Abstract
Background
The liver is one of the most frequently injured abdominal organs. Hepatic hemorrhage is a complex and challenging complication following hepatic trauma. Significant shifts in the treatment of hepatic hemorrhage, including the increasing use of angioembolization, are believed to have improved patient outcomes. We aimed to describe the efficacy of angioembolization in the setting of acute hepatic arterial hemorrhage, as well as the complications associated with this treatment modality.
Methods
A systematic review of published literature (MEDLINE, SCOPUS, and Cochrane Library) describing hepatic angioembolization in the setting of trauma was performed. Articles that fulfilled the predetermined inclusion and exclusion criteria were included. We analyzed the efficacy rate of angioembolization in the setting of traumatic hepatic hemorrhage as well as the complications associated with hepatic angioembolization.
Results
Four hundred and fifty nine articles were identified in the literature search. Of these, 10 retrospective studies and 1 prospective study met inclusion and exclusion criteria. Efficacy rate of angioembolization was 93%. The most frequently reported complications following hepatic angioembolization included hepatic necrosis (15%), abscess formation (7.5%), and bile leaks.
Conclusion
Although the outcomes of hepatic angioembolization were generally favorable with a high success rate, the treatment modality is not without associated morbidity. The most frequently associated major complication was hepatic necrosis. Rates of complications were affected by study heterogeneity and should be better defined in future studies.
Keywords: angioembolization, liver, trauma
BACKGROUND
The management of hepatic trauma is a dynamic field with significant paradigm shifts over the past several decades. The liver’s size and location make it one of the most commonly injured organs in the abdomen. The vast majority of hepatic injuries are secondary to blunt trauma sustained during motor vehicle collisions1. The possibility of uncontrolled hemorrhage and a myriad of delayed complications contribute to a high morbidity and mortality rate associated with hepatic trauma. Historically, operative management was the treatment option of choice for patients with hepatic injuries. In the 1980s, rapidly improving imaging with computed tomography (CT) allowed for noninvasive assessment of trauma patients and their associated injuries. The arrival of transarterial angioembolization (AE) of acute hemorrhage in the early 1970s2, 3, and the advances in catheter and microcatheter design coupled with widespread interventional training has created a viable option for acute arterial hepatic hemorrhage. By the mid 1990’s endovascular techniques became an integral part of the care of trauma patients. At the same time, a push for nonoperative management of hepatic trauma patients began, in part fueled by the success of non-surgical treatment of pediatric patients and the high rate of non-therapeutic operations4, 5. These advances in non-surgical intervention, combined with the contemporary use of AE, are believed to have played a decisive role in decreasing overall morbidity and mortality6. Today, algorithms for the operative and non-operative management of adult blunt hepatic trauma consider interventional radiologists and their support staff as integral team members in the treatment of hepatic trauma 7, 8. Non-operative management for hepatic trauma is regarded as the standard of care in hemodynamically stable patients, regardless of the grade of the injury9 and the majority of hepatic injuries are now managed non-surgically. Such is true even for higher-grade injuries where the operation rate remains less than 40%1. Success rates of nonoperative management, as defined as no surgical intervention required, are generally greater than 90%10.
Although there is a large body of literature supporting the use of angioembolization in the setting of hepatic trauma, the expected efficacy and complication rates of this treatment are not well characterized and the majority of reports consist of small numbers (<100) of patients. There have been several reports questioning its efficacy when combined with additional operative measures11–13. Other investigators have raised concern over the seemingly high rate of liver necrosis following hepatic embolization14 as well as the possibility of gallbladder infarction following occlusion of the right hepatic artery15. Furthermore, the ideal timing of angioembolization in the setting of hepatic trauma remains unanswered.
We conducted a systematic review of the literature in order to define the value of AE as a resuscitative measure in patients with hepatic lacerations secondary to trauma. The primary objective of this study is to determine the efficacy of AE in the setting of hepatic hemorrhage secondary to trauma. A secondary objective is to establish reported complication rates following AE of the liver.
METHODS
Search strategy
The MEDLINE, SCOPUS, and Cochrane Library databases were electronically searched for published papers on the use of AE in trauma patients with hepatic injuries. The search was conducted using the following search terms and BOOLEAN operators: “hepatic” OR “liver” AND “trauma” AND “embolization.” Prior to the search, inclusion and exclusion criteria were defined. Manuscripts were considered eligible for inclusion if they met the following criteria: 1) The study population consisted of patients with traumatic causes (blunt or penetrating) of hepatic hemorrhage; 2) AE was considered as an intervention for the treatment of hepatic hemorrhage 3) At least one outcome of interest was described; 4) A liver injury grade range was provided for embolized patients. The principle outcome of interest was the efficacy rate of AE in obtaining control of arterial hepatic hemorrhage. Secondary outcomes of interest included mortality rate, liver related mortality rate, and frequency of both AE and non-AE specific complications. Exclusion criteria included: 1) case reports; 2) case series with fewer than 10 consecutive patients; 3) papers describing the treatment of only iatrogenic causes of hepatic hemorrhage or papers in which patients suffering from iatrogenic causes of hemorrhage could not be separated from those suffering traumatic causes of liver injury; 4) papers limited to pediatric patients only. Search results were limited to humans, English language, and papers published after 1990. Two reviewers [CS, SK] independently scrutinized the titles and abstracts of the papers retrieved. Most of the search results could be excluded based on the title and abstract alone. The full-length articles of the remaining papers were reviewed for eligibility criteria. The references of these papers were also searched for additional relevant papers. Any discrepancy between the two reviewers was resolved by review of a self-made quality assessment form. The quality assessment form included the following questions: 1) Is the embolization technique clearly described; 2) Was the description of the outcomes of interest complete; 3) Was there an adequate description of other clinical factors that may impact the primary and secondary outcomes, such as description of additional injuries in polytrauma patients or review of the patient population Injury Severity Score (ISS); 4) Were additional clinical factors detailed such as transfusion requirements; 5) Can missing data be reliably obtained; 6) Can liver injury grade be determined for each embolized patient. A protocol does not exist for this systematic review.
