Abstract
Diagnostic computed tomography (CT) scans provide numerous opportunities for body composition analysis including quantification of abdominal circumference, abdominal adipose tissues (subcutaneous, visceral and intermuscular) and skeletal muscle (SM). CT scans are commonly performed for diagnostic purposes in clinical settings and methods for estimating abdominal circumference and whole-body SM mass from them have been reported. A supine abdominal circumference is a valid measure of waist circumference (WC). The valid correlation between a single cross sectional CT image (slice) at third lumbar (L3) for abdominal SM and whole body SM is also well established. Sarcopenia refers to the age-associated decreased in muscle mass and function. A single dimensional definition of sarcopenia using CT images that includes only assessment of low whole body SM has been validated in clinical populations and significantly associated with negative outcomes. However, despite the availability and precision of SM data from CT scans and the relationship between these measurements and clinical outcomes, they have not become a routine component of clinical nutrition assessment. Lack of time, training, and expense are potential barriers that prevent clinicians from fully embracing this technique. This tutorial presents a systematic, step-by-step guide to quickly quantify abdominal circumference as a proxy for WC and SM using a cross-sectional CT image from a regional diagnostic CT scan for clinical identification of sarcopenia. Multiple software options are available, however this tutorial utilizes ImageJ, a free public domain software developed by the National Institutes of Health (NIH).
Keywords: Computed tomography, abdominal circumference, skeletal muscle, sarcopenia
Background
Computed tomography (CT) imaging provides powerful diagnostic information and is a routine component of the medical care for many patient populations. In research settings, single-cross-sectional abdominal images from archived diagnostic CT have been assessed for abdominal circumference (cm), abdominal adipose tissue and skeletal muscle (SM) areas (cm2). A supine estimate of CT generated abdominal perimeter is a valid measure of standing WC1. Cross-sectional CT images at the L3 region provide precise estimates of body composition, including regional abdominal adipose tissue, SM2 and WC3. The cut-points associated with health or disease for the CT defined abdominal adipose depots have not been determined, rendering this information somewhat arbitrary and clinically uninterpretable at this time. However, CT measured WC and low SM or CT defined sarcopenia have clinical relevance. This tutorial provides a platform for assisting clinicians in understanding these techniques and utilizing these CT scans that have been used predominantly in research.
Low SM mass assessed by CT has been associated with extended mechanical ventilation, longer hospital stays and mortality4-8. Traditionally, sarcopenia is defined as a low appendicular SM mass (kg/m2) two standard deviations below the mean of young reference group coupled with a concomitant loss of muscle function9-12. Mourtzarkis et al13 developed and validated regression equations for extrapolating data from a single L3 cross-sectional CT image2 to predict whole body composition of adipose tissue and SM mass (kg) that formed the basis for subsequent work by Prado et al4 which established cut-points for SM mass using CT derived data. Specifically, Prado et al determined the gender-specific L3 skeletal muscle index (SM area adjusted for height) cut-points (cm2/m2; males ≤52.4 cm2/m2; females ≤38.5 cm2/m2) which correlate with whole body SM mass (kg) to classify sarcopenia cut-points related to mortality risk4. These cut-points have been linked to adverse outcomes in various populations including the ICU7, cancer5,14, and liver disease15. We recently reported, the prevalence of sarcopenia and sarcopenic obesity in critically ill patients requiring mechanical ventilation using these gender-specific cut-points16,17.
Despite its widespread availability and limited costs of using existing CT images, this assessment methodology is not a routine component of nutritional assessment. This may reflect the training required, the time needed for the analyses and the enhanced understanding and knowledge regarding the required software and techniques. This tutorial presents a systematic, step-by-step guide to successfully analyze a cross-sectional CT image taken from a regional diagnostic CT scan to overcome these barriers. Upon completion of this tutorial, the clinician will have an increased comprehension of basic abdominal anatomy and learn new skills that enable the quantification of abdominal circumference, a proxy for WC and SM area from a L3 cross-sectional CT image.
Methods
A detailed description of the essential tools needed to use the NIH ImageJ software followed by a step-by-step guide for analyzing a diagnostic single cross-sectional CT image (slice) with this program is described. Additionally, a website link that features a preprogrammed template in Microsoft Excel for conversion of the NIH ImageJ data to measures of WC and SM area and an in screen-capture format of this tutorial is also included.
Essential Resources and Tools
The resources, equipment and radiologic expertise needed to obtain body composition information from a cross-sectional CT image includes basic knowledge of CT scans and human anatomy, a compatible computer, specific imaging software (NIH ImageJ), collaboration with a radiologist and a CDROM or USB storage device (for CT image transport).
