Abstract
STAT3 is a member of STAT transcription activators. Aberration in STAT3 activity due to constitutive activation or mutations leads to diseases such as cancer and hyper immunoglobulin E syndrome (HIES). STAT3 contains several structured domains including the SH2 domain, linker domain (LD), DNA-binding domain (DBD) and the coil-coil domain (CCD). Here we report the discovery of inter-domain allosteric communications in STAT3 from studies using NMR and other methods. We found that pTyr-peptide interactions with the SH2 domain cause structural and dynamic changes in LD and DBD. The inter-domain allosteric effect is likely mediated by the flexibility in the hydrophobic core. In addition, a mutation in LD found in HIES (I568F) induces NMR chemical shift perturbation in SH2, DBD and CCD, suggesting conformational changes in these domains. Consistent with conformational changes in SH2, the I568F mutant reduces SH2’s binding affinity to a pTyr-containing peptide. This study provides an example of dynamics-dependent allosteric effects, and due to the structural conservation of the STAT family of proteins, the inter-domain allosteric communication observed in STAT3 likely occurs in other STATs.
Keywords: STAT3, NMR, HIES, SH2, dynamics, allostery
Graphical abstract
Introduction
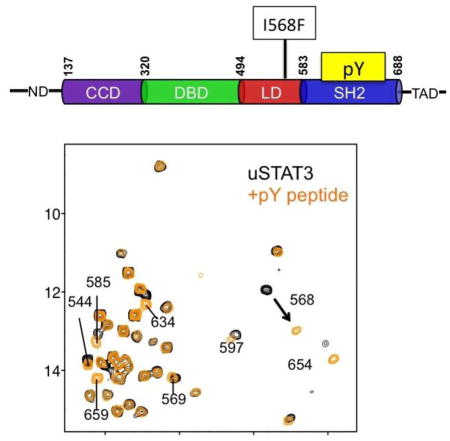
Since the description of allostery about 50 years ago 1, the allostery concept is found to be increasingly important in describing structure-function relationships of proteins and has a powerful and lasting impact on drug discovery. The role of allostery has not yet been investigated for the signal transducer and activator of transcription (STAT) family of proteins that play a key role in mediating cellular responses to extracellular stimuli. There are seven STAT proteins (1, 2, 3, 4, 5a, 5b and 6), which all share a similar domain architecture that includes an unstructured N-terminal domain (ND), coiled-coil domain (CCD), DNA-binding domain (DBD), linker domain (LD), Src homology 2 domain (SH2) and an unstructured transactivation domain (TAD) located at the C-terminus (Figs. 1A). The SH2 domains are involved in binding the phosphorylated membrane-receptors and their associated tyrosine kinases for phosphorylation of STAT 2; 3 (Fig. 1B). The phosphorylated STATs form hetero- or homodimers, also through the SH2, which lead to nuclear import and DNA-binding through their DBDs 4 (Fig. 1B). The interleukin-6 (IL-6) receptor is well known to interact with STAT3 and mediates STAT3-dependent signaling events in a broad range of cell types.
Fig. 1.
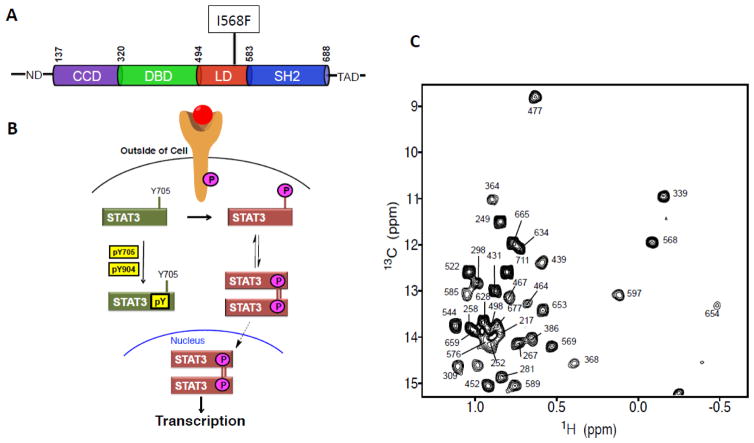
Overview of domain structure of STAT3, STAT3 signaling pathway and NMR spectrum. (A) Schematic illustration of domain architecture of STAT3. The residues at the domain boundaries are indicated at the top. The N-terminal domain (ND) and transactivation domain (TAD) are not well structured. The position of the HIES mutant I568F is indicated. (B) Schematic illustration of the various states and complexes of STAT3 investigated in this study by NMR methods and how the different states are involved in the signal transduction process of STAT3. (C) 1H-13C HMQC spectrum of the 68 kDa STAT3 encompassing residues 127-711. All Ile residues have been assigned and labeled.
Further investigation of the role of allostery in STAT function is needed, because currently available X-ray structures of STAT proteins do not provide sufficient understanding of the mechanisms underlying the conditions caused by some recently identified disease-inducing mutations 5. Aberrantly weakened STAT3 signaling due to mutations are linked to various diseases including the hyper immunoglobulin E syndrome (HIES) 6; 7. Some of these HIES-causing mutations are located in functionally unknown domains, such as the LD (Fig. 1A), and their molecular mechanism for disease pathogenesis is unclear based on our current knowledge of structure-function relationship of STAT3 8; 9; 10. Information on allostery in STAT’s function is not yet available, but is fundamentally important and necessary for understanding the etiology of mutant-induced diseases.