Data extraction and synthesis
Data extraction from the eligible articles was performed with a predefined template. The data extracted included year of publication, study time period, study type (prospective, retrospective), and both minor and major complications following embolization. Weighted means and ranges were calculated for variables of interest. Because of the heterogeneity of the data, meta-analysis was not performed.
RESULTS
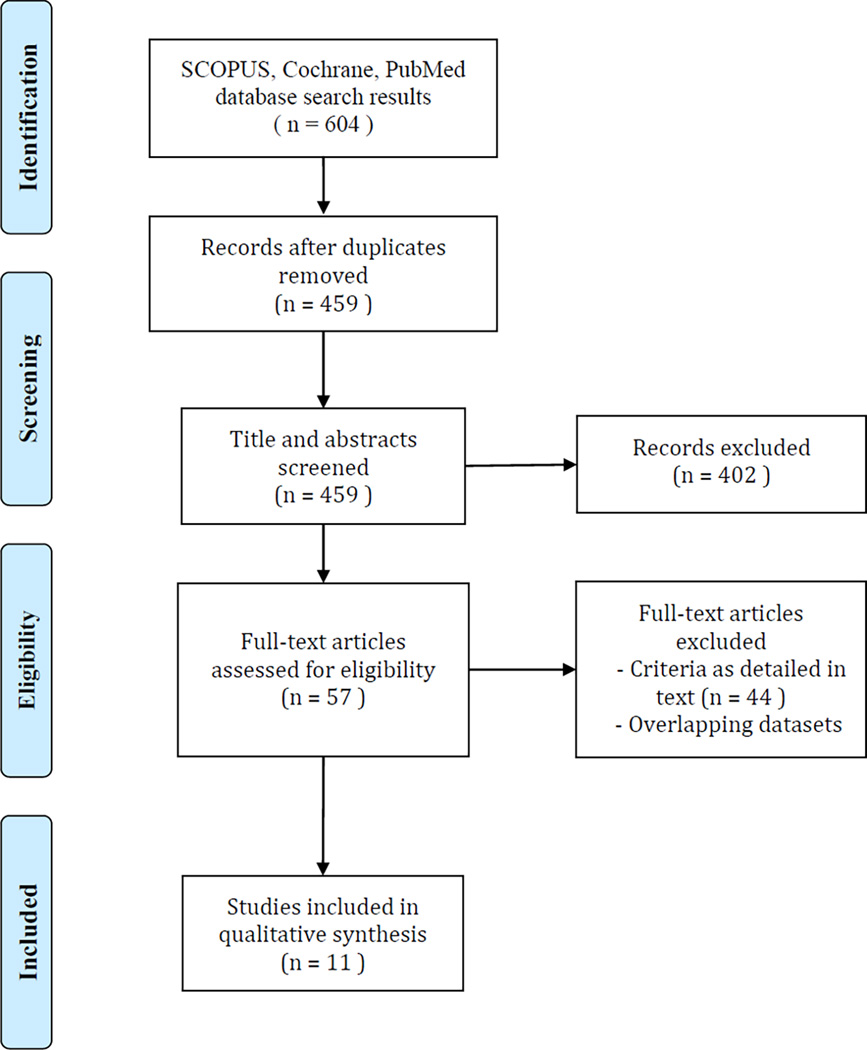
A total of 459 unique articles were identified in the search process. Of those, 402 articles were excluded through title and abstract filtering. No randomized controlled trials were identified. After review of the full-texts of the 57 remaining articles 46 were excluded, leaving a total of 11 articles in the study (Figure 1)15–25. A manual review of references did not identify any additional articles that met the inclusion criteria. All but one of the included articles was a retrospective case series25. The publication dates ranged from 2002–2014, with eight studies published in the last decade. The included studies are summarized in Table 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram showing the selection of articles for inclusion.
Table 1.
Characteristics of included studies, patient demographics, and embolic agents used.