1) Basics of CT imaging, CT Scan Acquisition and Human Anatomy
Detailed descriptions on CT scanners and CT imaging techniques have been described elsewhere 18-20 and should be reviewed for a comprehensive understanding of this technology. In general, CT is a relatively simple, non-invasive technique used classically for diagnostic and surveillance purposes. The CT imaging procedure scans the body like slices of bread producing cross-sectional images or ‘slices’ 20,21. The CT scanner differentiates body tissues (organs, adipose, skeletal muscle and bone), water and air based on tissue-specific attenuation values such as SM (-29, +150) and adipose tissue (-190, -30) as depicted in 22. These attenuation valuesare reported in Hounsfield Units (HU) named after Sir Godfrey Newbold Hounsfield, the scientist and electrical engineer who invented the CT scanner20,23,24. The use of CT imaging for quantifying anatomic detail using the CT attenuation values was described by Abrams and McNeil over 30 years ago25. Using the minimum and maximum values for these tissue specific thresholds or attenuation values, it is possible to distinguish body composition compartments such as SM and adipose tissue. In the research setting, use of CT imaging is limited by risks associated with radiation exposure, instrument availability and costs 26. Therefore most researches conducting large studies obtain this data via exploitation of existing CT scans completed for clinical purpose18,27,28.
The WC and SM can be assessed with regional CT scans that contain the L3 landmark CT image. These include chest, chest/abdominal, chest/abdominal/pelvic, or abdominal. Of note, contrast agents (ie. barium, iodine dyes) that are commonly administered during a routine CT scan do not interfere with assessment of SM or abdominal adipose tissues. The diagnostic CT scans are contained within a radiology database. A radiologist identifies the L3 landmark, extracts the corresponding single cross-sectional image contained within a CT study and copies the image to a storage device (ie., CDROM or USB flash drive).
The L3 region contains the spinal vertebrae, intestine, kidney and liver, in addition to visceral adipose, subcutaneous adipose and seven muscle groups (the psoas, erector spinae, quadratus lumborum, transversus abdominus, external and internal obliques and rectus abdominus). Images obtained from acute or chronically ill patients likely contain pathologic changes that are different from those of healthy individuals. Figure 1 shows a pictorial representation of the anatomical components of the lower spinal column and the location of the L3 vertebra of an individual. Figure 2 highlights the anatomical features (abdominal perimeter and skeletal muscle groups) at the L3 region of a CT cross-sectional L3 image that are important for this tutorial. The Further Reading list contains recommended books and publications on anatomy, CT of the body in medicine and body composition to use as resources and references during this learning process.
Figure 1.
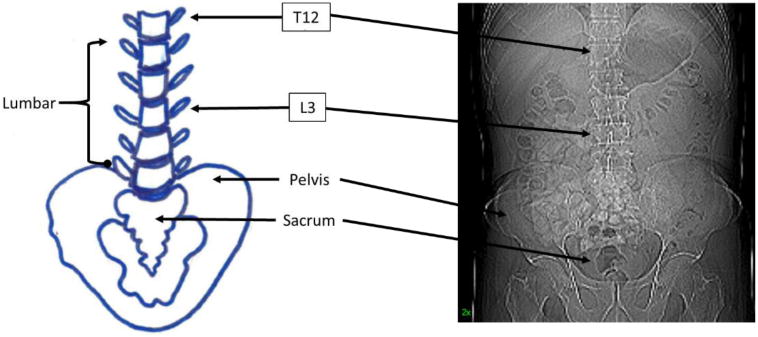
Partial spinal column highlighting various anatomical landmarks including the third lumbar vertebral body (L3).
Figure 2.
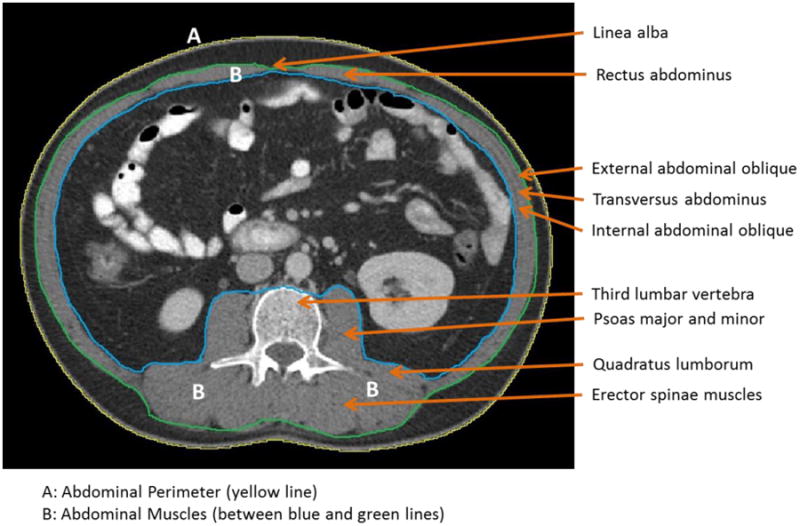
High quality third lumbar (L3) cross-sectional image with major skeletal muscles labeled.
2) Hardware and Software
A personal computer (i.e., desktop or laptop) with a Windows operating systems (32-bit or 64-bit bundle) with or without Java is needed to run NIH ImageJ. There are a variety of commercial software products available for 2D and CT imaging analysis, including Mimics® (Materialise HQ, Leuven, Belgium) and SliceOmatic (Tomovision, Magog, Canada) that have been extensively used in research4,13,27,29. This tutorial highlights the use and application of ImageJ, a free, public domain software developed by National Institutes of Health (NIH). The NIH ImageJ website (http://IMAGEJ.gov/ij) offers an instruction manual, numerous tutorials and many resources related to this software. It has been used extensively for imaging in various medical and biological fields and for body composition research to assess thigh and abdominal skeletal muscle and adipose tissues (subcutaneous, superficial and deep superficial adipose tissues)30-34. One important limitation of the NIH IMmageJ software has recently been raised for its lack of accuracy in assessment of visceral adipose tissue (VAT) because it includes the non-VAT fat within the intestines and other organs.35 Thus, if accurate assessment of VAT is desired, software that remove this fat from the estimated VAT area, such as sliceOmatic (Tomovision), is recommended.