In this study, using nuclear magnetic resonance (NMR) spectroscopy in conjunction with site-directed mutagenesis and other methods, we show that the STAT3 domains that have well-defined structures (SH2, LD, DBD and CCD) (Fig. 1A) are not structurally independent from each other. Allosteric communications between domains occur as shown by NMR chemical shift perturbation (CSP) and relaxation dispersion measurements. To examine the functional significance of the allosteric communication, we tested the hypothesis that a HIES-associated mutation (I568F) that is not located in domains of known functional importance (pTyr-peptide or DNA binding) reduces STAT3 signaling through allosteric effect (Fig. 1A). We found that the I568F mutation caused NMR CSP at SH2, DBD and CCD, and consistently, affects the SH2 activities. This result suggests the functional significance of the allosteric communication. In addition, this finding also provides an understanding of disease pathogenesis by mutations at STAT3 sites that are not directly involved in pTyr or DNA-binding activities. Because of the structural and mechanistic conservation within the STAT family, the allosteric mechanism in STAT3 identified here is likely applicable to other STATs.
Results
Resonance assignments of the 68kDa core region of STAT3
The recent advancement of NMR methods has enabled structural and dynamic studies of high-molecular-weight proteins and their complexes 11; 12; 13; 14; 15; 16; 17. Upon extensive optimization of expression constructs and soluble conditions, we obtained a well-dispersed two-dimensional (2D) 13C-1H heteronuclear multiple quantum coherence (HMQC) spectrum of the 68 kDa construct of the unphosphorylated STAT3 (uSTAT3) core region, encompassing residues 127–711 (Fig. 1C), excluding the unstructured ND and TAD. The protein sample was selectively 13C-1H-labeled at the Cδ1 methyl group of all 35 Ile, using previously described protocols 18; 19. We unambiguously assigned all Ile residues in STAT3 (Fig. 1C) by a combination of site-directed mutagenesis and 13C-edited NOESY spectrum based on the X-ray structure 10. The Ile residues are distributed throughout the entire protein, thus providing site-specific probes to gain structure and dynamic insights into STAT3.
Inter-domain allosteric communications involved in pTyr-binding
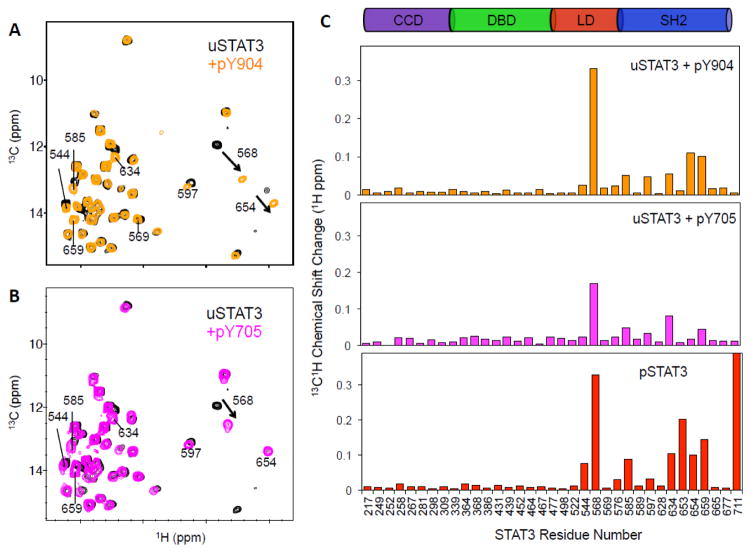
The function of the SH2 domain is to bind a pTyr-containing peptide segment of another protein, which can be the activated receptor (i.e., IL-6 receptor, also known as glycoprotein 130, gp130) or the pTyr-segment of another STAT molecule (Fig. 1B). Binding of uSTAT3 with a peptide derived from the phosphorylated IL-6 receptor, known as pY904 and which represents phosphorylated IL-6 receptor binding by STAT3 20, caused significant chemical shift perturbation (CSP) in SH2 at the peptide-binding groove (I634, I654 and I659) (Figs. 2A and 2C top panel). Unexpectedly, I544 and I568 in the LD showed substantial CSP (Fig. 2A). These two residues do not directly contact pTyr or residues in contact with pTyr, which include K591, R609, S611 and S613 10. In particular, I568 in LD showed the largest CSP (Figs. 2A, and 2C top panel). I544, which is 17 Å from I568 in the LD, showed significant CSP (Fig. 2A), suggesting overall structural perturbation of the LD upon binding of the pTyr-peptide to SH2. Similarly, binding of uSTAT3 with pY705, which was derived from phosphorylated STAT3 (pSTAT3) 21, resulted in similar CSP patterns, although with smaller amplitudes, consistent with lower affinity (Figs. 2B, and 2C middle panel). These results indicate that binding of pTyr-peptide at SH2 causes a significant cross-domain allosteric effect on the LD.
Fig. 2.
NMR CSP observed due to STAT3 interactions with pTyr-peptides and pSTAT3 dimer formation. (A and B) Superimposed 2D 13C-1H HMQC spectra of uSTAT3, free and in complex with pY904 (A) and pY705 (B). (C) Plots of CSP versus residue number for the different complexes: uSTAT3 + pY904 (top panel), uSTAT3 + pY705 (middle panel), and pSTAT3 dimer (bottom panel).
