Abstract
Background
To reduce colorectal cancer (CRC) mortality, positive fecal blood tests must be followed by colonoscopy.
Methods
We identified 62,384 individuals aged 50–89 years with a positive fecal blood test between 1/1/2011 and 12/31/2012 in four healthcare systems within the Population-Based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium. We estimated the probability of follow-up colonoscopy and 95% confidence intervals (CIs) using the Kaplan-Meier method. Overall differences in cumulative incidence of follow-up across health care systems were assessed with the log-rank test. Hazard ratios and 95% CIs were estimated from multivariate Cox proportional hazards models.
Results
Most patients who received a colonoscopy did so within six months of their positive fecal blood test, although follow-up rates varied across healthcare systems (p <0.001). Median days to colonoscopy ranged from 41 (95% CI, 40–41) to 174 (95% CI, 123–343); percent followed-up by 12-months ranged from 58.1% (95% CI, 51.6%–63.7%) to 83.8% (95% CI, 83.4%–84.3%) and differences across healthcare systems were also observed at 1, 2, 3, and 6 months. Increasing age and comorbidity score were associated with lower follow-up rates.
Conclusion
Individual characteristics and healthcare system were associated with colonoscopy after positive fecal blood tests. Patterns were consistent across healthcare systems, but proportions of patients receiving follow-up varied. These findings suggest there is room to improve follow-up of positive CRC screening tests.
Impact
Understanding the timing of colonoscopy after positive fecal blood tests and characteristics associated with lack of follow-up may inform future efforts to improve follow-up.
Keywords: colorectal cancer, screening, abnormal result, colonoscopy
INTRODUCTION
Annual testing for blood in the stool using high sensitivity guaiac fecal occult blood tests (gFOBT) or fecal immunochemical tests (FIT) is one of several recommended colorectal cancer (CRC) screening strategies for adults 50 to 75 years old (1, 2). Fecal blood testing requires colonoscopy to evaluate positive test results, but reported follow-up colonoscopy rates vary substantially, from less than 50% to 90% within one year of a positive test (3–11). Little is known about the variability in time to follow-up colonoscopy and how this may differ among individuals and across healthcare systems.
Understanding the variability in follow-up colonoscopy after a positive fecal blood test may help healthcare providers and systems identify patients in need of targeted interventions to complete follow-up. Research on determinants of follow-up, including age and gender, has yielded conflicting results, and few studies have examined other characteristics such as race or comorbidity. Also, most prior studies have been restricted to healthcare settings such as the Veterans Administration (VA) (3, 5, 9, 12–14), individual health maintenance organizations (HMOs) (6, 7), or international screening programs (4, 5, 10, 11) whose results may not be more broadly generalizable within the United States (U.S.). There is increasing interest in studying follow-up to abnormal screening tests in a multi-level context (15, 16), but to date, few studies have compared follow-up times across healthcare systems. Such studies are needed to lay the groundwork for future research on improving the effectiveness of cancer screening.
Our aims, therefore, were to characterize time to follow-up colonoscopy after a positive fecal blood test and to identify factors associated with timing of follow-up across four U.S. healthcare systems, which provided a geographically and ethnically diverse study population.
MATERIALS AND METHODS
Setting
This study was conducted as part of the NCI-funded consortium Population-Based Research Optimizing Screening through Personalized Regiments (PROSPR). The overall aim of PROSPR is to conduct multi-site, coordinated, transdisciplinary research to evaluate and improve cancer screening processes. The seven PROSPR Research Centers reflect the diversity of U.S. delivery system organizations. This paper’s data originate from Group Health (GH), Kaiser Permanente Northern California (KPNC), Kaiser Permanente Southern California (KPSC), and Parkland Health and Hospital System – University of Texas Southwestern Medical Center (PHHS-UTSW). Details of the Research Centers’ populations and screening practices have been described elsewhere (17).
The current analysis was restricted to individuals with a positive gFOBT (≥1 positive card) or FIT between January 1, 2011 and December 31, 2012 (N=74,754). A patient’s first positive fecal blood test during this time window was considered the index test. Participants in the integrated healthcare delivery systems (KPNC, KPSC, and GH) were required to have been continuously enrolled (with no more than a 90 day enrollment gap) in their healthcare system from January 1st of the calendar year prior to the index test (i.e., 2010 or 2011). The GH population was limited to patients who were covered by GH and selected or were assigned to one of its Medical Centers for their primary care. Participants from the safety-net health system (PHHS-UTSW) were required to have had at least one primary care visit in the above time frame to indicate that PHHS-UTSW was their medical home. We excluded patients who had a colonoscopy or positive fecal blood test in the calendar year prior to their index fecal blood test (N=2,521); patients not enrolled during the calendar year before their index test (N=7,719) or whose index test was an in-office or single specimen guaiac test and thus not considered adequate for screening (N=2,091); and patients missing the end of follow-up date (N=41). The final analytic cohort consisted of 62,384 subjects.