| Article | Publication Year |
Study Type | Study period |
Number of patients with AE (non- iatrogenic) |
M/F ratio for AE patients |
Age range for AE patients (non- iatrogenic) |
AAST Average |
AAST Grade I |
AAST Grade II |
AAST Grade III |
AAST Grade IV |
AAST Grade IV |
Embolization agents used |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dabbs, D. N., et al |
2009 | Retrospective | 2002– 2007 |
71 | 2.4 | 34 ± 14 | 3.9 | 0 | 0 | 16 | 44 | 11 | Coils, Gelatin sponge |
| Hagiwara, A., et al |
2002 | Prospective | 1996– 2000 |
32 | nr | nr | 3.5 | 0 | 0 | 18 | 13 | 1 | Coils, Gelatin sponge, PVA particles |
| Kong, Y. L., et al |
2014 | Retrospective | 2002– 2011 |
70 | 2.9 | 36.3 (16–62) | 3.4 | 0 | 13 | 25 | 23 | 9 | Coils |
| Kozar, R. A., et al |
2005 | Retrospective | 2000– 2003 |
12 | nr | nr | 4.3 | 0 | 0 | 0 | 8 | 4 | nr |
| Lee, Y. H., et al |
2014 | Retrospective | 2009– 2012 |
48 | 1.6 | 31.5 (8–64) | 3.6 | 0 | 5 | 14 | 25 | 4 | Coils, Gelatin sponge |
| Li, M., et al |
2014 | Retrospective | 2007– 2012 |
24 | 3.2 | 35.9± 10.8 (17–69) |
3.9 | 0 | 1 | 5 | 13 | 5 | nr |
| Mohr, A. M., et al |
2003 | Retrospective | 1995– 2002 |
26 | nr | 33 (16–85) | 3.9 | 0 | 0 | 6 | 17 | 3 | Coils, Gelatin sponge, PVA particles |
| Monnin, V., et al |
2008 | Retrospective | 2000– 2005 |
14 | 6 | 37 (7–77) | 4.1 | 0 | 0 | 2 | 9 | 3 | Coils, Gelatin sponge, PVA particles |
| Saltzherr, T. P., et al |
2011 | Retrospective | 1995– 2008 |
23 | nr | nr | nr | nr | nr | nr | nr | nr | Coils, PVA particles |
| Tzeng, W. S., et al |
2005 | Retrospective | 1996– 2003 |
15 | 2.6 | nr | 3.9 | 1 | 0 | 3 | 6 | 5 | Coils, Tissue adhesive |
| Wahl, W. L., et al |
2002 | Retrospective | 1997– 2001 |
12 | nr | 47 | 3.7 | 0 | 0 | 5 | 6 | 1 | nr |
Nr, not reported; AAST, American Association for the Surgery of Trauma; AE, angioembolization; PVA, polyvinyl alcohol
Patient demographics
A total of 998 patients were included in the patient study populations. The study population age range was 3–84 years. The median ISS score for the study populations was 24 (range 16.9–36.9). Six studies did not record ISS scores15, 17, 19–21, 23. A total of 347 patients with hepatic hemorrhage were embolized from 1992 to 2012, accounting for 34.8% of the total study patients. The mean age ± SD of embolized patients per study was 31 ± 21.9 (range 12–71). Seven studies recorded the number of patient’s undergoing angiography15, 16, 19, 20, 23–25. Over two thirds of patients, 72%, undergoing angiography proceeded to embolization. A total of 10 articles reported individual liver injury grade scores for patients15–21, 23–25. Embolized patients had an average injury grade of score of 3.73 with range of (I-V). One study recorded only injury grade range for embolized patients22. Blunt trauma accounted for 92% of injuries, with motor vehicle collision as the most common cause.
Indications for embolization
A total of 6 studies reported the indications for embolization15–17, 20, 21, 25. A contrast blush on CT was the most common indication. The next most common indications included failure of nonoperative management and control of continued hemorrhage following damage control laparotomy.
Technique
Of the articles describing the embolization protocol, all reported use of microcatheter systems with selective and superselective embolization techniques. Gelatin sponge and microcoils were the most commonly used embolization materials.
Efficacy
The angioembolization success rate ranged from 77–100%. The weighted average efficacy rate was 93%. Two studies reported a failure to embolize three patients secondary to technical factors such as stenotic arteries or sharp branching limiting cannulation of the bleeding vessel24, 25. One patient’s neurological status declined prior to embolization attempts and the procedure was terminated24. Three studies including 51 patients reported on the impact of embolization timing with respect to transfusion requirements15, 21, 24. A total of 26 patients underwent immediate embolization following CT, while 25 were embolized following failure of conservative management, following damage control laparotomy, or for hemobilia. Among the early embolizations, an average of 5.8 units of PRBC were required in the first 24 hours. An average of 11.1 units of PRBCs were utilized in the late embolization group.
Mortality
Details regarding deaths among embolized patients were obtained from all but one study, and are summarized in Table 220. There were a total of 31 deaths accounting for a death rate of 9.6% among patients undergoing embolization (range 0–27%). There were 18 liver related deaths for a total liver related death rate among embolized patients of 5.6% (range 0–19.2%).
Table 2.
Outcomes of angioembolization.
| Article | Number of patients with AE (non- iatrogenic) |
Immediate rebleeding | Efficacy rate | Death | Liver related death |
|---|---|---|---|---|---|
| Dabbs, D. N., et al | 71 | 2 | 97.2% | 10 | 8 |
| Hagiwara, A., et al | 32 | 2 | 93.8% | 2 | 2 |
| Kong, Y. L., et al | 70 | 0 | 100.0% | 0 | 0 |
| Kozar, R. A., et al | 12 | 0 | 100.0% | 0 | 0 |
| Lee, Y. H., et al | 48 | 11 | 77.1% | 5 | 0 |
| Li, M., et al | 24 | 2 | 91.7% | nr | nr |
| Mohr, A. M., et al | 26 | 2 | 92.3% | 7 | 5 |
| Monnin, V., et al | 14 | 0 | 100.0% | 1 | 0 |
| Saltzherr, T. P., et al | 23 | 2 | 91.3% | 0 | 0 |
| Tzeng, W. S., et al | 15 | 2 | 86.7% | 0 | 0 |
| Wahl, W. L., et al | 12 | 1 | 91.7% | 6 | 3 |
nr, not reported; AE, angioembolization
Morbidity
The most commonly reported complication was hepatic necrosis (Table 3). There were a total of 48 cases of hepatic necrosis accounting for 14.9% of embolized patients (range 0–43%). A single study accounted for 30 cases (63%) of hepatic necrosis16. Details on abscess formation were obtained from 9 studies15–19, 21, 22, 24, 25. A total of 23 patients (7.5%) developed hepatic abscesses or infected hepatic collections post embolization. There were 17 cases of gallbladder infarction following embolization and 37 reported bile leaks/bilomas. There was only one reported groin hematoma following embolization15. Although complications were reported in the studies by Li et al.20 and Tzeng et al.23, these complications could not be definitively assigned specifically to patients who underwent angioembolization, and therefore these complications were not included in the calculations.