NIH ImageJ can be downloaded as a ZIP archive for Windows, which enables use of the software on institution owned computers. Mac users can download NIH ImageJ as a Mac OS X application. Instructions for both of these options are available at http://IMAGEJ.gov/ij/. Briefly, after arriving at the NIH ImageJ home page, select the ‘DOWNLOAD’ link to get to the webpage listing the available platforms for installation and other software requirements. The download and installation require less than 5 minutes. If a Macintosh operating system is used an older version of Java (Java SE 6) may need to be downloaded prior to opening NIH ImageJ. Java SE 6 may be acquired at https://support.apple.com/kb/DL1572 free of charge.
To improve overall tracing control and delineation of smaller details a graphics tablet with a stylus pen rather than a computer mouse should be used for NIH ImageJ body composition analyses. Graphics tablets range from $25 to $1000 (http://www.intellireview.com/Top-Digitizing-Tablets/); however, a simple tablet with dimensions of 4×5 or 6×8, (average cost of $45-$50) will suffice. We currently use the Turcom Graphic Drawing Tablet (6×8; Turcom USA, San Diego, CA) ($50) and the WACOM Graphire4 (4×5; Wacom Technology Corporation, Vancouver, WA) Tablet ($60-150). Becoming comfortable with the graphics tablet and stylus pen takes time but ultimately improves the accuracy and precision of the tissue delineation process. As with any tool or new procedure, practice is necessary to increase precision, proficiency and reproducibility. NIH ImageJ software requires manual tracing of the various regions within the CT image. As shown in Figure 2, the outer (green line) and inner (blue line) abdominal musculature perimeters are visible. It is recommended that the user practice tracing the outer and inner perimeters of a CT image 5 or more times following NIH ImageJ installation.
Step-by-step Guidelines for Analyzing a Single Cross-sectional Image
The following section describes the step-by-step process for using NIH ImageJ for estimation of WC and SM area. The use of abdominal circumference as an estimate for waist circumference and techniques for tracing abdominal ROIs for body composition analysis have been detailed and described for NIH ImageJ and other body composition medical imaging software36-38. Similar methods will be implemented in this tutorial, modified specifically for use with NIH ImageJ for SM area assessment. As noted earlier, the Hounsfield unit (HU) measures the attenuation levels of body tissues, air and water and is the standard unit of measurement for CT imaging. The CT scanner is calibrated on the premise that the radiodensity of water is 0 HU and air is -1000 HU at standard pressure and temperature. The attenuation thresholds for muscle applied in this tutorial are -29 to +150HU13,28. The ImageJ software allows demarcation and differentiation of these tissues using these specific thresholds.
A) Extraction of CT images
Search the electronic medical record to determine if the patient of interest has a CT study that includes the L3 region during the desired time interval. Once the CT study has been verified, the patient medical record number, CT study type, scan date and storage device (if transporting images) should be provided to the radiologist.
Request the cross-sectional image at the midpoint of the L3.
Ask the radiologist to save the CT image as a DICOM (.dcm file extension) which stands for ‘Digital Imaging and Communication in Medicine.’ The DICOM format is standard for sharing and viewing medical images and compatible with ImageJ.
B) Assessment of CT image quality
High quality images are required for accurate measurement of the parameters described in this tutorial. Unfortunately diagnostic CT scans are not always of high quality; thus each L3 image must be reviewed for quality prior to compositional analysis. Assess the initial quality of CT image using the four step process described below:
Open the NIH ImageJ software (click microscope icon) and left click on FILE tab, move the mouse cursor to OPEN and left click. A new window called OPEN will pop up which allows any NIH ImageJ compatible file to be opened such as a DICOM as previously discussed.
Select the directory where DICOM L3 image files are stored on computer or storage device.
Pick the desired L3 image DICOM file and click “OPEN” tab to open the image. 4. Evaluate your image. An example of a high-quality image for abdominal body composition assessment is provided in Figure 2. Precise estimate of waist circumference requires image to include a complete abdominal perimeter (Figure 2). Additionally, the abdominal skeletal muscles, particularly the obliques and transversus abdominus, should be visible all the way around the abdominal cavity. Figure 3 depicts various examples of poor quality images. Poor quality images occur due to patient movement, fluid overload, or external (i.e., cardiac leads) or internal (i.e., bullet fragments) metal artifacts. The use of such images are limited with NIH ImageJ and results may not be interpretable.
Figure 3.