Data collection
Data were collected from automated data systems, including the electronic health record (EHR) and administrative databases at each healthcare system (17). Variables included demographic characteristics, diagnoses, and procedures that were available as PROSPR common data elements and had been examined in prior studies (3, 4, 7, 10, 14). We also included body mass index (BMI) because of its association with receipt of CRC screening in certain subgroups (18). Age was computed at the time of the index exam and BMI was calculated from height and weight recorded in the year before the index test. Charlson comorbidity scores (19) were computed with a standardized algorithm at each center using ICD-9-CM codes from care episodes in the calendar year before the index fecal blood test. Prior CRC screening was defined as having a record of a fecal blood test, colonoscopy, or sigmoidoscopy before the index fecal blood test. Information on prior screening was available beginning in 2006 for GH, 1999 for KPNC and KPSC, and 2009 for PHHS-UTSW.
Analysis
Our primary outcome was time from index fecal blood test to colonoscopy. Participants with no colonoscopy during follow-up were censored at the earliest of death, health plan disenrollment, or end of study (December 31, 2012).
We calculated the probability of follow-up colonoscopy at time-points following a positive fecal test using the Kaplan-Meier method, accounting for censoring. Cumulative incidence of colonoscopy curves were generated by plotting the estimated probability versus follow-up time for each healthcare system. We compared cumulative incidence of follow-up curves using the log-rank test (20). We also used the Kaplan-Meier method to estimate the probability of follow-up by 1, 2, 3, 6, and 12 months and median follow-up times with 95% confidence intervals (CI). We supplemented primary analyses with an exploration of clinic-level variation in follow-up times. We computed the interquartile range (IQR) of the proportion of patients within clinics who received a follow-up colonoscopy within six months of a positive fecal blood test. Description of between-clinic variability was limited to the two healthcare systems that provided clinic-level identifiers, GH (26 clinics, with 5 to 424 positive fecal blood tests) and PHHS-UTSW (12 clinics, with 2 to 128 positive fecal blood tests).
We used multivariate Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs. First, we evaluated healthcare system as an effect modifier. For each covariate of interest, we tested for effect modification by healthcare system with a Cox regression model including an interaction between that covariate and healthcare system, adjusting for all other covariates. The likelihood ratio test for the interaction was calculated comparing the models with and without the interaction terms. We repeated this for each variable of interest. Because we did not find strong evidence for healthcare system as an effect modifier, our final model did not include interaction terms. The final model included age, BMI, Charlson comorbidity score, gender, race/ethnicity, and healthcare system as covariates. Persons with missing covariate values (N=14,557) were excluded from the full model, leaving 47,827 subjects.
Tests of proportional hazards assumption based on Schoenfeld residuals (21) indicated that the assumption was violated for healthcare system (p<0.001). To address this, we estimated proportional hazards models that were stratified by healthcare system. This relaxed the proportional hazards assumption for healthcare system by allowing baseline hazards to vary across systems. Results for other covariates were nearly identical (not shown). We therefore only report the results from the main Cox model, which provides the averaged association with healthcare system over the full follow-up period. As a secondary analysis, we fit separate multivariate Cox models (including healthcare system as a covariate) where follow-up periods were restricted to three and six months to evaluate how the associations varied by time.
Because age eligibility varied by health system, we conducted sensitivity analyses in which we separately considered models for persons <65 years old (including all four healthcare systems) and persons aged 65 to 89 (restricted to the three healthcare systems with participants in this range).
All activities were approved by the institutional review boards associated with each Research Center and the Statistical Coordinating Center. Analyses were conducted using R version 3.1.1(http://www.r-project.org/) and SAS version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Demographics
The study population consisted of 62,384 subjects (Table 1), 61% of whom were younger than 65 years old. About half were male (52%), had a Charlson comorbidity score of 0 (52%), and were non-Hispanic white (54%). Nearly 1 in 5 (19%) was Hispanic. Most participants had been screened for CRC previously (78%) and were members of Kaiser Permanente in the northern or southern California regions (95%). Nearly all index tests were FITs (98%). The 47,827 persons with no missing data who were included in the multivariate model (Supplementary Table S1) were generally similar to persons with some missing data, except they were more likely to have previously been screened for CRC.
Table 1.
