treatment outcome that is sensitive and appropriate for this developmental period. In adolescent addiction, the recommendation is to move away from a focus on relapse as the metric of successful treatment response, which is widely the outcome of interest in adult addiction studies (e.g., percent days abstinent). Because substance use experimentation is normative and typical in this age range (Shedler and Block, 1990), abstinence is rarely a clinically useful (or tenable) treatment target. Instead, when treating adolescents, many clinicians target consumption at less hazardous levels and in less harmful ways (e.g., harm reduction) (Winstock et al., 2010) as their target treatment outcome.
Step 4 includes rigorously subjecting these theories to empirical examination by developing neuroimaging and behavioral paradigms that directly test the association of these proposed mechanisms with youth psychosocial treatment response (Naqvi and Morgenstern, 2015). While not always feasible, the strongest design approaches (Naqvi and Morgenstern, 2015) include randomization to active control treatment conditions (e.g., motivational interviewing vs. cognitive behavioral therapy), and active comparisons within the neuroimaging paradigm (e.g., therapist complex reflections vs. therapist closed questions) (Feldstein Ewing et al., 2015b). Pre-post-designs, which examine the modifiable factor prior to treatment exposure and following treatment outcome, are critical to obtain a temporally accurate estimate of how treatment affected the proposed neural mechanism of change (Schacht and Hutchison, 2015). Once these data have been generated, updating the research team’s working model to reflect treatment response is critical. Feldstein Ewing and colleagues actively empirically test and use these data to refine their theoretical model of treatment response in this very manner (Feldstein Ewing et al., 2011b, 2015b).
Step 5 is potentially the most important step, which includes leaving the laboratory to share the translational data with the larger field of clinicians and scientists, to disseminate these findings, and to generate dialogue in this area. Feldstein Ewing and colleagues have taken on this challenge by actively publishing their research findings in both clinical as well as neuroimaging journals, discussing empirical outcomes in integrative clinical–neuroscience forums (including www.scienceofchange.org, which targets both clinicians and neuroscientists), sharing the translation of “how these data matter for clinicians” in national and international clinical trainings, and integrating their empirical brain data into their clinical approach and supervision.
In terms of developmental caveats, it is critical to note that the adolescent brain is changing in structure and function throughout this period. Thus, these examinations must occur with an eye to age, gender, and pubertal development status, in order to assess which neural mechanisms of treatment response are most important, and/or have the most impact at different points in adolescents’ developmental trajectory.
6. Conclusions
There are compelling scientific reasons to merge basic neuroscience with treatment development; however, innovative integrative work can be daunting and difficult. We believe that these practical steps might help form the groundwork for successful collaboration. For example, we encourage teams to begin by engaging in multi-disciplinary collaboration to translate methodologies, data, and interpretations between neuroscientists and clinical researchers. Next, when collaborating across the fields of clinical treatment and neuroimaging, it is integral to learn and translate one another’s jargon. By this, we encourage teams to sit down together to ensure that any familiar and unfamiliar terminology has the correct interpretation for both fields. For example, terms that are widely used but mean different things in the neuroimaging and clinical literatures include measures of “behavior” or “behavioral response”, and/or “impulsivity”. Next, collaborative teams are encouraged to ask questions, as no well-worn path exists in synergistic clinical neuroscience inquiry. Openly querying any terms that are new or unfamiliar is a worthwhile endeavor, and will help establish successful clinical research collaboration. In this sense, it is important to be comfortable with sounding like a novice in the unfamiliar field. For example, it is okay to query what a “regressor”, “covariate” or “controlling for” means in the context of neuroimaging analyses. Fundamentals that are clear to neuroimagers may not be clear to clinicians (e.g., “BOLD response”, “white matter”, “gray matter”, “DTI”, “prepotent response”). Similarly, neuroimagers may not know terms or concrete examples of commonly accepted jargon in clinical communities (“therapist behaviors”, “in-session response”, “clinically significant change”). Additionally, we must brainstorm methods to effectively pave an open road for communication of study findings between clinicians and neuroimagers. One clear arena to facilitate dynamic communication is via scientific meeting; in one active effort, the Drs. T. Chung and S.W. Feldstein Ewing have initiated an annual forum for both fields to share scientific advances (www.scienceofchange.org). This has proven to be a highly fruitful avenue to begin dialogue and communication, with attention to the practical relevance of findings across the aisle to both fields (clinician to neuroimager; neuroimager to clinician). However, more work is needed to develop an ongoing and accessible platform for neuroimagers to disseminate their findings to clinicians and for clinicians to share their clinical observations to neuroimagers. With this active communication, this type of scientific synthesis will catalyze the field.
Ultimately, understanding brain structure and function subserving treatment response, and using methodologies that more effectively fuse meaningful clinical targets with neuroimaging methodology, should push treatment innovation further. Through these efforts, improved treatment response, both acute and long-term, should result.
Acknowledgments
This work was supported by National Institutes of Health (United States) funding to the first author (1R01AA023658-01) and to Dr. Molina (MH101096; AA011873; DA039881; DA035464; DA040213). The authors would like to thank Karen Hudson for her contribution to this manuscript. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of interest
The authors have no competing financial or other conflicts of interest relating to the data included in the manuscript.
Contributor Information
Sarah W. Feldstein Ewing, Email: feldstei@ohsu.edu.
Susan F. Tapert, Email: stapert@ucsd.edu.
Brooke S.G. Molina, Email: molinab@upmc.edu.