pSTAT3 dimer formation
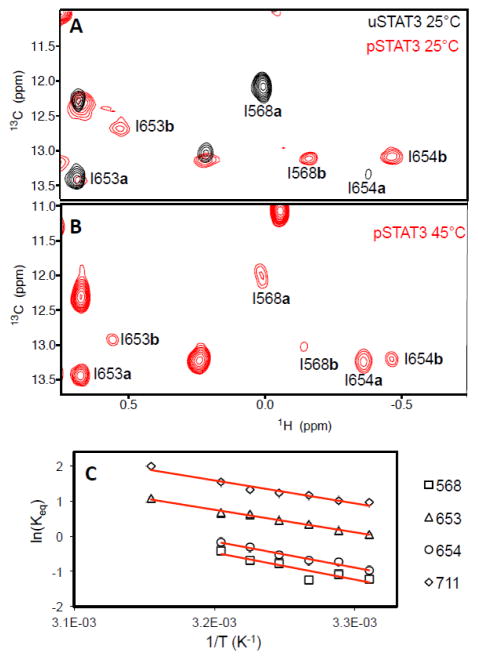
Next, we investigated STAT3 dimerization, which occurs through reciprocal binding of pTyr-containing segments via the SH2 domain and induces translocation of STAT3 to the nucleus for high affinity DNA-binding to initiate gene expression (Fig. 1B) 4. Methyl-13C-labeled pSTAT3 was produced by expressing the protein in TKB1 cells (Agilent Technologies) that contained a tyrosine kinase that was also expressed upon induction with indoleacrylic acid. Using the methyl-13C-labeled pSTAT3, we observed a dominant set of pSTAT3 resonances that followed the same trend as uSTAT3 in complex with pTyr-containing peptides (labeled with “b” following residue numbers, such as I568b, in Fig. 3A). Additional CSPs unique to pSTAT3 were observed at I653 and I711. Besides pTyr-dependent interactions through the SH2 domain, residues 643–644, 647–648, 706, 708, and 710 are also involved in the homodimer formation10. Therefore, I711 is located near the binding interface of the pSTAT3 dimer, and its CSP reflects the contacts unique to the pSTAT3 dimer. However, I653 is not at the dimer interface, and thus its CSP likely reflects allosteric effects induced by the dimer formation 9; 10.
Fig. 3.
NMR studies of pSTAT3 homodimer. (A) Representative section of the overlay of the uSTAT3 (black) and pSTAT3 (red) 13C-1H HMQC spectra at 25°C. (B) Temperature-dependent changes of pSTAT3 peaks (labeled) between monomer (labeled with a following the residue number) and dimer (labeled with b following the residue number). (C) Van’t Hoff plots for the exchanging LD and SH2 resonances in pSTAT3 spectra. Keq is the ratio of the exchanging peak volumes for the labeled residues (black symbols) at each temperature (T). The solid red lines are fits to the linear van’t Hoff equation.
When temperature increased, the peaks corresponding to that of uSTAT3 appeared (labeled with “a” following residue numbers, such as I568a, Fig. 3B). The intensity of this set of peaks increased as temperature increased, with a corresponding decrease in the peaks corresponding to the pSTAT3 dimer (Fig. 3B). This suggested that this set of peaks was due to pSTAT3 dissociation to monomer (no pTyr binding), whose population increased with increasing temperature. Therefore, this result suggests that pSTAT3 undergoes monomer-dimer exchange at the NMR timescale.
We conducted van’t Hoff analyses for the exchange observed in the HMQC spectra recorded at several temperatures ranging from 25 °C to 45 °C (Fig. 3C) to further confirm the monomer-dimer equilibrium. The intensity ratios between monomer and dimer states vary slightly among different residues, likely due to the differences in local dynamics in the different states. Individual fits of the linear van’t Hoff plots showed that I653 and I711 at the SH2 dimer interface shared the same ΔH° and ΔS° values (12.8 ± 0.5 kcal/mol and 42.2 ± 1.6 cal/mol•K, respectively) as I568 in the LD and I654 in the SH2 domain (ΔH0 and ΔS0 values of 14.8 ± 1.1 kcal/mol and 47.0 ± 3.7 cal/mol•K, respectively), further supporting that the two sets of peaks observed for residues in SH2 and LD are due to the monomer-dimer equilibrium (Fig. 3C).
Structural and dynamic changes associated with allosteric coupling
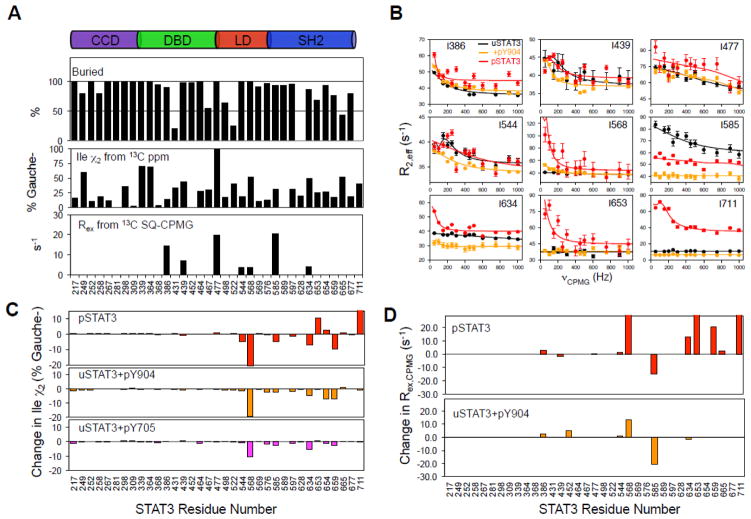
We investigated the conformational shift of the allosteric effect. The Ile chi-2 (χ2) rotamer population distributions calculated from 13C chemical shifts 22 indicated that most uSTAT3 residues in the hydrophobic core adopt more than a single χ2 configuration (Fig. 4A, middle panel). In particular, the sidechains of I439, I568, and I634 are populated approximately equally between the Trans and Gauche- states (50 ± 10 %), and yet these residues are fully buried in the hydrophobic core of STAT3 (Fig. 4A, top panel). To provide quantitative insights on dynamics, we conducted 13C single-quantum (SQ) Carr-Purcell-Meiboom-Gill (CPMG) relaxation dispersion experiments 23; 24 (Fig. 4B, Fig. S1) for which changes in the 13C chemical shift resulting from conformational exchange cause an increase in the effective transverse relaxation rate R2,eff 23. For uSTAT3, we observed significant Rex in seven Ile methyl groups at the DBD (I386, I439 and I477), LD (I544 and I568), and SH2 (I585 and I634) (Fig. 4A, bottom panel). These residues are all fully buried (Fig. 4A, top panel) in the hydrophobic core. Taken together, these data indicate that the hydrophobic core residues of the various STAT3 domains are highly dynamic.