Characteristics of PROSPR participants with a positive fecal occult blood test, 2011–2012 (N=62,384)
| Characteristic | Overall N=62,384 | GH N=2,707 (4.34%) | KPNC N=32,263 (51.72%) | KPSC N=27,013 (43.30%) | PHHS-UTSW N=401 (0.64%) |
|---|---|---|---|---|---|
|
| |||||
| Col. % | Col. % | Col. % | Col. % | Col. % | |
| Age (years) at positive test | |||||
| 50–54 | 20.39 | 14.85 | 20.03 | 21.19 | 33.17 |
| 55–59 | 20.21 | 17.33 | 20.11 | 20.40 | 34.41 |
| 60–64 | 20.51 | 20.58 | 21.16 | 19.54 | 32.42 |
| 65–69 | 17.35 | 17.55 | 17.89 | 16.94 | 0 |
| 70–75 | 16.29 | 16.11 | 16.46 | 16.36 | 0 |
| 76–84 | 4.22 | 10.53 | 3.52 | 4.49 | 0 |
| 85–89 | 1.03 | 3.07 | 0.83 | 1.07 | 0 |
| Gender | |||||
| Female | 47.76 | 48.69 | 47.20 | 48.12 | 62.84 |
| Male | 52.24 | 51.31 | 52.80 | 51.88 | 37.16 |
| Unknown | 0 | 0 | 0.01 | 0 | 0 |
| Charlson comorbidity score in calendar year before positive test | |||||
| Unknown | 10.21 | 6.91 | 9.03 | 11.26 | 56.36 |
| 0 | 51.78 | 48.13 | 55.90 | 47.71 | 19.20 |
| 1 | 17.32 | 16.00 | 17.77 | 16.95 | 14.96 |
| 2 | 9.53 | 11.34 | 9.22 | 9.81 | 3.99 |
| 3+ | 11.16 | 17.62 | 8.09 | 14.26 | 5.49 |
| Race/ethnicity | |||||
| NH White | 54.29 | 76.41 | 57.75 | 48.56 | 14.46 |
| Hispanic | 18.50 | 4.21 | 12.49 | 26.95 | 29.43 |
| NH Asian/Pacific Islander | 13.11 | 8.64 | 15.71 | 10.58 | 4.24 |
| NH Black | 8.14 | 4.25 | 6.91 | 9.36 | 51.12 |
| NH Other | 0.63 | 3.92 | 0.66 | 0.28 | 0.25 |
| Unknown/Missing | 5.33 | 2.84 | 6.48 | 4.26 | 0.50 |
| Body mass index (kg/m2) in calendar year before positive test | |||||
| <25 | 19.42 | 19.87 | 20.40 | 18.28 | 14.46 |
| 25 to <30 | 30.20 | 32.03 | 29.97 | 30.27 | 31.17 |
| 30 to <35 | 20.86 | 23.01 | 20.32 | 21.21 | 26.18 |
| 35+ | 16.43 | 21.98 | 15.77 | 16.49 | 27.43 |
| Missing | 13.09 | 3.10 | 13.53 | 13.75 | 0.75 |
| Type of fecal blood test | |||||
| FIT | 98.05 | 68.86 | 98.84 | 100.00 | 99.75 |
| Guaiac | 1.95 | 31.14 | 1.16 | 0.00 | 0.25 |
| Prior colorectal cancer screening | |||||
| No | 22.36 | 37.46 | 20.83 | 22.37 | 43.14 |
| Yes | 77.64 | 62.54 | 79.17 | 77.63 | 56.86 |
FIT: fecal immunohistochemical test; GH: Group Health; KPNC: Kaiser Permanente Northern California; KPSC: Kaiser Permanente Southern California; NH: non-Hispanic; PHHS-UTWS: Parkland Health and Hospital System – University of Texas Southwestern Medical Center; PROSPR: Population-Based Research Optimizing Screening through Personalized Regimens
Time to follow-up by healthcare system
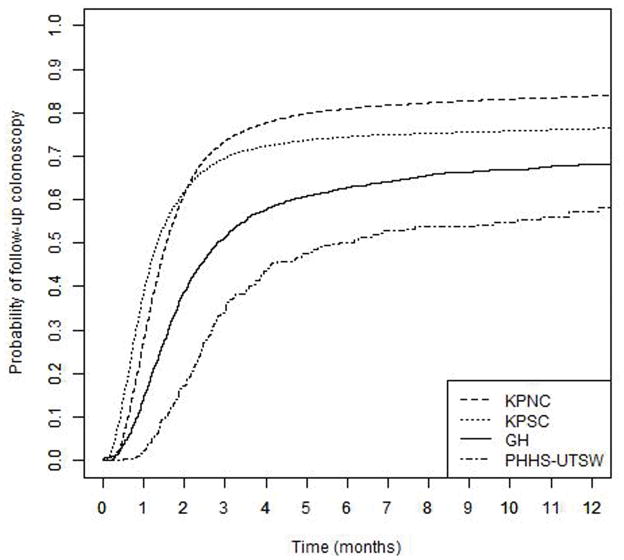
In all four healthcare systems, most patients who received a follow-up colonoscopy did so within six months of their positive fecal blood test (Figure 1). However, the estimated probability of receiving a follow-up colonoscopy varied across healthcare systems (log-rank p-value <0.001). For example, estimated 12-months follow-up probabilities ranged from 58.1% (95% CI, 51.6%–63.7%) to 83.8% (95% CI, 83.4%–84.3%); and differences across healthcare systems were also observed at 1, 2, 3, and 6 months (Table 2). The follow-up probabilities increased sharply initially then leveled off around three months (for the integrated healthcare delivery systems) to six months (for the safety net system). Of note, the KPNC and KPSC curves cross at approximately three months. The median number of days to colonoscopy follow-up differed across systems: 41 (95% CI, 40–41) at KPSC, 47 (95% CI, 46–47) at KPNC, 84 (95% CI, 80–92) at GH, and 174 (95% CI, 123–343) at PHHS-UTSW. The shapes of the follow-up probability curves were similar when restricted to patients with full covariate information available (results not shown).
Figure 1.
Time to follow-up colonoscopy after positive fecal occult blood test, by PROSPR healthcare system, 2011–2012 (GH: Group Health; KPNC: Kaiser Permanente Northern California; KPSC: Kaiser Permanente Southern California; PHHS-UTSW: Parkland Health and Hospital System – University of Texas Southwestern Medical Center; PROSPR: Population-Based Research Optimizing Screening through Personalized Regimens)
Table 2.