Table 3.
Complications following angioembolization.
| Article | Number of patients with AE (non-iatrogenic) |
Hepatic necrosis | Abscess | Gall bladder infarction |
Bile leak/Biloma |
|---|---|---|---|---|---|
| Dabbs, D. N., et al | 71 | 30 | 12 | 5 | 14 |
| Hagiwara, A., et al | 32 | None reported | None reported | None reported | None reported |
| Kong, Y. L., et al | 70 | 11 | None reported | 5 | 6 |
| Kozar, R. A., et al | 12 | None reported | None reported | None reported | 1 |
| Lee, Y. H., et al | 48 | None reported | None reported | None reported | None reported |
| Li, M., et al | 24 | None reported | Not reported for AE patients |
None reported | Not reported for AE patients |
| Mohr, A. M., et al | 26 | 4 | 2 | 4 | 7 |
| Monnin, V., et al | 14 | 1 | 2 | 2 | 6 |
| Saltzherr, T. P., et al | 23 | 2 | 5 | 1 | 2 |
| Tzeng, W. S., et al | 15 | 0 | Not reported for AE patients |
0 | 0 |
| Wahl, W. L., et al | 12 | 0 | 2 | 0 | 1 |
AE, angioembolization
DISCUSSION
The management of traumatic hepatic injuries has benefited from a significant paradigm shift over the past four decades. Advances in diagnosis, management, and treatment have lead to a multidisciplinary approach to the treatment of complex hepatic hemorrhage. Currently, there is substantial body of evidence in support of nonoperative management of hemodynamically stable patients with hepatic injuries5, 26–29. Success with nonoperative management of patients has lead to significant decreases in mortality rates6. As a result of the compelling improvements in patient outcomes, nonoperative management is the standard of care in hemodynamically stable patients with traumatic liver injuries. Angiography and angioembolization are essential components of successful nonoperative management of hepatic trauma patients, as well as a critical component of hemorrhage control following laparotomy9, 13, 30–34. Indications for conventional hepatic angiography include active extravasation identified by computed tomography, evidence of ongoing bleeding despite conservative resuscitative measures, hemobilia, and high-grade liver injuries.
The demographics of this study’s patient population are similar to those of multiple published large retrospective reviews, with a mean patient age in the early 30s and a significant male predominance. Like other studies, blunt hepatic injury was more common than penetrating, with motor vehicle collisions as the most common cause of hepatic injury35. Although only three studies recorded the ISS score for embolized patients, the ISS range was consistent with major traumatic and multisystem injuries15, 16, 24.
Hepatic transarterial embolization was 93% effective in stopping arterial hemorrhage. Lee et al. reported 11 cases of incomplete embolization24. Ten of these cases were secondary to a persistent contrast blush without an identifiable vessel or a blush supplied by multiple collaterals that could not be embolized. There was one reported failure secondary to a stenotic celiac artery. Both Lee et al. and Hagiwara et al.25 reported failures of nonoperative management despite technically successful embolization. Many of the patients who failed conservative management despite successful embolization were found to have significant juxatahepatic venous injuries. These types of injuries can be difficult to identify during angiography, however they should be suspected in patients with high-grade liver lacerations who require ongoing fluid resuscitation despite successful embolization. Cross sectional imaging can aid in detection of retrohepatic caval and juxtahepatic venous injuries and ongoing venous hemorrhage may require operative packing. Failure to identify these types of injuries is an important explanation for the failure of nonoperative treatment. Despite successful embolization, delayed hemorrhage can still occur and has been documented in 5–12% of patients36–38. More recent advent of hybrid operating suites may allow for near simultaneous treatment of arterial hemorrhage with angioembolization and juxthepatic venous injuries with laparotomy.
Several articles have suggested that early angiography and embolization improve outcomes in patients with high-grade hepatic injuries12, 13, 31, 32, 39–41. Similar improved outcomes with earlier embolization have also been documented with both traumatic pelvic and splenic injuries 42–46. Only three articles in this study sufficiently separated outcomes for early versus late embolization patients15, 21, 24. In each study, there was a trend towards reduced transfusion requirements for those patients undergoing early AE. However, higher transfusion requirements in the late AE could be confounded by greater severity of injury in this group, as these patients could have been more likely to require damage control laparotomy. Given the small and heterogeneous patient samples, no definitive conclusions could be drawn about mortality and morbidity rates.
One of the principle advantages of AE is that is generally well tolerated, even among critically ill patients. In this study, the average liver injury grade of patients undergoing embolization was 3.73, which is consistent with a major traumatic event. Not surprisingly, high-grade hepatic injuries are frequently associated with polytrauma and elevated injury severity scores, complicating a patient’s hospital course. Despite impressively high injury grades and ISS ranges among the study populations, the overall mortality rate for embolized patients remained just below 10%, and the liver related mortality rate was less than 6%. There were no reported procedure related mortalities. The overall mortality rate is within the range of previously published data evaluating patients with high-grade liver injuries and below that of the National Trauma Data Bank, despite an overall higher weighted average organ injury score1.