Fig. 4.
Structural and dynamic changes associated with STAT3 dimerization and pTyr-peptide binding. (A) The top panel shows the buried surface area of Ile in the crystal structure (pdbid: 3CWG). Ile chi-2 (χ2) rotamer populations of uSTAT3 in solution (middle panel) are indicated by the Gauche- conformer distribution (100% minus Trans). Quantitative μs-ms dynamics are indicated by Rex values (R2,eff [0 Hz] minus R2,eff [1000 Hz]) obtained from fits in (B). (B) Relaxation dispersion of STAT3 in different states. Overlays of representative 13C SQ-CPMG relaxation dispersion profiles (R2,eff vs. νCPMG) at 700 MHz and 35 °C for uSTAT3 (black), uSTAT3+pY904 complex (orange) and pSTAT3 homo-dimer (red). The solid lines are from the individual fits to a two-state exchange model. Error bars were estimated from duplicated measurements. (C) Bar graphs of the changes in the Ile χ2 rotamers of uSTAT3 upon pSTAT3 dimerization (red) or binding pTyr-peptide (orange and magenta). (D) Bar graphs of the dynamic changes upon pSTAT3 dimer formation (red) or pY904 binding to uSTAT3 (orange).
Binding of pTyr-containing peptides and pSTAT3 dimerization through the SH2 domain involve structural changes. χ2 rotameric analysis indicated that binding of the pTyr-peptide at the SH2 domain induced a significant change in rotameric distribution at the LD (Fig. 4C). In particular, the buried I568 showed the largest change in its χ2 rotamer among all Ile residues, consistent with it showing the largest CSP upon binding of pTyr-peptides (Fig. 2C). The allosteric effect from binding pTyr-containing peptides and pSTAT3 dimerization through the SH2 domain also involves dynamic changes. Binding of the pY904 peptide quenched the μs-ms timescale dynamics at the SH2, as indicated by decreased Rex at the SH2 residues I585 and I634. However, the interaction increased the μs-ms timescale dynamics at LD, as shown by the increased Rex of the LD residue I568 (Fig. 4B and Fig. 4D). Larger increases in the Rex of I568 were observed for the dimeric pSTAT3 than for pY904-bound uSTAT3. Increase in Rex upon forming pSTAT3 may reflect monomer-dimer equilibrium for some residues, such as that of residue I711. For other residues, such as I568, the increase in Rex likely reflects both dynamics changes with allosteric coupling and monomer-dimer equilibrium, and displayed larger increases in Rex upon forming pSTAT3 dimer than forming complex with the pY904 peptide. These results provided substantial insights into the mechanism of the allosteric effects.
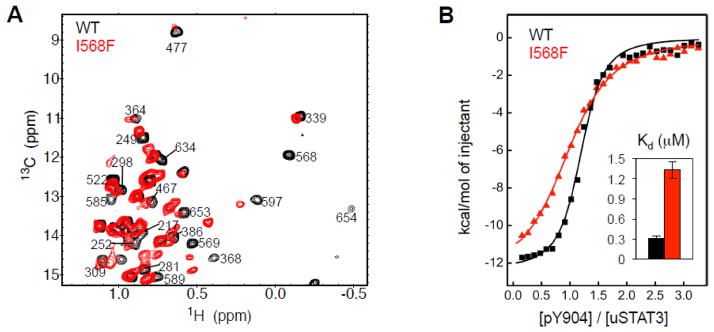
Studies of a HIES-associated STAT3 with mutations in the LD
As discussed above, I568F is a HIES associated mutation in the LD 25; 26. Coincidently, I568 undergoes the largest changes in structure and dynamics among all measured residues when the SH2 domain forms a complex with a pTyr-containing peptide (Figs. 4C and 4D). Because the LD is not known of functional importance, the influence of I568F in the development of HIES was unknown. NMR chemical shift dispersion suggests that I568F is folded and maintains a hydrophobic core (Fig. 5A). However, its NMR spectrum showed significant CSP relative to WT protein in all structured domains of STAT3, from the CCD to SH2, suggesting that this mutation causes cross-domain allosteric effects (Fig. 5A). Consistent with structural perturbation in SH2, isothermal titration calorimetry (ITC) measurements indicated that I568F showed an approximately 5-fold reduction in the binding affinity for the pY904 peptide (Fig. 5B). This result further illustrates the inter-domain allosteric communications in STAT3.
Fig 5.
Allosteric effects on structure and function of the HIES mutant I568F in the LD. (A) Superposition of the 2D 13C-1H HMQC Ile spectra of WT (black) and I568F (red) STAT3. Peak labels are on WT STAT3. (B) ITC binding curves for the interaction of WT (black) and I568F (red) STAT3 with pY904 peptide. The binding affinities (Kd) shown in the insert demonstrated altered function of the SH2 domain by the mutation in LD.