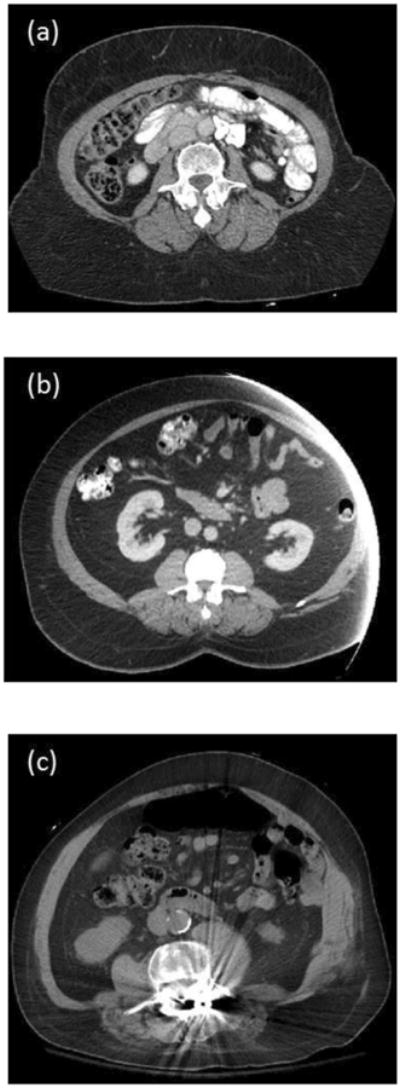
Examples of poor quality cross-sectional third lumbar (L3) images. (a) Incomplete abdominal perimeter will yield inaccurate waist circumference. (b) Incomplete abdominal perimeter and abdominal skeletal muscle mass. (c) Metal artifact interferes with thresholding.
C) Technique for Assessment of WC and SM
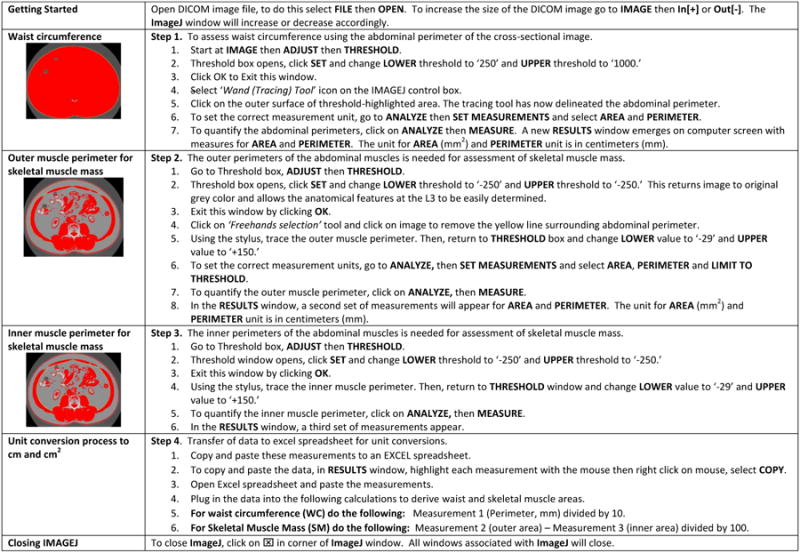
Once the L3 image is obtained and determined to be of good quality, the following CT imaging protocol can be used for assessment of WC and SM area at the L3 region. A one-page, easy to use format which summarizes the body composition analysis protocol for NIH ImageJ is presented in Figure 4. Attach the graphics tablet to the computer and launch NIH ImageJ (i.e., microscope icon). The NIH ImageJ tabs FILE, IMAGE, and ANALYZE will be employed for this tutorial.
Figure 4. Summary of steps for body composition analysis using National Institutes of Health ImageJ.
Open the DICOM image as previously described.
Under the tab headings there are a series of boxes representing drawing tools to trace ROIs on medical and scientific images. Move the mouse over the different tools to see the types and names of these tools. The ‘Freehand selections’ and ‘Wand (tracing) tool’ will be used in the following CT body composition analysis protocol presented in this tutorial.
Figure 5 provides an example of the NIH ImageJ software of a CT image and the four NIH ImageJ windows: IMAGEJ (main menu window), ‘BRIGHTNESS & CONTRAST,’ ‘THRESHOLDS’ and ‘RESULTS’. Follow step 1 below for delineation of the abdominal perimeter of the CT image as depicted in Figure 6 to obtain a proxy measure of WC.
Figure 5.
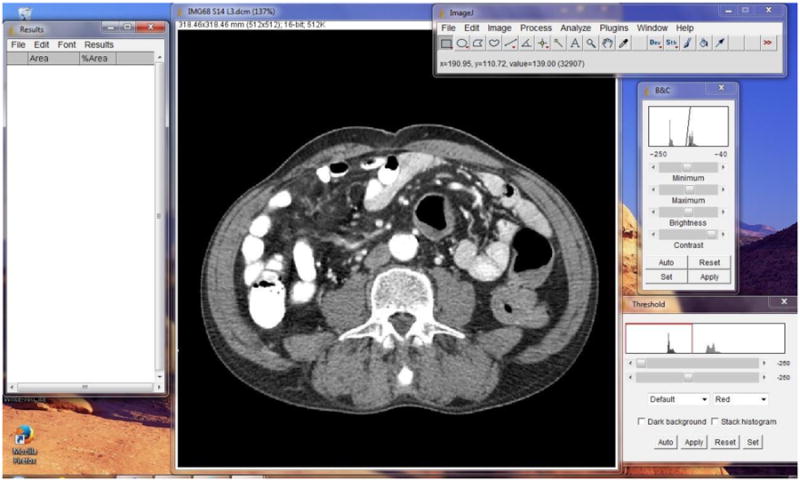
ImageJ software launched on computer screen according to imaging protocol.