Kaplan-Meier estimates with 95% confidence intervals of percent with follow-up colonoscopy at 1, 2, 3, 6, and 12 months after a positive fecal blood test in 2011–2012, by PROSPR healthcare system (N=62,384)
| Healthcare system | 1 month | 2 months | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|
| Group Health | 14.9 (13.5–16.2) | 38.9 (36.9–40.8) | 51.3 (49.3–53.3) | 62.8 (60.7–64.7) | 68.2 (66.0–70.1) |
| Kaiser Permanente Northern California | 28.3 (27.8–28.8) | 61.5 (61.0–62.0) | 73.4 (72.9–73.9) | 80.9 (80.5–81.3) | 83.8 (83.4–84.3) |
| Kaiser Permanente Southern California | 39.3 (38.7–39.9) | 61.9 (61.3–62.5) | 69.6 (69.0–70.2) | 74.4 (73.8–74.9) | 76.4 (75.8–76.9) |
| Parkland Health and Hospital System – University of Texas Southwestern Medical Center | 2.4 (0.8–3.9) | 17.0 (13.1–20.8) | 34.7 (29.4–39.5) | 50.2 (44.3–55.5) | 58.1 (51.6–63.7) |
PROSPR: Population-Based Research Optimizing Screening through Personalized Regimens
Even after adjustment for patient-level variables, healthcare system remained associated with follow-up time (Table 3). The association between healthcare system and receipt of follow-up varied slightly over time. For example when we restricted follow-up time to three months the HR for KPNC (with KPSC as the reference health system) was 0.92 (95% CI, 0.90–0.95), compared to 0.97 (95% CI, 0.95–0.99) for six months of follow-up (Supplementary Table S2). The variation in the association over time is consistent with the crossing follow-up probability curves in Figure 1.
Table 3.
Associations between patient characteristics and time to colonoscopy follow-up after positive fecal occult blood test in PROSPR healthcare systems, 2011–2012 (47,827)a
| Characteristic at positive fecal occult blood test | Hazard ratio (95% confidence interval) |
|---|---|
| Age (years) | |
| 50–54 | Reference |
| 55–59 | 1.02 (0.98–1.05) |
| 60–64 | 0.98 (0.95–1.02) |
| 65–69 | 0.98 (0.95–1.02) |
| 70–75 | 0.90 (0.87–0.94) |
| 76–84 | 0.65 (0.61–0.69) |
| 85–89 | 0.34 (0.29–0.39) |
| Gender | |
| Female | Reference |
| Male | 1.03 (1.00–1.05) |
| Charlson comorbidity score | |
| 0 | Reference |
| 1 | 0.93 (0.91–0.96) |
| 2 | 0.87 (0.84–0.90) |
| ≥3 | 0.70 (0.67–0.72) |
| Body mass index (kg/m2) | |
| <25 | Reference |
| 25 to <30 | 1.06 (1.03–1.09) |
| 30 to <35 | 1.07 (1.04–1.10) |
| ≥35 | 1.01 (0.97–1.04) |
| Race/ethnicity | |
| White, non-Hispanic | Reference |
| Hispanic (any race) | 1.01 (0.98–1.04) |
| Asian/Pacific Islander | 0.97 (0.94–1.00) |
| Black | 0.98 (0.94–1.02) |
| Other | 0.96 (0.84–1.09) |
| Prior colorectal cancer screening | |
| No | Reference |
| Yes | 1.30 (1.26–1.34) |
| Healthcare system | |
| Kaiser Permanente Southern California | Reference |
| Group Health | 0.64 (0.61–0.68) |
| Kaiser Permanente Northern California | 0.99 (0.97–1.01) |
| Parkland Health and Hospital System – University of Texas Southwestern Medical Center | 0.24 (0.18–0.32) |
Multivariate regression limited to persons with complete covariate information
PROSPR: Population-Based Research Optimizing Screening through Personalized Regimens
Follow-up rates varied across clinics within the healthcare systems that had data available for this analysis. At GH, the IQR of the proportion of patients receiving follow-up within six months of a positive fecal blood test was 0.58 to 0.65. At PHHS-UTSW, the IQR was 0.41 to 0.53.
Patient-level risk factors
Time to colonoscopy follow-up varied by patient characteristics, even after adjustment in the multivariate regression model (Table 3). Compared to patients 50 to 54 years old, patients 55 to 69 years old had similar follow-up rates, but older patients (70 to 89 years old) were at a higher risk of not receiving follow-up. Follow-up rates were lower in patients with higher comorbidity scores and not previously screened for CRC. In comparison with age and comorbidity, there was relatively little variation in rates by gender, BMI, or race/ethnicity, though some small associations were statistically significant due to the study’s large sample size. Results for all covariates were similar in a model where baseline hazards were allowed to vary by healthcare system (results not shown).