Discussion
In this study, we have provided the first evidence of allosteric communications between the SH2 and LD domains associated with activation and formation of a transcriptional active complex for a STAT transcription factor. Binding of pTyr-containing peptides to the SH2 domain caused significant CSP and changes in dynamics on residues in LD. In particular, the LD residue I568 showed the largest change in its χ2 rotamer among all Ile residues, consistent with it showing the largest CSP upon binding of pTyr-peptides. In addition, binding of the pY904 peptide increased the μs-ms timescale dynamics of I568 (Fig. 4B and Fig. 4D). I568 does not directly interact with pTyr or residues in the pTyr-binding pocket 10. I568 is located at the LD-SH2 interface, and along with V572, contacts F610 in SH2. However, F610 is not part of the pTyr-binding pocket, but could mediate the allosteric communication between LD and SH2. Additional support of the allosteric communication is indicated by the HIES mutant I568F (Fig. 5). Although located in LD, this mutation not only caused CSP in LD, but also CSP in SH2, DBD and CCD, and reduced the SH2 domain’s ability to bind the pTyr-containing peptide pY904. Therefore, this mutation confirms the allosteric communications between LD and SH2, and suggests allosteric communications between LD and DBD and CCD.
The allosteric communications identified from this study is consistent with a recent finding using hydrogen-deuterium exchange (HDX) mass spectrometry to analyze changes in HDX rates due to binding small molecule inhibitors to the SH2 domain of STAT3 27. This mass spectrometry analysis found that small molecule inhibitors bound to the SH2 domain of STAT3 caused allosteric effects in other domains of STAT3, suggesting cross-domain allosteric effects. The mass spectrometry approach is complementary to our studies, because the mass spectrometry study did not investigate whether an allosteric effect is involved in the physiological functions of STAT3, and our studies have provided such information. In addition, NMR studies have provided molecular insights into the allosteric effects.
The conformational dynamics of the hydrophobic core likely facilitates the inter-domain allosteric effects and molecular interactions. Several hydrophobic core residues in the various domains of uSTAT3 contained at least two significant sidechain rotamer populations that are in equilibrium (Fig. 4A). CPMG relaxation dispersion experiments quantitatively demonstrated the existence of μs-ms conformational dynamics at residues buried in the STAT3 hydrophobic core of various domains (Figs. 4A and 4B). The flexibility likely allowed structural and dynamic changes upon molecular complex formation at the direct contact site and propagation of these changes to distant sites, such as from SH2 to LD. Binding of pTyr-containing peptide to uSTAT3 and pSTAT3 dimer formation resulted in structural changes at the SH2 along with a quench of dynamics in this domain (Figs. 4C and 4D). In addition, the interactions increased dynamics and induced structural changes at LD (Figs. 4C and 4D). The increased dynamics in LD upon binding pTyr-containing peptide would compensate for the entropy loss due to the interaction, and thus such allosteric effect would facilitate the interaction. This finding is distinct from other findings of dynamic-driven allosteric effects; for example it was found previously that ligand binding reduces fast motions but enhance slow motions 28. Therefore, the findings described here contributes to our understanding of dynamic-driven allosteric effects by providing an example that the direct interaction site is rigidified coupled with increased dynamics at a distal site upon ligand binding.
The functional significance of allosteric inter-domain communication is indicated by the HIES mutant I568F (Fig. 5). I568F mutation occurs in LD (Fig. 1A), a domain without a previously known function. Here we show that the mutation reduced the SH2 domain’s ability to bind the pTyr-containing peptide through allosteric effect. Therefore, characterization of this mutation has provided an understanding of the molecular mechanism of this HIES-causing mutation, whose etiology could not be explained by previous structural information of STAT3. Taken together, studies described here not only indicated the functional significance of the allosteric effect, but also provided an understanding, at the molecular level, of how the I568F mutation reduces STAT3 signaling. It is possible that other HIES or other disease causing STAT3 mutants that are not located at the pTyr- or DNA-binding sites alter STAT3 functions through allosteric effects.
In summary, the studies described here have provided novel insights into how inter-domain allosteric communications control STAT3 function, regulation and the change of STAT3 functions by disease-inducing mutants that are not located at sites involved in direct binding activities. Because of the structural and mechanistic conservation within the STAT family, the findings described here improve our understanding of signal transduction by the JAK-STAT pathway as well as of diseases caused by dysregulation of this pathway. In addition, the information on allosteric effects of STAT proteins would help to accelerate the development of therapeutic strategies targeting aberrant activities of STATs.
Materials and Methods
STAT3 expression and purification
A portion of the cDNA encoding human STAT3β (127–711) was inserted into the pET28+ expression vector between the NcoI site and the histidine tag and verified by DNA sequencing. All mutations were performed using the QuikChange Site-directed Mutagenesis Kit (Agilent) by following the manufacturer’s suggested procedure. The primer sequences for the mutagenic generation of all constructs used in bacterial expressions are listed in SI Table S1.