Figure 6.
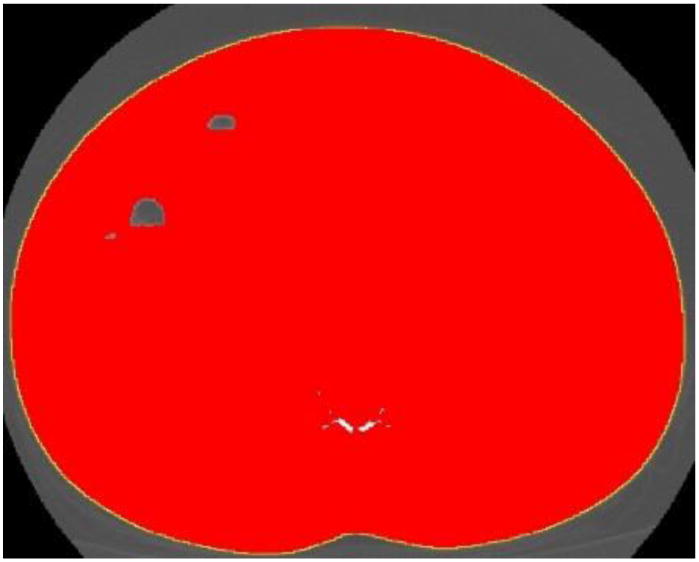
Abdominal perimeter delineated with the ‘wand’ tool. The yellow line represents the abdominal perimeter (waist circumference).
Step 1. Abdominal perimeter for WC
Go to Image>Adjust>Threshold. A Threshold window opens.
Click SET to change the LOWER and UPPER thresholds to -250 and 1000, respectively.
Click OK to close this window.
Select ‘Wand Tracing Tool’ icon on IMAGEJ control box. Click on the outer surface of the threshold-highlighted area. A yellow line should appear surrounding the entire abdominal perimeter.
Go to ANALYZE>SET MEASUREMENTS and select AREA and PERIMETER from the list provided and unselect any other measurements.
Click OK to close this window.
Go to ANALYZE>MEASURE for estimates of AREA and PERIMETER. A RESULTS window opens listing first measurement of area and perimeter (Measurement 1).
Follow these steps for delineating outer abdominal musculature (Figure 7). The outer abdominal musculature perimeters are needed for calculation of SM.
Figure 7.
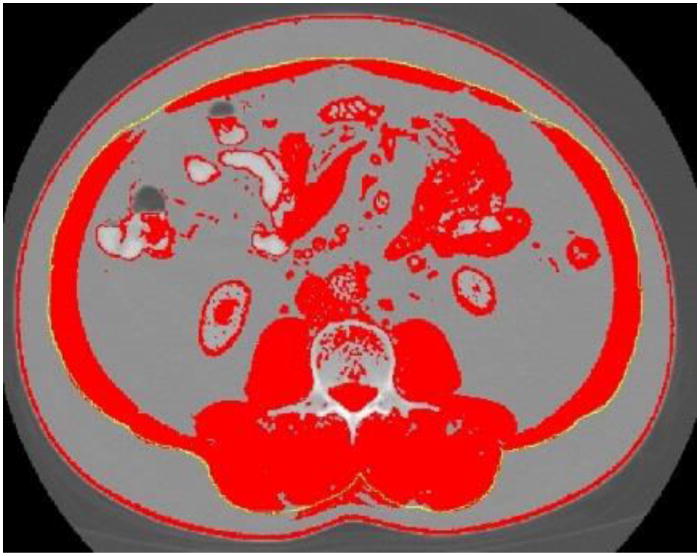
Outer abdominal musculature delineated with ImageJ “freehands selection” tool.
Step 2. Outer Musculature Perimeter
Return to the THRESHOLD window again and adjust the LOWER and UPPER thresholds to -250.
Select the ‘Freehands selection,’ tool then click on image to remove abdominal perimeter line and using the stylus, carefully trace the outer perimeter of the abdominal musculature. If hand slips or if tracing line is not directly over the perimeter, click the pen over the line to make it disappear and repeat again. Repeat the trace around the outer musculature perimeter as many times as needed in order to get it as close as possible to the actual perimeter. Once the best line is drawn, be careful not to touch the image again or the line will be erased.
Return to THRESHOLD window and adjust LOWER and UPPER thresholds to -29 and +150, respectively.
Go to ANALYZE>SET MEASUREMENTS and select AREA, PERIMETER and LIMIT TO THRESHOLD.
Click OK to close this window.
Go to ANALYZE>MEASURE for estimates of AREA, PERIMETER and LIMIT TO THRESHOLD. A RESULTS window opens listing these measurements (Measurement 2).