DISCUSSION
Variations by healthcare system
This large cohort study conducted within four diverse healthcare systems demonstrates that differences exist across healthcare systems in the time to follow-up colonoscopy and in the proportions of patients who receive follow-up colonoscopy within 12 month of a positive fecal blood test. All four healthcare systems had some patients with a positive test who did not receive a follow-up colonoscopy within one year. The system-level variation in percent of patients who remained in the cohort but did not receive follow-up colonoscopy within 12 months of a positive fecal blood test is consistent with the wide range reported in the literature (3, 4, 6, 7, 9, 10, 22).
The two healthcare systems in our study with the highest colonoscopy follow-up rates (KPNC and KPSC) are integrated healthcare delivery systems that, in 2011, had ambitious targets for time to colonoscopy after a positive fecal blood tests (KPNC: target of 80% within four weeks; KPSC: target of 100% within 14 days), prospective appointment supply monitoring (KPSC only), performance monitoring via monthly dashboards and reports shared with leadership (e.g., chiefs, managers, and medical directors), and organizational targets for screening. At GH – also an integrated healthcare delivery system – primary care providers follow-up on positive fecal blood tests. GH also maintained a registry of patients who had not received followed up colonoscopy and contacted them approximately four months after a positive fecal blood test and every three months thereafter if the positive fecal test had not been resolved. However, at GH, reports on follow-up testing were not routinely sent to leaders. Also GH contracted out many colonoscopies, and so may have had less direct control over colonoscopy capacity. The system with the longest follow-up time was a county-wide public, safety net health system with limited colonoscopy capacity whose patients may face greater personal and system-level barriers to successfully completing follow-up colonoscopy. Like other studies (23), we observed some variation in follow-up time across clinics within healthcare systems, which may reflect differences in patient populations or local practices.
Previous research has suggested a variety of reasons for lack of follow-up after an abnormal fecal test, including physician decision not to follow-up (24), lack of referral (9), and patient non-adherence (9). These factors, in addition to patient characteristics, may vary across healthcare systems and may also account for some of the differences in colonoscopy follow-up that were observed between systems in our study.
Our findings are consistent with recent research demonstrating the importance of organizational structures and processes in follow-up of positive fecal blood tests. A VA study of 98 facilities found that 60-day colonoscopy follow-up rates were associated with direct notification of gastroenterology providers by gastroenterology staff and with colonoscopy appointment availability not being identified as a barrier (23). Follow-up rates were also related to the how instructions for bowel preparation were delivered. Randomized controlled trials and observational studies of quality improvement initiatives have also suggested that organizational processes can improve follow-up, at least in VA and managed care settings. For instance, evidence suggests that interventions that automatically notify gastroenterology of a positive fecal blood test are effective (25), (26). Providing educational outreach to PCPs and reminding them when patients have gone 60 days without follow-up (27) may also increase follow-up rates. Combining provider education, reduction of gastroenterology backlog, and electronic fecal blood test result notification may be effective (28). The effect of patient navigation is less clear but also promising (29, 30). Thus, in general, data support the importance of organizational factors in the completion of diagnostic work-up of positive fecal blood tests. This existing body of literature helps explain our observation that in a multi-institutional setting, the healthcare systems with the shortest time to follow-up and highest percent follow-up were those with the most extensive organizational systems in place to facilitate follow-up colonoscopy.
Timing of follow-up
A unique contribution of our study is its presentation of time to follow-up colonoscopy as a continuous measure with cumulative incidence of follow-up curves to visualize trajectories. We report that most persons who received follow-up did so within six months of their positive fecal blood test, though the median follow-up time differed by healthcare system. The percentages of patients followed-up by two months (range across healthcare systems:17–62%) and six months (range across healthcare systems: 50–81%) in our study were similar to but slightly higher than those from Partin et al.’s study of VA hospitals during a similar time period. Partin et al. reported that 30% of positive fecal blood tests were followed up by 60 days (range across facilities: 10–57%) and 49% by six months (range across facilities: 30–70%) (23). VA studies from earlier time periods reported lower 60-day (24.5%, interquartile range: 13.8% to 40.7%) (31) and one year follow-up rates (around 40%) (9, 14) compared to our study. A 2009 report from a multi-specialty group practice found approximately two thirds of positive fecal blood tests were followed-up within one year. Like us, they observed that most follow-up occurred within six months (87% of colonoscopies performed) and additional 7% were completed between six months and one year) (7).
Our results do not provide evidence about optimal timing of follow-up colonoscopy outreach efforts, but they do suggest that patients without a follow-up colonoscopy within approximately six months of a positive fecal blood test are unlikely to complete a diagnostic evaluation in the absence of additional outreach beyond what the healthcare systems in our study had already implemented. We cannot be sure why six months was a notable time-point; however, the consistency across healthcare systems suggests that it might be a generalizable feature of patient behavior rather than system-level factors. Health plans and practices may wish to consider studying the effect of additional interventions for patients, providers, and healthcare systems at six months to increase follow-up rates. Ideally, intervention design would also be informed by data on the relationship between timing of follow-up and colorectal cancer outcomes. It will be important for future studies to assess whether follow-up delays are associated with adverse clinical outcomes. Understanding this association may help inform follow-up recommendations, intervention design, and evidence-based metrics for healthcare systems.