The E. coli BL21 (DE3) strains (CodonPlus-RIL and TKB1) harboring the STAT3 expression plasmid were initially grown in 1L terrific broth (TB) at 37 °C into the end of the log phase, washed with 1L M9 media lacking NMR isotopes and then rehydrated in 250 mL M9 media containing 0.25 g 15NH4Cl, 1 g [D7]-glucose, 100% D2O, 100 mg [13CH3]-L-methionine, 800 mg [3-13CH3; 2-D]-L-alanine, 2.5 g [D4]-succinic acid, and the sodium salts of 60 mg [13CH3; 3,3-D2]-alpha-ketobutyric acid and 100 mg [3-13CH3; 3,4,4,4-D4]-alpha-ketoisovaleric acid 18; 29; 30. All isotopes were purchased from Cambridge Isotope Laboratories. Mutant samples used for isoleucine assignments were expressed in a 2 to 5-fold reduction of the M9 media recipe and with isotope labeling of isoleucines only using the alpha-ketobutyrate precursor. Cells were allowed to recover for 1 hour in the M9 media prior to addition of 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 24 hours at 20 °C to induce uSTAT3 expression. To generate pSTAT3, uSTAT3 was simultaneously expressed with the tyrosine kinase induced with 53 μM indoleacrylic acid in TKB1 cultures. To produce unlabeled samples for ITC, the M9 step was omitted and protein expression followed previous protocols 31; 32.
Soluble His-tagged uSTAT3 or pSTAT3 (127-711-His6) was purified by nickel affinity chromatography (Ni-NTA agarose from Qiagen) using previously described protocols 19; 33. The STAT3 eluent was exchanged into sample buffer at pH 5.5 containing 10 mM MES buffer, 2 mM TCEP and 0.03% sodium azide. NMR samples contained deuterated versions of all buffer reagents in 100% D2O. Protein sample concentrations were determined by Bradford Assay (BioRad Laboratories) and by UV absorbance at 280 nm. The ExPASy ProtParam tool was used to estimate the extinction coefficient from the protein sequence 34.
Peptides
The phosphotyrosine peptides derived from STAT3 (pY705) and gp130 (pY904) were synthesized at the City of Hope Synthetic and Biopolymer Chemistry core facility and were dissolved in STAT3 sample buffer. The concentration of each peptide was calibrated with the standard DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid) using 1D 1H NMR.
Nuclear magnetic resonance (NMR) spectroscopy
NMR spectra were acquired at multiple temperatures ranging from 25 °C to 45 °C using the IconNMR module of Topspin 3.1 on a Bruker Ascend 700 MHz spectrometer equipped with a TCI cryo-probe head and a Sample Jet automation unit (Bruker BioSpin, Karlsruhe, Germany) or a TXI cryo-probe equipped Bruker Avance 600 MHz spectrometer. 1H-13C correlation spectra for methyl-labeled STAT3 samples at 25 to 200 μM were obtained using the 2D 1H-13C HMQC experiment 13. Spectra were processed with Topspin (Bruker) and analyzed with Sparky (T. D. Goddard and D. G. Kneller, SPARKY 3, University of California, San Francisco). Isoleucines were unambiguously assigned by comparison of wt STAT3 or pSTAT3 spectra with Ile-to-Leu mutants in the absence or presence of pY904 peptide. Addition of peptide at 3-fold molar excess relative to STAT3 concentration ensured protein saturation as observed with both NMR and ITC titrations. Chemical shift perturbations (CSP) of assigned Ile Cδ1 methyls were calculated using the relation:
| [1] |
where, Δδ1H and Δδ13C were the constituent chemical shift differences.
13C single-quantum (SQ) Carr-Purcell-Meiboom-Gill (CPMG) relaxation dispersion experiments 23; 24 were recorded on ~ 170 μM wt pSTAT3 or uSTAT3 samples in the absence or presence of a saturating amount of pY904 peptide (~ 220 μM) at 35 °C on 600 MHz and 700 MHz instruments using [U-2H; IleCδ1-13CH3; Met-13CH3]-labeled protein samples. A series of 2D (13C, 1H) methyl spectra were collected on samples labeled with Ile Cδ1 and Met Cε1 methyls only and with varying CPMG frequencies (from 50 Hz to 1000 Hz) as defined by delays (2τ) between 13C refocusing pulses under a fixed total relaxation delay of 20 ms (Trelax). The effective decay rate (R2,eff) at each frequency (νCPMG) was extracted by the relation 35:
| [2] |
where, I(νCPMG) and I(0) are the peak intensities in the presence and absence of the CPMG spin-lock, respectively. The statistical errors in R2,eff were estimated by Monte Carlo simulations using duplicated spectra (50 Hz and 1000 Hz). The dispersion profiles were fit to the generalized expression of Carver-Richards for two exchanging states A and B 36 using the software GUARDD 37 to extract the adjustable site-specific exchange parameters: exchange rate (kex); populations (pA, pB); absolute 13C chemical shift change (|ΔδCPMG|); and the non-exchange transverse relaxation rate (R2,0). The Carver-Richards expression is:
| [3] |
where, τcp is the delay between consecutive 180° pulses in the CPMG spin-lock (2τ),
Δω = γCB0(|ΔδCPMG|), and γCB0 is the 13C Larmor frequency x 10−6.
The exchange contribution to R2,eff is defined by the term Rex, an indicator of the magnitude of the dispersion and thus dynamics. Rex was estimated from the dispersion fit by calculating the difference between R2,eff values at ~ 0 Hz and ~ 1,000 Hz.
For van’t Hoff analysis, a series of HMQC spectra were recorded for pSTAT3 on the 700 MHz spectrometer at 25, 30, 32, 34, 36, 38, 40 and 45 °C. Enthalpies (ΔH0) and entropies (ΔS0) were estimated from linear regression fits in plots of ln (Keq) vs. 1/T to the van’t Hoff relation:
| [4] |
where, Keq is the population ratio of exchanging pSTAT3 species (a) and (b) and T is the temperature.