Step 3. Inner Musculature Perimeter
Delineate the inner abdominal musculature perimeter (Figure 8). The inner abdominal musculature perimeter is also needed for calculation of SM.
Return to THRESHOLD window and adjust LOWER and UPPER values to -250.
Repeat tracing the inner abdominal musculature perimeter as many times as needed to get the delineation as accurate as possible.
Once the inner perimeter muscle wall is outlined, return to the THRESHOLD window and set the LOWER and UPPER thresholds to -29 and +150, respectively.
Go to ANALYZE>MEASURE. A third set of measurements (Measurement 3) will be added to the RESULTS box.
Figure 8.
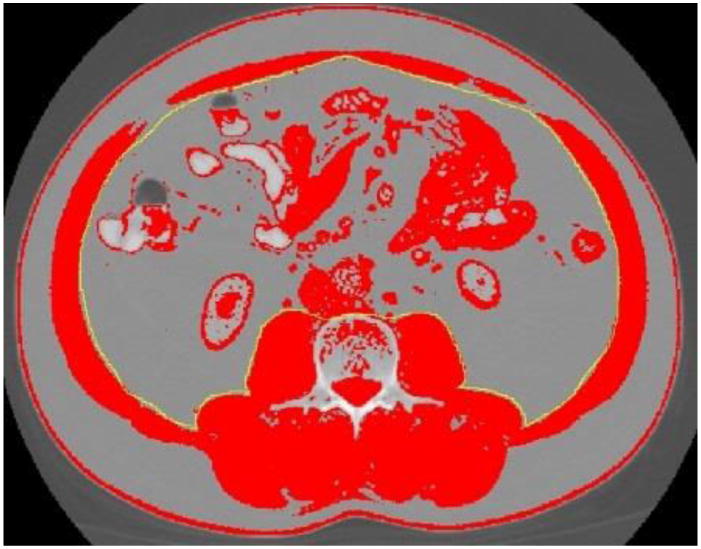
Inner abdominal musculature delineated with ImageJ ‘freehands selection’ tool.
Steps for outer and inner abdominal musculature perimeters can be repeated as many times as desired.
Step 4. Unit conversion process (cm and cm2)
Copy and paste these measurements to a Microsoft EXCEL spreadsheet.
To copy and paste the measurements, return to RESULTS window, highlight the measurements and then right click on mouse and select COPY.
Open a Microsoft Excel spreadsheet and paste the measurements.
Step 5. Closing ImageJ
To close ImageJ, simply click on ☒ in corner of the ImageJ window.
All windows will close.
D) Calculating WC and SM estimates
Insert the results copied to a Microsoft Excel spreadsheet into the following calculations to derive WC and SM area:
For WC, take Measurement 1 (Perimeter, mm) and divide by 10. For example, Measurement 1 equals 1058.832 mm, divide this number by 10 to get 105.832 cm. This is the WC measurement.
For SM area, take Measurement 3 (inner area, mm2) and subtract it from Measurement 2 (outer area mm2) and divide the result by 100. For example, subtract 7144.093 (Measurement 3) from 24280.911 (Measurement 2) and divide by 100 to get 171.37 cm2. This is the SM area measurement.
A preprogrammed template (Figure 9) of this spreadsheet and a screen-capture tutorial may be downloaded at www.slgperez.net. To gain confidence in using the graphics pen, tablet and body composition analysis protocol, repeat the steps numerous times (about 5-10 times) with one cross-sectional image. Using the ‘Wand (Tracing) Tool’ provides a degree of automation to the procedure, producing an exact line around the abdominal perimeter. This step does not need to be repeated because it is the same every time and can simply be copied for future measurements. However, the ‘Freehand Selections’ step requires manual delineation of the inner and outer abdominal musculature membranes so these measurements will vary. Confidence, ease and accuracy will increase with practice. To test consistency between repeated attempts, a coefficient of variation may be obtained from the preprogrammed spreadsheet available at slgperez.net.
Figure 9.
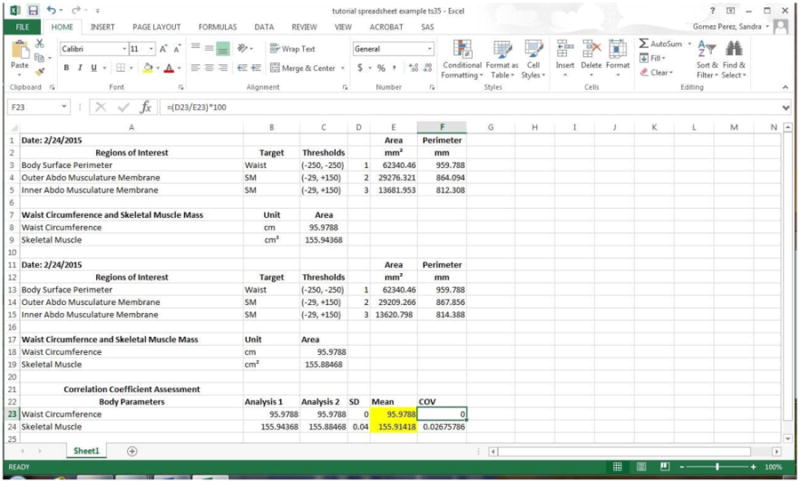
Example of CT body composition imaging results entered on preprogrammed template.