Complications are common following significant hepatic injuries. Not surprisingly, the number of complications increases with a higher degree of liver injury18, 37, 47. One of the major criticisms of angioembolization in the setting of hepatic trauma is the apparent high morbidity rate. A major concern is hepatic necrosis following embolization, as it can be associated with longer hospital stays, increased transfusion requirements, and the need for multiple operations in what was otherwise a planned nonoperative treatment course. Hepatic necrosis occurs following the death of a large number of contiguous hepatocytes. In the setting of trauma, hepatic necrosis is caused by major devascularization of a portion of the liver through a traumatic insult, therapeutic embolization, or a combination of the two. The liver’s dual arterial and portal venous blood supply confers protection against ischemic insults. However, despite this robust dual supply, the combined insult of trauma and embolization has been shown to cause significant hepatic necrosis. The included studies report a hepatic necrosis rate that ranged from 0–42%, with a weighted mean rate of 15%. However, nearly 2/3 of cases of hepatic necrosis were documented in a single study by Dabbs et al., which had a notably high rate of necrosis compared with the other studies (42% vs. 0–16%)16. The degree of arterial selectivity during embolization in this study was not clear but it is generally thought that reduced necrosis rates may be achieved by use of microcatheter systems and superselective embolization. Additionally, the high rate of necrosis may be secondary to the higher injury grade and ISS scores for the patients in that study. This is turn may further exacerbate injury to the liver because of higher rates of damage control laparotomy. It is notable that in the study by Dabbs et al., nearly 97% of patients with major hepatic necrosis underwent operative management including perihepatic packing. If this study is excluded as an outlier, the mean hepatic necrosis rate falls to 6.2%.
Similar to prior studies, abscess formation and bile leak/biloma were the next two most common complications18, 48. These complications are not AE specific and have been documented following both operative and nonoperative management of liver trauma49–51. These complications can often be managed through minimally invasive techniques such as percutaneous drainage with a nominal impact on the patient’s hospital course. Identification of biliary injuries is important, as bile leaks may be an important contributor to delayed bleeding. Gallbladder infarction is an important complication that is generally identified following non-target embolization of the cystic artery during embolization of the right hepatic artery.
This current study is limited by the quality of the available published studies. Most of the included articles were retrospective without comparative groups. There is currently no standardization for patient selection or reporting, resulting in heterogeneity in the data. If incomplete embolization was described, it was considered an AE failure. If the details of rebleeding following AE were not sufficiently described, it was considered embolization failure. If a complication was not reported, then a complication was assumed to not occur; this assumption could have impacted our results. Lee et al. reported no AE-related complications, but did not describe non-AE specific complications such as abscess formation or bile leak19. It seems unlikely that none of these complications occurred in the third largest study population. Similarly the available published studies could be affected by publication bias, although this could have had either positive or negative impacts on AE outcomes. When not specifically stated, organ injury scoring was assumed reported using the AAST classification. One study reported organ injury grade using the Mirvis scoring system52. The numerical values from this study were included in the average orange injury grade.
To date, there are no consensus guidelines on appropriate patient selection criteria for those who would benefit from angiography and angioembolization. For patients who are hemodynamically stable, contrast-enhanced computed tomography (CT) has been shown to identify those at risk for impending failure of non-operative management, with high risk seen in those with intraperitoneal contrast extravasation in the peritoneum, hemoperitoneum involving multiple abdominal compartments, or contrast extravasation into ruptured liver parenchyma53–55. However, low-grade hepatic injuries with contained, intraparenchymal contrast pooling may benefit from observation alone55. After laparotomy, persistent transfusion requirements usually suggest need for angiography and embolization. In this setting additional imaging can be helpful, as early post-operative CT has been found to determine which patients would require post-laparotomy angioembolization with high sensitivity and specificity56.
In summary, the present review demonstrates that hepatic angioembolization is an effective and important component in the management of traumatic hepatic hemorrhage. However, serious complications such as hepatic necrosis can occur following embolization and the rates of these complications should be better defined in future studies. The poor quality of currently available studies limits establishment of additional clinically relevant conclusions. Questions remain regarding patient selection and the ideal timing of embolization.
Acknowledgments
Dr. Kwan receives salary support from the National Institutes of Health, National Center for Advancing Translational Sciences, grant KL2 TR000421.
Footnotes
None of the authors report a conflict of interest.
This paper has not been presented at a meeting to date.
Author contribution statement:
Christopher S. Green, M.D., M.B.A.: Study design, literature search, data collection, data analysis, data interpretation, writing, critical revision
Eileen M. Bulger, M.D.: Data interpretation, writing, and critical revision
Sharon W. Kwan, M.D.: Study design, literature search, data collection, data analysis, data interpretation, writing, and critical revision
Contributor Information
Christopher S. Green, Department of Radiology, University of California, Irvine School of Medicine, 101 The City Drive South, Building 56, Suite 300, Orange, CA 92868, greenc@uci.edu.
Eileen M. Bulger, Division of Trauma and Critical Care, Department of Surgery, University of Washington Medical Center, 1959 NE Pacific Street, Seattle, Washington 98195, ebulger@uw.edu.
Sharon W. Kwan, Interventional Radiology Section, Department of Radiology, University of Washington Medical Center, 1959 NE Pacific Street, Seattle, Washington 98195 and Comparative Effectiveness, Cost, and Outcomes Research Center, University of Washington, Campus Box 359455, Seattle, Washington 98195, shakwan@uw.edu, Fax: (206) 598-6406, Telephone number: (206) 598-1454.