Isothermal titration calorimetry (ITC)
The thermodynamic parameters for the binding of unphosphorylated wt and I568F STAT3 to the phosphotyrosine peptide pY904 was obtained from titration experiments using a Microcal VP-ITC calorimeter (GE Healthcare) at 25 °C. We used a similar protocol from a previous study 38 with the sample concentrations at 10 μM for STAT3 in the sample cell and ligand titrant at 150 μM pY904, respectively. Both the ligand and protein were dissolved in the MES sample buffer at pH 5.5. Following subtraction of heats of dilution obtained from the titration of ligand into buffer, the binding isotherms were fit to models for single site binding of pY904 to extract thermodynamic parameters using the software ORIGIN (Microcal). The fitted parameters include the stoichiometric ratio (N), the directly measured enthalpy (ΔH), and the association equilibrium constant (1/Kd). The entropy of binding (ΔS) was calculated by using the relation: ΔG = ΔH TΔS, where ΔG = −RT Ln (1/Kd).
Supplementary Material
Highlights.
Demonstrated the feasibility of studying STAT transcription factors by NMR spectroscopy
Discovered allosteric communications between STAT3 domains
Revealed the mechanism of an HIES mutation that is not located at direct binding sites
Acknowledgments
We thank the various core facilities at City of Hope for their services: DNA sequencing, Synthetic and Biopolymer Chemistry and Nuclear Magnetic Resonance. This work was supported by the National Institutes of Health grants K01CA168958 (A.N.), R01GM086717 (Y.C.) and R01GM102538 (Y.C.).
Abbreviations
- STAT
signal transducer and activator of transcription
- NMR
nuclear magnetic resonance
- HIES
hyper immunoglobulin E syndrome
- HMQC
heteronuclear multiple quantum coherence
- CPMG
Carr-Purcell-Meiboom-Gill
- ND
N-terminal domain
- CCD
coiled-coil domain
- DBD
DNA-binding domain
- LD
linker domain
- SH2
Src homology 2 domain
- TAD
transactivation domain
- CSP
chemical shift perturbation
- gp130
glycoprotein 130
- ITC
isothermal titration calorimetry
- pY705
phosphotyrosine peptide derived from STAT3
- pY904
phosphotyrosine peptide derived from gp130
- pTyr
phospho-tyrosine
- uSTAT3
unphosphorylated STAT3
- pSTAT3
Tyr-705-phosphorylated STAT3
- RT-PCR
reverse transcriptase polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monod J, Wyman J, Changeux JP. On the Nature of Allosteric Transitions: A Plausible Model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 2.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 3.Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–4. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–73. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Kim JH, Shin YK, Lee SI, Ahn KM. A novel mutation in the linker domain of the signal transducer and activator of transcription 3 gene, p.Lys531Glu, in hyper-IgE syndrome. J Allergy Clin Immunol. 2009;123:956–8. doi: 10.1016/j.jaci.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 6.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 7.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schäffer AA, Puck JM, Grimbacher B. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 8.Ren ZY, Mao X, Mertens C, Krishnaraj R, Qin J, Mandal PK, Romanowski MJ, McMurray JS, Chen XM. Crystal structure of unphosphorylated STAT3 core fragment. Biochemical and Biophysical Research Communications. 2008;374:1–5. doi: 10.1016/j.bbrc.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 9.Nkansah E, Shah R, Collie GW, Parkinson GN, Palmer J, Rahman KM, Bui TT, Drake AF, Husby J, Neidle S, Zinzalla G, Thurston DE, Wilderspin AF. Observation of unphosphorylated STAT3 core protein binding to target dsDNA by PEMSA and X-ray crystallography. Febs Letters. 2013;587:833–839. doi: 10.1016/j.febslet.2013.01.065. [DOI] [PubMed] [Google Scholar]
- 10.Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3 beta homodimer bound to DNA. Nature. 1998;394:145–151. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 11.Pervushin K, Riek R, Wider G, Wüthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci U S A. 1997;94:12366–71. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiaux J, Bertelsen EB, Horwich AL, Wuthrich K. NMR analysis of a 900K GroEL GroES complex. Nature. 2002;418:207–11. doi: 10.1038/nature00860. [DOI] [PubMed] [Google Scholar]
- 13.Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-correlated relaxation enhanced 1H[bond]13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc. 2003;125:10420–8. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- 14.Popovych N, Sun S, Ebright RH, Kalodimos CG. Dynamically driven protein allostery. Nat Struct Mol Biol. 2006;13:831–8. doi: 10.1038/nsmb1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprangers R, Kay LE. Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature. 2007;445:618–22. doi: 10.1038/nature05512. [DOI] [PubMed] [Google Scholar]
- 16.Namanja AT, Wang XJ, Xu B, Mercedes-Camacho AY, Wilson KA, Etzkorn FA, Peng JW. Stereospecific gating of functional motions in Pin1. Proc Natl Acad Sci U S A. 2011;108:12289–94. doi: 10.1073/pnas.1019382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CH, Namanja AT, Chen Y. Conformational flexibility and changes underlying activation of the SUMO-specific protease SENP1 by remote substrate binding. Nat Commun. 2014;5:4968. doi: 10.1038/ncomms5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE. A robust and cost-effective method for the production of Val, Leu, Ile (delta 1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR. 1999;13:369–74. doi: 10.1023/a:1008393201236. [DOI] [PubMed] [Google Scholar]