References
- Allen NB, Dahl RE. Multi-level models of internalizing disorders and translational developmental science: seeking etiolgoical insights that can inform early intervention strategies. J Abnorm Child Psychol. 2015;43:875–883. doi: 10.1007/s10802-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annual Rev Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Griffith VE, Simon AB, Moradi F, Shmuel A. Variability of the coupling of blood flow and oxygen metabolism responses in the brain: a problem for interpreting BOLD studies but potentially a new window on the underlying neural activity. Front Neurosci. 2014;11:139. doi: 10.3389/fnins.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canterberry M, Hanlon CA, Hartwell KJ, Li X, Owens M, LeMatty T, et al. Sustained reduction of nicotine craving with real-time neurofeedback: exploring the role of severity of dependence. Nicotine Tob Res. 2013;15:2120–2124. doi: 10.1093/ntr/ntt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev Sci. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration OS. Estimating the reproduciblity of psychological science. Science. 2015;349:aac4716. doi: 10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: emerging translational approaches that bridge biology and behavior. 2013;27:1–2. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. How psychosocial alcohol interventions work: a preliminary look at what fMRI can tell us. Alcohol Clin Exp Res. 2011a;35(4):1–9. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Hendershot CS, McEachern AD, Hutchison KE. A proposed model of the neurobiolgocial mechanisms underlying psychosocial alcohol interventions: the example of motivational interviewing and functional magnetic resonance imaging. J Stud Alcohol Drugs. 2011b;72:903–916. doi: 10.15288/jsad.2011.72.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescents’ response to a cannabis intervention. Psychol Addict Behav. 2013;27:510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Houck JM, Yezhuvath U, Shokri Kojori E, Truitt D, Filbey FM. The impact of therapists’ words on the adolescent brain: in the context of addiction treatment. Behav Brain Res. 2015a doi: 10.1016/j.bbr.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Karoly H, Houck JM. Deconstructing the neural substrates of motivational interviewing: a new look at an unresolved question. In: Feldstein Ewing SW, Witkiewitz K, Filbey FM, editors. Neuroimaging and Psychosocial Addiction Treatment. Guilford; London, UK: 2015b. pp. 231–243. [Google Scholar]
- Feldstein Ewing SW, Gaume J, Ernst DB, Rivera L, Houck JM. Do therapist behaviors differ with Hispanic youth? A brief look at within-session therapist behaviors and youth treatment response. Psychology of Addictive Behaviors. 2015;29(3):779–786. doi: 10.1037/adb0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Yezhuvath U, Houck JM, Filbey FM. Brain-based origins of change language: A beginning. Addictive Behaviors. 2014;39:1904–1910. doi: 10.1016/j.addbeh.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. 2015;85:11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The digital revolution and adolescent brain evolution. J Adolesc Health. 2012;51:101–105. doi: 10.1016/j.jadohealth.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd The amazing teen brain. Sci Am. 2015;312:32–37. doi: 10.1038/scientificamerican0615-32. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Rev Clin Psychol. 2005;1(1):91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta-analytic review. J Consult Clin Psychol. 2011;79:433–440. doi: 10.1037/a0023992. [DOI] [PubMed] [Google Scholar]
- Logothetis N. What we can do and what we cannot do with fMRI. Nature. 2008;453(12):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lipka J, Hoffman M, Miltner WH, Straube T. Effects of cognitive-behavioral therapy on brain responses to subliminal and supraliminal threat and their functional significance in specific phobia. Biol Psychiatry. 2014;76:869–877. doi: 10.1016/j.biopsych.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Magnan RE, Callahan TJ, Ladd BO, Claus ED, Hutchison KE, Bryan AD. Evaluating an integrative theoretical framework for HIV sexual risk among juvenile justice involved adolescents. J AIDS Clin Res. 2013;4(217) http://dx.doi.org/10.4172/2155-6113.1000217. [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Nakao T, Matsunaga M, Kasuya Y, Shinoda J, Yamada J, et al. The structure of mindful brain. PLoS One. 2012;7:e46377. doi: 10.1371/journal.pone.0046377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill M, Longabaugh R. The stakes are high. Addiction. 2013;108:882–884. [Google Scholar]
- Merikangas KR, Calkins ME, Burstein M, He JP, Chiavacci R, Lateef T, et al. Comorbidity of physical and mental disorders in the neurodevelopmental genomics cohort study. Pediatrics. 2015;135:e927–e938. doi: 10.1542/peds.2014-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Morgenstern J. Cognitive neuroscience approaches to understanding behavior change in alcohol use disorder treatments. Alcohol Res. 2015;37:29–38. [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Hutchison KE. In: Using clinical neuroscience to understand addictions treatment. Feldstein Ewing SW, Witkiewitz K, Filbey FM, editors. Palgrave; London: 2015. pp. 29–47. [Google Scholar]
- Shedler J, Block J. Adolescent drug use and psychological health: a longitudinal inquiry. Am Psychol. 1990;45(5):612–630. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol. 2013;41 C:51–59. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. Addiction science: uncovering neurobiological complexity. Neuropharmacology. 2014;76:235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. The neuroscience of addiction. Nat Neurosci. 2005;8(11):1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69(11):1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Tapert SF. Adolescent brain development, substance use, and psychotherapeutic change. Psychol Addict Behav. 2013;27:393–402. doi: 10.1037/a0029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol Psychiatry. 2014;75:746–748. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Ford C, Witton J. Assessment and management of cannabis use disorders in primary care. Br Med J. 2010;340:c1571. doi: 10.1136/bmj.c1571. [DOI] [PubMed] [Google Scholar]