Individual-level risk factors
In our study, older age and higher comorbidity burden were independent risk factors for lack of follow-up. Increasing age was previously associated with lack of follow-up in some (4, 7, 10) but not other studies (3, 5, 6, 9, 32) and, in contrast to the current study, two prior studies that also used Charlson comorbidity scores did not observe an association with receipt of follow-up (3, 14). The discrepancy between findings from these studies and ours might be explained by the fact that both other studies were conducted in the VA nearly 15 years ago and had overall lower follow-up rates (around 40% within one year). To our knowledge, studies have not directly investigated whether individual-level predictors of follow-up completion differ by time interval (e.g., within two, three, six, and 12 months).
Consistent with our study, several others have not reported strong associations between gender and complete diagnostic follow-up (5, 7, 8, 13), but others have suggested women are less likely than men to receive follow-up testing (6, 11, 32). The four studies conducted in the VA were all (3) or almost all male (5, 9, 14) and therefore were limited in their ability to analyze gender as a risk factor. In previous studies, rates of follow-up in African-Americans have been comparable to (3, 9) or higher (7) than in whites.
Our age and comorbidity findings may offer a basis for further detailed studies of these areas and for intervention research. These data also suggest opportunities for research into patient preferences regarding the goals and outcomes of screening tests for all cancers. For example, if, at the time screening is to be initiated, there is no intention (either due to patient preference or provider concerns regarding the appropriateness of more invasive procedures) of performing a complete diagnostic exam if the test is positive, shared decision-making about whether or not to screen may be worthwhile. Although the current data suggest failure to follow-up is a concern for fecal blood testing, this issue is relevant for other cancers where effective screening tests are available, such as breast and cervical cancers. Comparisons between different approaches within PROSPR and in other settings may inform these investigations. In general, studies on screening decision aids have not focused on “no screening” as an option or when to stop screening (33). Such decision aids may be useful given that the 2008 U.S. Preventive Services Task Force recommendations for CRC screening are age-specific with a C recommendation for adults aged 76 to 85 years. Additionally or alternatively, future research could focus on targeted interventions for specific populations to ensure colonoscopy follow-up as appropriate.
Strengths and limitations
PROSPR provides a unique opportunity to study fecal blood screening in a large, diverse subset of the U.S. population. A major strength of this study is that we were able to adjust for potential confounders that many other studies have not been able to account for, such as comorbidity. Other strengths of our study include that the racial and ethnic distribution of the population was similar to that of the U.S. general population (34) and that we used administrative and clinical data sources to ascertain completion of follow-up testing, rather than self-report, thereby reducing the likelihood of bias due to differential reporting.
Although our study identifies important opportunities for future intervention research, it has several important limitations. First, the patient characteristics we studied were generally not modifiable; thus, our study highlights patients to target rather than characteristics that can be intervened upon. Second, about one quarter of participants were missing data on one or more covariates and were excluded from multivariate analysis. However, those that were excluded due to missing covariate data were similar to those included in analyses with respect to all variables except prior screening history. Third, 100% follow-up may not be an appropriate target. We cannot be certain that all of the fecal blood tests were for CRC screening. We excluded single specimen guaiac tests and in-office tests but not those conducted in inpatient settings as there were no separate procedure codes to identify these. There also may be patients for whom subsequent colonoscopy is not recommended based on comorbidities or age. At two sites, these patients may have received a FIT kit through a mass mailed outreach program. Thus, there may be an opportunity to more selectively target patients to receive outreach. Fourth, a limitation of this study is that we did not include socioeconomic status (SES) variables in our model, which may have resulted in residual confounding, particularly of the association between healthcare system and time to follow-up (35). Finally, we did not have provider- or clinic-level identifiers for patients at KPNC or KPSC, which precluded us from characterizing the structure of the data and examining variability in follow-up time within healthcare systems. Exploratory analyses with data from GH and PHHS-UTWS suggested that the variability among clinics within a healthcare system was smaller than the observed between-system variability in time to follow-up. Future studies that provide a more detailed characterization of sources of variability could provide additional insight into gaps in care and opportunities for intervention.
Summary
In summary, we found that even when patients have access to coordinated healthcare, many do not receive follow-up colonoscopies after abnormal fecal blood tests. Understanding the timing of follow-up, as well as characteristics associated with lack of follow-up, may inform future efforts to tailor and test interventions to improve follow-up after positive a fecal blood test. Our finding that both individual-level factors as well as healthcare system were associated with follow-up strengthens the rationale for investigating multilevel interventions to improve follow-up after abnormal screening tests (15).
Supplementary Material
Acknowledgments
Financial Support: A. Burnett-Hartman was funded by National Center for Advancing Translational Sciences KL2 TR000421 and National Cancer Institute at the National Institutes of Health U01CA163304.
J. Chubak, A. Kamineni, B.B. Green, C.M. Rutter were funded by National Cancer Institute at the National Institutes of Health U54CA163261
D.A. Corley, T.R. Levin, C.A. Doubeni, V.P. Quinn, J.E. Schottinger were funded by National Cancer Institute at the National Institutes of Health U54CA163262
A.G. Singal, E.A. Halm were funded by National Cancer Institute at the National Institutes of Health U54CA163308
M.P. Garcia, Y. Zheng were funded by National Cancer Institute at the National Institutes of Health U01CA163304.