REFERENCES
- 1.Tinkoff G, Esposito TJ, Reed J, Kilgo P, Fildes J, Pasquale M, Meredith JW. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. Journal of the American College of Surgeons. 2008;207(5):646–655. doi: 10.1016/j.jamcollsurg.2008.06.342. [DOI] [PubMed] [Google Scholar]
- 2.Bookstein JJ, Goldstein HM. Successful management of postbiopsy arteriovenous fistula with selective arterial embolization. Radiology. 1973;109(3):535–536. doi: 10.1148/109.3.535. [DOI] [PubMed] [Google Scholar]
- 3.Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102(2):303–306. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- 4.Oldham KT, Guice KS, Ryckman F, Kaufman RA, Martin LW, Noseworthy J. Blunt liver injury in childhood: evolution of therapy and current perspective. Surgery. 1986;100(3):542–549. [PubMed] [Google Scholar]
- 5.Carrillo EH, Platz A, Miller FB, Richardson JD, Polk HC., Jr Non-operative management of blunt hepatic trauma. The British journal of surgery. 1998;85(4):461–468. doi: 10.1046/j.1365-2168.1998.00721.x. [DOI] [PubMed] [Google Scholar]
- 6.Richardson JD, Franklin GA, Lukan JK, Carrillo EH, Spain DA, Miller FB, Wilson MA, Polk HC, Jr, Flint LM. Evolution in the management of hepatic trauma: A 25-year perspective. Annals of surgery. 2000;232(3):324–330. doi: 10.1097/00000658-200009000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozar RA, Feliciano DV, Moore EE, Moore FA, Cocanour CS, West MA, Davis JW, McIntyre RC., Jr Western Trauma Association/critical decisions in trauma: operative management of adult blunt hepatic trauma. The Journal of trauma. 2011;71(1):1–5. doi: 10.1097/TA.0b013e318220b192. [DOI] [PubMed] [Google Scholar]
- 8.Kozar RA, Moore FA, Moore EE, West M, Cocanour CS, Davis J, Biffl WL, McIntyre RC., Jr Western trauma association critical decisions in trauma: Nonoperative management of adult blunt hepatic trauma. Journal of Trauma - Injury, Infection and Critical Care. 2009;67(6):1144–1148. doi: 10.1097/TA.0b013e3181ba361f. [DOI] [PubMed] [Google Scholar]
- 9.Stassen NA, Bhullar I, Cheng JD, Crandall M, Friese R, Guillamondegui O, Jawa R, Maung A, Rohs TJ, Sangosanya A, et al. Nonoperative management of blunt hepatic injury: An eastern association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5) SUPPL.4:S288–S293. doi: 10.1097/TA.0b013e318270160d. [DOI] [PubMed] [Google Scholar]
- 10.Hurtuk M, Reed RL, 2nd, Esposito TJ, Davis KA, Luchette FA. Trauma surgeons practice what they preach: The NTDB story on solid organ injury management. The Journal of trauma. 2006;61(2):243–254. doi: 10.1097/01.ta.0000231353.06095.8d. discussion 54–5. [DOI] [PubMed] [Google Scholar]
- 11.Duane TM, Como JJ, Bochicchio GV, Scalea TM. Reevaluating the management and outcomes of severe blunt liver injury. The Journal of trauma. 2004;57(3):494–500. doi: 10.1097/01.ta.0000141026.20937.81. [DOI] [PubMed] [Google Scholar]
- 12.Asensio JA, Demetriades D, Chahwan S, Gomez H, Hanpeter D, Velmahos G, Murray J, Shoemaker W, Berne TV. Approach to the management of complex hepatic injuries. The Journal of trauma. 2000;48(1):66–69. doi: 10.1097/00005373-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Asensio JA, Roldan G, Petrone P, Rojo E, Tillou A, Kuncir E, Demetriades D, Velmahos G, Murray J, Shoemaker WC, et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. The Journal of trauma. 2003;54(4):647–653. doi: 10.1097/01.TA.0000054647.59217.BB. discussion 53–4. [DOI] [PubMed] [Google Scholar]
- 14.Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. The Journal of trauma. 2009;66(3):621–627. doi: 10.1097/TA.0b013e31819919f2. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 15.Mohr AM, Lavery RF, Barone A, Bahramipour P, Magnotti LJ, Osband AJ, Sifri Z, Livingston DH. Angiographic embolization for liver injuries: low mortality, high morbidity. The Journal of trauma. 2003;55(6):1077–1081. doi: 10.1097/01.TA.0000100219.02085.AB. discussion 81–2. [DOI] [PubMed] [Google Scholar]
- 16.Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high-grade liver injuries. The Journal of trauma. 2009;66(3):621–627. doi: 10.1097/TA.0b013e31819919f2. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 17.Kong YL, Zhang HY, He XJ, Zhao G, Liu CL, Xiao M, Zhen YY. Angiographic embolization in the treatment of intrahepatic arterial bleeding in patients with blunt abdominal trauma. Hepatobiliary Pancreatic Dis Int. 2014;13(2):173–178. doi: 10.1016/s1499-3872(14)60027-8. [DOI] [PubMed] [Google Scholar]
- 18.Kozar RA, Moore JB, Niles SE, Holcomb JB, Moore EE, Cothren CC, Hartwell E, Moore FA. Complications of nonoperative management of high-grade blunt hepatic injuries. The Journal of trauma. 2005;59(5):1066–1071. doi: 10.1097/01.ta.0000188937.75879.ab. [DOI] [PubMed] [Google Scholar]
- 19.Lee YH, Wu CH, Wang LJ, Wong YC, Chen HW, Wang CJ, Lin BC, Hsu YP. Predictive factors for early failure of transarterial embolization in blunt hepatic injury patients. Clin Radiol. 2014;69(12):e505–e511. doi: 10.1016/j.crad.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Yu WK, Wang XB, Ji W, Li JS, Li N. Non-operative management of isolated liver trauma. Hepatobiliary & pancreatic diseases international : HBPD INT. 2014;13(5):545–550. doi: 10.1016/s1499-3872(14)60049-7. [DOI] [PubMed] [Google Scholar]