Discussion
CT images for assessment of body composition are accessible, ubiquitous for many patient populations and have been used in many research studies. These investigations have not only consistently demonstrated the clinical significance of quantifying SM for prediction of untoward clinical outcomes, but they have also exposed the widespread prevalence of patients with sarcopenia, particularly in obese individuals4,39. CT defined sarcopenia was strongly associated with longer hospital stays and bacterial infections in patients following liver transplantation15. Patients with hepatocellular carcinoma and CT defined sarcopenia had significantly higher mortality risks compared to those without sarcopenia 40. In a heterogeneous sample of obese cancer patients, those classified as sarcopenic had significantly lower functional status and lower survival than non-sarcopenic patients4. Finally, among women receiving capecitabine treatment for metastatic breast cancer, those with sarcopenia detected by CT imaging had greater likelihood for chemotherapy toxicity and shorter time to disease progression than women without sarcopenia27.
In clinical nutrition practice, a primary goal of nutritional assessment is to discern the adequacy or inadequacy of whole body SM and to develop nutritional treatments tailored to address these findings. Unfortunately clinical tools currently available rely predominantly on subjective measures which are often not sensitive to detect depletion of SM mass, particularly in patients with excessive adiposity. In line with other clinical fields, nutrition professionals need to embrace and incorporate the use of validated imaging technologies into our standard assessment methods to move our field forward. Archived CT scans reflect an opportunity to do so by providing an accessible, accurate measurement of SM mass via the equations developed for classification of sarcopenia. Sarcopenic breast cancer patients behave as if ‘overdosed’ when chemotherapy agents are prescribed using surrogate measures of body composition (e.g., body surface area, body weight), as opposed to the more precise determinants of body composition; a concept introduced by Prado et al 4. Similarly, we propose that the CT measured SM holds promise for improved accuracy in dosing the energy and protein requirements of patients receiving enteral and parenteral nutrition, in addition to the evaluation of such therapies and outcomes. Thus, incorporating the information on SM contained in these cross-sectional L3 CT images can provide clinicians with a much needed objective data for nutrition assessment and objective evaluation.
Conclusion
The use of cross-sectional CT images for SM area and SM mass measurement and abdominal circumference is an objective method of body composition assessment which continues to gain attention and clinical momentum. We speculate this is the wave of the future. To incorporate these measurements into guidelines the utility of these techniques need to be confirmed in varied populations and validation studies testing their accuracy need to be performed. The purpose for this tutorial was to provide a step-by-step guide to facilitate and demystify its use by clinicians as a routine component of nutrition assessment. The quantification of SM area and SM mass has been used to: 1) describe and quantify abdominal circumference, which is often difficult to assess in our bed-ridden patients, 2) quantify L3 SM area and determine SM mass and detect sarcopenia4, and 3) determine the relationship between these measures and various clinical outcomes18,28,41. Nutrition professionals need to acknowledge the potential for implementing imaging techniques in clinical practice to provide much needed objective data for modern, comprehensive nutrition assessment.
Clinical Relevancy Statement.
The purpose of this tutorial is to provide clinicians with detailed methodology for the utilization of diagnostic computed tomography scans to quantify waist circumference and abdominal skeletal muscle area for use in clinical nutrition assessments.
Acknowledgments
We thank Sarah Peterson, Caitlin Levey and Marla Williams for testing this tutorial. We thank Dr. Vickie Baracos, Dr. Carla Prado, Dr. Nina Esfandiari, and Crystal Beaumont at the University of Alberta (Canada) for expertise and training on CT body composition analysis. We would like to thank Dr. Thomas PJ Solomon and Dr. John P Kirwan (Cleveland Clinic Foundation) for providing details for using NIH ImageJ for body composition analysis. The project described was supported by Grant Number 5R25-CA057699 from the National Cancer Institute and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
- 1.Waninge A, Ligthart KA, Kramer J, Hoeve S, van der Schans CP, Haisma HH. Measuring waist circumference in disabled adults. Research in developmental disabilities. 2010 May-Jun;31(3):839–847. doi: 10.1016/j.ridd.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. Journal of applied physiology (Bethesda, Md: 1985) 2004;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Neeland IJ, Turer AT, Vega GL. Waist circumference as measure of abdominal fat compartments. Journal of obesity. 2013;2013 doi: 10.1155/2013/454285. 454285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. The lancet oncology. 2008 Jul;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 5.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. British journal of cancer. 2012;107(6):931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weijs PJ, Looijaard WG, Dekker IM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Critical care (London, England) 2014;18(1):R12. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moisey LL, Mourtzakis M, Cotton BA, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Critical care (London, England) 2013;17(5):R206. doi: 10.1186/cc12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013 Apr 20;31(12):1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010 Jul;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr 15;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 11.Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. The American journal of clinical nutrition. 1989 Nov;50(5 Suppl):1121–1235. [PubMed] [Google Scholar]