C.N. Klabunde is an employee of National Cancer Institute at the National Institutes of Health.
Parkland-UT Southwestern Colorectal PROSPR Research Center Acknowledgement:
Principal Investigators: Celette Sugg Skinner, PhD and Ethan A. Halm, MD, MPH
Co-Investigators: Chul Ahn, PhD, Ruben Amarasingham, MD, MBA, Bijal Balasubramanian, MBBS, PhD, Stephen Inrig, PhD, Simon Craddock Lee, PhD, Milton Packer, MD, Noel Santini, MD, Amit Singal, MD, MSCS, and Jasmin Tiro, PhD, MPH;
Management: Wendy Bishop, MS and Katharine McCallister;
Data: Adam Loewen and Joanne Sanders, MS;
Consultant: Samir Gupta, MD
Group Health Research Institute Colorectal PROSPR Research Center Acknowledgement:
Principal Investigator: Jessica Chubak, PhD, (Original PI: Carolyn M. Rutter, PhD);
Co-Investigators: Beverly B. Green, MD, MPH, Aruna Kamineni, PhD, MPH, Carolyn M. Rutter, PhD, and Karen Wernli, PhD;
Management: Kristina Hansen and Christine Mahoney, MA;
Staff: Kevin P. Filocamo, Ann Kelley, MHA, Kilian Kimbel, Renee Remedios, and Leslie Sizemore;
Data: Scott Halgrim, MA, Eric Johnson, MS, Malia Oliver, Chester Pabiniak, MS, and Diem-Thy Tran
Kaiser Permanente Research Institute Colorectal PROSPR Research Center Acknowledgement
KP Northern California:
Principal Investigator: Douglas A. Corley, MD, PhD, Chyke A. Doubeni, MD, MPH (University of Pennsylvania) and Ann G. Zauber, PhD (Memorial Sloan Kettering Cancer Center);
Co-Investigator: Theodore R. Levin, MD;
Management: Christopher D. Jensen, MPH, PhD;
Data: Wei K. Zhao, MPH
KP Southern California:
Principal Investigator: Virginia P. Quinn, PhD, MPH;
Co-Investigators: Alexander T. Lee, MD and Joanne E. Schottinger, MD;
Management: Nirupa R. Ghai, PhD, MPH;
Data: Richard Contreras, MS
Fred Hutchinson PROSPR Research Coordinating Center Acknowledgement:
Principal Investigators: William Barlow, PhD and Mark Thornquist, PhD;
Co-Investigators: Andrea Burnett-Hartman, PhD, John Inadomi, MD, Connie Lehman, MD, Chris Li, PhD, Connie Mao, MD, Rachel Winer, PhD, and Yingye Zheng, PhD;
Management: Stephanie Page-Lester, Suzanna Reid, PhD;
Staff: Hallie Pritchett;
Data: Elisabeth Beaber, PhD, MPH, Michael Garcia, MS, Dale McLerran, MS, and Deanna Stelling
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.U S. Preventive Services Task Force. Screening for colorectal cancer: U.S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 3.Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med. 2011;171:249–56. doi: 10.1001/archinternmed.2010.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi KS, Lee HY, Jun JK, Shin A, Park EC. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. J Gastroenterol Hepatol. 2012;27:1070–7. doi: 10.1111/j.1440-1746.2011.06944.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferrat E, Le Breton J, Veerabudun K, Bercier S, Brixi Z, Khoshnood B, et al. Colorectal cancer screening: factors associated with colonoscopy after a positive faecal occult blood test. Br J Cancer. 2013;109:1437–44. doi: 10.1038/bjc.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miglioretti DL, Rutter CM, Bradford SC, Zauber AG, Kessler LG, Feuer EJ, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008;46:S91–6. doi: 10.1097/MLR.0b013e31817946c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SK, Schilling TF, Sequist TD. Challenges in the management of positive fecal occult blood tests. J Gen Intern Med. 2009;24:356–60. doi: 10.1007/s11606-008-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields HM, Weiner MS, Henry DR, Lloyd JA, Ransil BJ, Lamphier DA, et al. Factors that influence the decision to do an adequate evaluation of a patient with a positive stool for occult blood. Am J Gastroenterol. 2001;96:196–203. doi: 10.1111/j.1572-0241.2001.03475.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15:1232–5. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 10.Morris S, Baio G, Kendall E, von Wagner C, Wardle J, Atkin W, et al. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107:765–71. doi: 10.1038/bjc.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paszat L, Rabeneck L, Kiefer L, Mai V, Ritvo P, Sullivan T. Endoscopic follow-up of positive fecal occult blood testing in the Ontario FOBT Project. Can J Gastroenterol. 2007;21:379–82. doi: 10.1155/2007/569689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steele RJC, Kostourou I, McClements P, Watling C, Libby G, Weller D, et al. Effect of gender, age and deprivation on key performance indicators in a FOBT-based colorectal screening programme. Journal of Medical Screening. 2010;17:68–74. doi: 10.1258/jms.2010.009120. [DOI] [PubMed] [Google Scholar]