- 21.Monnin V, Sengel C, Thony F, Bricault I, Voirin D, Letoublon C, Broux C, Ferretti G. Place of arterial embolization in severe blunt hepatic trauma: A multidisciplinary approach. Cardiovascular and interventional radiology. 2008;31(5):875–882. doi: 10.1007/s00270-007-9277-1. [DOI] [PubMed] [Google Scholar]
- 22.Saltzherr TP, Van Der Vlies CH, Van Lienden KP, Beenen LFM, Ponsen KJ, Van Gulik TM, Goslings JC. Improved outcomes in the non-operative management of liver injuries. HPB. 2011;13(5):350–355. doi: 10.1111/j.1477-2574.2011.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzeng WS, Wu RH, Chang JM, Lin CY, Koay LB, Uen YH, Tian YF, Fong Y. Transcatheter arterial embolization for hemorrhage caused by injury of the hepatic artery. J Gastroenterol Hepatol. 2005;20(7):1062–1068. doi: 10.1111/j.1440-1746.2005.03768.x. [DOI] [PubMed] [Google Scholar]
- 24.Wahl WL, Ahrns KS, Brandt MM, Franklin GA, Taheri PA. The need for early angiographic embolization in blunt liver injuries. The Journal of trauma. 2002;52(6):1097–1101. doi: 10.1097/00005373-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara A, Murata A, Matsuda T, Matsuda H, Shimazaki S. The efficacy and limitations of transarterial embolization for severe hepatic injury. The Journal of trauma. 2002;52(6):1091–1096. doi: 10.1097/00005373-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Croce MA, Fabian TC, Menke PG, Waddle-Smith L, Minard G, Kudsk KA, Patton JH, Jr, Schurr MJ, Pritchard FE. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg. 1995;221(6):744–753. doi: 10.1097/00000658-199506000-00013. discussion 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knudson MM, Lim RC, Jr, Oakes DD, Jeffrey RB., Jr Nonoperative management of blunt liver injuries in adults: the need for continued surveillance. The Journal of trauma. 1990;30(12):1494–1500. doi: 10.1097/00005373-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Pachter HL, Knudson MM, Esrig B, Ross S, Hoyt D, Cogbill T, Sherman H, Scalea T, Harrison P, Shackford S, et al. Status of nonoperative management of blunt hepatic injuries in 1995: a multicenter experience with 404 patients. The Journal of trauma. 1996;40(1):31–38. doi: 10.1097/00005373-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Raza M, Abbas Y, Devi V, Prasad KV, Rizk KN, Nair PP. Non operative management of abdominal trauma - a 10 years review. World journal of emergency surgery : WJES. 2013;8:14. doi: 10.1186/1749-7922-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaarder C, Naess PA, Eken T, Skaga NO, Pillgram-Larsen J, Klow NE, Buanes T. Liver injuries-Improved results with a formal protocol including angiography. Injury. 2007;38(9):1075–1083. doi: 10.1016/j.injury.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Carrillo EH, Spain DA, Wohltmann CD, Schmieg RE, Boaz PW, Miller FB, Richardson JD. Interventional techniques are useful adjuncts in nonoperative management of hepatic injuries. The Journal of trauma. 1999;46(4):619–622. doi: 10.1097/00005373-199904000-00010. discussion 22–4. [DOI] [PubMed] [Google Scholar]
- 32.Ciraulo DL, Luk S, Palter M, Cowell V, Welch J, Cortes V, Orlando R, Banever T, Jacobs L. Selective hepatic arterial embolization of grade IV and V blunt hepatic injuries: an extension of resuscitation in the nonoperative management of traumatic hepatic injuries. The Journal of trauma. 1998;45(2):353–358. doi: 10.1097/00005373-199808000-00025. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 33.Clemente N, Di Saverio S, Giorgini E, Biscardi A, Villani S, Senatore G, Filicori F, Antonacci N, Baldoni F, Tugnoli G. Management and outcome of 308 cases of liver trauma in Bologna Trauma Center in 10 years. Annali italiani di chirurgia. 2011;82(5):351–360. [PubMed] [Google Scholar]
- 34.Misselbeck TS, Teicher EJ, Cipolle MD, Pasquale MD, Shah KT, Dangleben DA, Badellino MM. Hepatic angioembolization in trauma patients: indications and complications. The Journal of trauma. 2009;67(4):769–773. doi: 10.1097/TA.0b013e3181b5ce7f. [DOI] [PubMed] [Google Scholar]
- 35.Scollay JM, Beard D, Smith R, McKeown D, Garden OJ, Parks R. Eleven years of liver trauma: the Scottish experience. World journal of surgery. 2005;29(6):744–749. doi: 10.1007/s00268-005-7752-x. [DOI] [PubMed] [Google Scholar]
- 36.van der Wilden GM, Velmahos GC, Emhoff T, Brancato S, Adams C, Georgakis G, Jacobs L, Gross R, Agarwal S, Burke P, et al. Successful nonoperative management of the most severe blunt liver injuries: a multicenter study of the research consortium of new England centers for trauma. Arch Surg. 2012;147(5):423–428. doi: 10.1001/archsurg.2012.147. [DOI] [PubMed] [Google Scholar]
- 37.Kozar RA, Moore FA, Cothren CC, Moore EE, Sena M, Bulger EM, Miller CC, Eastridge B, Acheson E, Brundage SI, et al. Risk factors for hepatic morbidity following nonoperative management: Multicenter study. Archives of Surgery. 2006;141(5):451–459. doi: 10.1001/archsurg.141.5.451. [DOI] [PubMed] [Google Scholar]