- 19.Hu W, Namanja AT, Wong S, Chen Y. Selective editing of Val and Leu methyl groups in high molecular weight protein NMR. J Biomol NMR. 2012;53:113–24. doi: 10.1007/s10858-012-9629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren Z, Cabell LA, Schaefer TS, McMurray JS. Identification of a high-affinity phosphopeptide inhibitor of Stat3. Bioorg Med Chem Lett. 2003;13:633–6. doi: 10.1016/s0960-894x(02)01050-8. [DOI] [PubMed] [Google Scholar]
- 21.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–55. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 22.Hansen DF, Neudecker P, Kay LE. Determination of isoleucine side-chain conformations in ground and excited states of proteins from chemical shifts. J Am Chem Soc. 2010;132:7589–91. doi: 10.1021/ja102090z. [DOI] [PubMed] [Google Scholar]
- 23.Lundstrom P, Vallurupalli P, Religa TL, Dahlquist FW, Kay LE. A single-quantum methyl 13C-relaxation dispersion experiment with improved sensitivity. J Biomol NMR. 2007;38:79–88. doi: 10.1007/s10858-007-9149-7. [DOI] [PubMed] [Google Scholar]
- 24.Namanja AT, Wang XDJ, Xu BL, Mercedes-Camacho AY, Wilson BD, Wilson KA, Etzkorn FA, Peng JW. Toward Flexibility-Activity Relationships by NMR Spectroscopy: Dynamics of Pin1 Ligands. Journal of the American Chemical Society. 2010;132:5607-+. doi: 10.1021/ja9096779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, Thumerelle C, Oksenhendler E, Boutboul D, Thomas C, Hoarau C, Lebranchu Y, Stephan JL, Cazorla C, Aladjidi N, Micheau M, Tron F, Baruchel A, Barlogis V, Palenzuela G, Mathey C, Dominique S, Body G, Munzer M, Fouyssac F, Jaussaud R, Bader-Meunier B, Mahlaoui N, Blanche S, Debré M, Le Bourgeois M, Gandemer V, Lambert N, Grandin V, Ndaga S, Jacques C, Harre C, Forveille M, Alyanakian MA, Durandy A, Bodemer C, Suarez F, Hermine O, Lortholary O, Casanova JL, Fischer A, Picard C. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore) 2012;91:e1–19. doi: 10.1097/MD.0b013e31825f95b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Kim JH, Shin YK, Lee SI, Ahn KM. A novel mutation in the linker domain of the signal transducer and activator of transcription 3 gene, p.Lys531Glu, in hyper-IgE syndrome. Journal of Allergy and Clinical Immunology. 2009;123:956–958. doi: 10.1016/j.jaci.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 27.Resetca D, Haftchenary S, Gunning PT, Wilson DJ. Changes in signal transducer and activator of transcription 3 (STAT3) dynamics induced by complexation with pharmacological inhibitors of Src homology 2 (SH2) domain dimerization. J Biol Chem. 2014;289:32538–47. doi: 10.1074/jbc.M114.595454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo J, Zhou HX. Dynamically Driven Protein Allostery Exhibits Disparate Responses for Fast and Slow Motions. Biophys J. 2015;108:2771–4. doi: 10.1016/j.bpj.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayala I, Sounier R, Use N, Gans P, Boisbouvier J. An efficient protocol for the complete incorporation of methyl-protonated alanine in perdeuterated protein. J Biomol NMR. 2009;43:111–9. doi: 10.1007/s10858-008-9294-7. [DOI] [PubMed] [Google Scholar]
- 30.Gelis I, Bonvin AM, Keramisanou D, Koukaki M, Gouridis G, Karamanou S, Economou A, Kalodimos CG. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell. 2007;131:756–69. doi: 10.1016/j.cell.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker S, Corthals GL, Aebersold R, Groner B, Muller CW. Expression of a tyrosine phosphorylated, DNA binding Stat3 beta dimer in bacteria. Febs Letters. 1998;441:141–147. doi: 10.1016/s0014-5793(98)01543-9. [DOI] [PubMed] [Google Scholar]
- 32.Buettner R, Corzano R, Rashid R, Lin J, Senthil M, Hedvat M, Schroeder A, Mao A, Herrmann A, Yim J, Li H, Yuan YC, Yakushijin K, Yakushijin F, Vaidehi N, Moore R, Gugiu G, Lee TD, Yip R, Chen Y, Jove R, Horne D, Williams JC. Alkylation of cysteine 468 in Stat3 defines a novel site for therapeutic development. ACS Chem Biol. 2011;6:432–43. doi: 10.1021/cb100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namanja AT, Li YJ, Su Y, Wong S, Lu JJ, Colson LT, Wu CG, Li SSC, Chen Y. Insights into High Affinity Small Ubiquitin-like Modifier (SUMO) Recognition by SUMO-interacting Motifs (SIMs) Revealed by a Combination of NMR and Peptide Array Analysis. Journal of Biological Chemistry. 2012;287:3231–3240. doi: 10.1074/jbc.M111.293118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–52. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- 35.Mulder FA, Mittermaier A, Hon B, Dahlquist FW, Kay LE. Studying excited states of proteins by NMR spectroscopy. Nat Struct Biol. 2001;8:932–5. doi: 10.1038/nsb1101-932. [DOI] [PubMed] [Google Scholar]
- 36.Carver JPaRRE. A general two-site solution for the chemical exchange produced dependence of T2 upon the Carr-Purcell pulse separation. J Magn Reson. 1972;6:89–105. [Google Scholar]
- 37.Kleckner IR, Foster MP. GUARDD: user-friendly MATLAB software for rigorous analysis of CPMG RD NMR data. J Biomol NMR. 2012;52:11–22. doi: 10.1007/s10858-011-9589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang XL, Yue PB, Page BDG, Li TS, Zhao W, Namanja AT, Paladino D, Zhao JH, Chen Y, Gunning PT, Turkson J. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9623–9628. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.