- 13.Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54:2497–502. doi: 10.1007/s10620-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garman KS, Jeffreys A, Coffman C, Fisher DA. Colorectal cancer screening, comorbidity, and follow-up in elderly patients. Am J Med Sci. 2006;332:159–63. doi: 10.1097/00000441-200610000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Zapka JM, Edwards HM, Chollette V, Taplin SH. Follow-up to abnormal cancer screening tests: Considering the multilevel context of care. Cancer Epidemiol Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-14-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tosteson AN, Beaber EF, Tiro J, McCarthy AM, Quinn VP, Doria-Rose P, et al. Variation in cancer screening abnormality rates and follow-up witin the PROSPR Consortium: a comparison of breast, cervical and colorectal cancer screening. doi: 10.1007/s11606-015-3552-7. in progress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiro JA, Kamineni A, Levin TR, Zheng Y, Schottinger JS, Rutter CM, et al. The CRC screening process in community settings: A conceptual model for the Population-based Research Optimizing Screening through Personalized Regimens Consortium. Cancer Epidemiol Biomarkers Prev. 2014 doi: 10.1158/1055-9965.EPI-13-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruthur NM, Bolen S, Gudzune K, Brancati FL, Clark JM. Body mass index and colon cancer screening: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:737–46. doi: 10.1158/1055-9965.EPI-11-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2. Vol. 2002 John Wiley & Sons; 2002. [Google Scholar]
- 21.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;8:515–26. [Google Scholar]
- 22.Church TR, Yeazel MW, Jones RM, Kochevar LK, Watt GD, Mongin SJ, et al. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst. 2004;96:770–80. doi: 10.1093/jnci/djh134. [DOI] [PubMed] [Google Scholar]
- 23.Partin MR, Burgess DJ, Burgess JF, Jr, Gravely A, Haggstrom D, Lillie SE, et al. Organizational predictors of colonoscopy follow-up for positive fecal accult blood test results: an observational study. Cancer Epidemiol Biomarkers Prev. 2015;24:422–34. doi: 10.1158/1055-9965.EPI-14-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimbo M, Myers RE, Meyer B, Hyslop T, Cocroft J, Turner BJ, et al. Reasons patients with a positive fecal occult blood test result do not undergo complete diagnostic evaluation. Ann Fam Med. 2009;7:11–6. doi: 10.1370/afm.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Kleek E, Liu S, Conn LM, Hoadley A, Ho SB. Improving the effectiveness of fecal occult blood testing in a primary care clinic by direct colonoscopy referral for positive tests. J Healthc Qual. 2010;32:62–9. doi: 10.1111/j.1945-1474.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey LL, Shannon J, Partin MR, O’Malley J, Chen Z, Helfand M. Improving the follow-up of positive hemoccult screening tests: an electronic intervention. J Gen Intern Med. 2011;26:691–7. doi: 10.1007/s11606-011-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers RE, Turner B, Weinberg D, Hyslop T, Hauck WW, Brigham T, et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38:375–81. doi: 10.1016/j.ypmed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Singh H, Kadiyala H, Bhagwath G, Shethia A, El-Serag H, Walder A, et al. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am J Gastroenterol. 2009;104:942–52. doi: 10.1038/ajg.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green BB, Anderson ML, Wang CY, Vernon SW, Chubak J, Meenan RT, et al. Results of nurse navigator follow-up after positive colorectal cancer screening test: a randomized trial. J Am Board Fam Med. 2014;27:789–95. doi: 10.3122/jabfm.2014.06.140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paskett ED, Katz ML, Post DM, Pennell ML, Young GS, Seiber EE, et al. The Ohio Patient Navigation Research Program: Does the American Cancer Society Patient Navigation Model Improve Time to Resolution in Patients with Abnormal Screening Tests? Cancer Epidemiol Biomarkers Prev. 2012;21:1620–8. doi: 10.1158/1055-9965.EPI-12-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell AA, Gravely AA, Ordin DL, Schlosser JE, Partin MR. Timely follow-up of positive fecal occult blood tests strategies associated with improvement. Am J Prev Med. 2009;37:87–93. doi: 10.1016/j.amepre.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Turner B, Myers RE, Hyslop T, Hauck WW, Weinberg D, Brigham T, et al. Physician and patient factors associated with ordering a colon evaluation after a positive fecal occult blood test. J Gen Intern Med. 2003;18:357–63. doi: 10.1046/j.1525-1497.2003.20525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimbo M, Rana GK, Hawley S, Holmes-Rovner M, Kelly-Blake K, Nease DE, et al. What is lacking in current decision aids on cancer screening? CA: A Cancer Journal for Clinicians. 2013;63:193–214. doi: 10.3322/caac.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Census Bureau. State and County QuickFacts. 2014 Dec 3;2014 [cited 2014 December 31] Available from: http://quickfacts.census.gov/qfd/states/00000.html. [Google Scholar]
- 35.Krok J, Kurta M, Weier R, Young G, Carey A, Tatum C, et al. Clinic type and patient characteristics affecting time to resolution after an abnormal cancer-screening exam. Cancer Epidemiol Biomarkers Prev. 2014;23:563. doi: 10.1158/1055-9965.EPI-14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.