- 12.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. The Journal of nutrition. 1993 Feb;123(2 Suppl):465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- 13.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2008;33(5):997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 14.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009 Nov 15;15(22):6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 15.Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014 Jun;20(6):640–648. doi: 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 16.Sheean PM, Peterson SJ, Gomez Perez S, et al. The prevalence of sarcopenia in patients with respiratory failure classified as normally nourished using computed tomography and subjective global assessment. JPEN Journal of parenteral and enteral nutrition. 2014 Sep;38(7):873–879. doi: 10.1177/0148607113500308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braunschweig CA, Sheean PM, Peterson SJ, et al. Exploitation of diagnostic computed tomography scans to assess the impact of nutrition support on body composition changes in respiratory failure patients. JPEN Journal of parenteral and enteral nutrition. 2014 Sep;38(7):880–885. doi: 10.1177/0148607113500505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN Journal of parenteral and enteral nutrition. 2014 Nov;38(8):940–953. doi: 10.1177/0148607114550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annual Review of Nutrition. 1997;17:527–558. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 20.Goldman LW. Principles of CT and CT technology. Journal of nuclear medicine technology. 2007;35(3):115–128. doi: 10.2967/jnmt.107.042978. quiz 129-130. [DOI] [PubMed] [Google Scholar]
- 21.Lee GH, Cohen AJ. CT imaging of abdominal hernias. AJR American journal of roentgenology. 1993;161(6):1209–1213. doi: 10.2214/ajr.161.6.8249727. [DOI] [PubMed] [Google Scholar]
- 22.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Current opinion in supportive and palliative care. 2009 Dec;3(4):269–275. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 23.Lee JK, Evens RG. Computed tomography of the body. Disease-a-month: DM. 1980 Apr;26(7):1–56. doi: 10.1016/s0011-5029(80)80008-3. [DOI] [PubMed] [Google Scholar]
- 24.Rossner S, Bo WJ, Hiltbrandt E, et al. Adipose tissue determinations in cadavers--a comparison between cross-sectional planimetry and computed tomography. International journal of obesity. 1990;14(10):893–902. [PubMed] [Google Scholar]
- 25.Abrams HL, McNeil BJ. Medical implications of computed tomography (“CAT scanning”) (first of two parts) The New England journal of medicine. 1978 Feb 2;298(5):255–261. doi: 10.1056/NEJM197802022980506. [DOI] [PubMed] [Google Scholar]
- 26.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Archives of internal medicine. 2009;169(22):2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. The American Journal of Clinical Nutrition. 2009;89(4):1173–1179. doi: 10.3945/ajcn.2008.27273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prado CM. Body composition in chemotherapy: the promising role of CT scans. Curr Opin Clin Nutr Metab Care. 2013 Sep;16(5):525–533. doi: 10.1097/MCO.0b013e328363bcfb. [DOI] [PubMed] [Google Scholar]
- 29.Prado CM, Lieffers JR, Bowthorpe L, Baracos VE, Mourtzakis M, McCargar LJ. Sarcopenia and physical function in overweight patients with advanced cancer. Canadian journal of dietetic practice and research: a publication of Dietitians of Canada = Revue canadienne de la pratique et de la recherche en dietetique : une publication des Dietetistes du Canada. 2013 Summer;74(2):69–74. doi: 10.3148/74.2.2013.69. [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. Journal of applied physiology (Bethesda, Md: 1985) 2000;89(1):104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 32.Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haus JM, Solomon TP, Marchetti CM, et al. Decreased visfatin after exercise training correlates with improved glucose tolerance. Medicine and science in sports and exercise. 2009;41(6):1255–1260. doi: 10.1249/MSS.0b013e318195bad5. [DOI] [PubMed] [Google Scholar]
- 34.Solomon TP, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. The Journal of clinical endocrinology and metabolism. 2011;96(5):1377–1384. doi: 10.1210/jc.2010-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casaer MP, Langouche L, Coudyzer W, et al. Impact of Early Parenteral Nutrition on Muscle and Adipose Tissue Compartments During Critical Illness. Critical care medicine. 2013 Jul 15; doi: 10.1097/CCM.0b013e31828cef02. [DOI] [PubMed] [Google Scholar]
- 36.Ciudin A, Salvador R, Budoy A, et al. Measurement of waist circumference for retrospective studies - prospective validation of use of CT images to assess abdominal circumference. Endocrinologia y nutricion: organo de la Sociedad Espanola de Endocrinologia y Nutricion. 2014 Mar;61(3):147–152. doi: 10.1016/j.endonu.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Bredella MA, Ghomi RH, Thomas BJ, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 2010 Nov;18(11):2227–2233. doi: 10.1038/oby.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geraghty EM, Boone JM. Determination of height, weight, body mass index, and body surface area with a single abdominal CT image. Radiology. 2003 Sep;228(3):857–863. doi: 10.1148/radiol.2283020095. [DOI] [PubMed] [Google Scholar]
- 39.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012 Oct;31(5):583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a Prognostic Index of Nutritional Status in Concurrent Cirrhosis and Hepatocellular Carcinoma. Journal of clinical gastroenterology. 2013 Jun 6; doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 41.Baracos V, Kazemi-Bajestani SM. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. The international journal of biochemistry & cell biology. 2013 Oct;45(10):2302–2308. doi: 10.1016/j.biocel.2013.06.016. [DOI] [PubMed] [Google Scholar]