- 38.Huang YC, Wu SC, Fu CY, Chen YF, Chen RJ, Hsieh CH, Wang YC, Huang HC, Huang JC, Lu CW. Tomographic findings are not always predictive of failed nonoperative management in blunt hepatic injury. Am J Surg. 2012;203(4):448–453. doi: 10.1016/j.amjsurg.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 39.Denton JR, Moore EE, Coldwell DM. Multimodality treatment for grade V hepatic injuries: Perihepatic packing, arterial embolization, and venous stenting. J TRAUMA INJ INFECT CRIT CARE. 1997;42(5):964–968. doi: 10.1097/00005373-199705000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Carrillo EH, Richardson JD. Delayed surgery and interventional procedures in complex liver injuries. The Journal of trauma. 1999;46(5):978. doi: 10.1097/00005373-199905000-00041. [DOI] [PubMed] [Google Scholar]
- 41.Johnson JW, Gracias VH, Gupta R, Guillamondegui O, Reilly PM, Shapiro MB, Kauder DR, Schwab CW. Hepatic angiography in patients undergoing damage control laparotomy. The Journal of trauma. 2002;52(6):1102–1106. doi: 10.1097/00005373-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Dent D, Alsabrook G, Erickson BA, Myers J, Wholey M, Stewart R, Root H, Ferral H, Postoak D, Napier D, et al. Blunt splenic injuries: high nonoperative management rate can be achieved with selective embolization. The Journal of trauma. 2004;56(5):1063–1067. doi: 10.1097/01.ta.0000123037.66867.f2. [DOI] [PubMed] [Google Scholar]
- 43.Brugere C, Arvieux C, Dubuisson V, Guillon F, Sengel C, Bricault I, Regimbeau JM, Pilleul F, Menegaux F, Letoublon C. Early embolization in the non-operative management of blunt splenic injuries: a retrospective multicenter study. Journal de chirurgie. 2008;145(2):126–132. doi: 10.1016/s0021-7697(08)73721-9. [DOI] [PubMed] [Google Scholar]
- 44.Panetta T, Sclafani SJ, Goldstein AS, Phillips TF, Shaftan GW. Percutaneous transcatheter embolization for massive bleeding from pelvic fractures. The Journal of trauma. 1985;25(11):1021–1029. [PubMed] [Google Scholar]
- 45.Tanizaki S, Maeda S, Matano H, Sera M, Nagai H, Ishida H. Time to pelvic embolization for hemodynamically unstable pelvic fractures may affect the survival for delays up to 60 min. Injury. 2014;45(4):738–741. doi: 10.1016/j.injury.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Tanizaki S, Maeda S, Hayashi H, Matano H, Ishida H, Yoshikawa J, Yamamoto T. Early embolization without external fixation in pelvic trauma. The American journal of emergency medicine. 2012;30(2):342–346. doi: 10.1016/j.ajem.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Zafar SN, Rushing A, Haut ER, Kisat MT, Villegas CV, Chi A, Stevens K, Efron DT, Zafar H, Haider AH. Outcome of selective non-operative management of penetrating abdominal injuries from the North American National Trauma Database. The British journal of surgery. 2012;99(Suppl 1):155–164. doi: 10.1002/bjs.7735. [DOI] [PubMed] [Google Scholar]
- 48.Bala M, Gazalla SA, Faroja M, Bloom AI, Zamir G, Rivkind AI, Almogy G. Complications of high grade liver injuries: management and outcomewith focus on bile leaks. Scandinavian journal of trauma, resuscitation and emergency medicine. 2012;20:20. doi: 10.1186/1757-7241-20-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh CH, Chen RJ, Fang JF, Lin BC, Hsu YP, Kao JL, Kao YC, Yu PC, Kang SC, Wang YC. Liver abscess after non-operative management of blunt liver injury. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2003;387(9–10):343–347. doi: 10.1007/s00423-002-0337-3. [DOI] [PubMed] [Google Scholar]
- 50.Krige JE, Bornman PC, Terblanche J. Therapeutic perihepatic packing in complex liver trauma. The British journal of surgery. 1992;79(1):43–46. doi: 10.1002/bjs.1800790114. [DOI] [PubMed] [Google Scholar]
- 51.Carmona RH, Peck DZ, Lim RC., Jr The role of packing and planned reoperation in severe hepatic trauma. The Journal of trauma. 1984;24(9):779–784. doi: 10.1097/00005373-198409000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Mirvis SE, Whitley NO, Vainwright JR, Gens DR. Blunt hepatic trauma in adults: CT-based classification and correlation with prognosis and treatment. Radiology. 1989;171(1):27–32. doi: 10.1148/radiology.171.1.2928537. [DOI] [PubMed] [Google Scholar]
- 53.Fang JF, Chen RJ, Wong YC, Lin BC, Hsu YB, Kao JL, Kao YC. Pooling of contrast material on computed tomography mandates aggressive management of blunt hepatic injury. Am J Surg. 1998;176(4):315–319. doi: 10.1016/s0002-9610(98)00196-2. [DOI] [PubMed] [Google Scholar]
- 54.Fang JF, Wong YC, Lin BC, Hsu YP, Chen MF. The CT risk factors for the need of operative treatment in initially hemodynamically stable patients after blunt hepatic trauma. The Journal of trauma. 2006;61(3):547–553. doi: 10.1097/01.ta.0000196571.12389.ee. discussion 53–4. [DOI] [PubMed] [Google Scholar]
- 55.Fang JF, Chen RJ, Wong YC, Lin BC, Hsu YB, Kao JL, Chen MF. Classification and treatment of pooling of contrast material on computed tomographic scan of blunt hepatic trauma. The Journal of trauma. 2000;49(6):1083–1088. doi: 10.1097/00005373-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 56.Kutcher ME, Weis JJ, Siada SS, Kaups KL, Kozar RA, Wawrose RA, Summers JI, Eriksson EA, Leon SM, Carrick MM, et al. The role of computed tomographic scan in ongoing triage of operative hepatic trauma: A Western Trauma Association multicenter retrospective study. J Trauma Acute Care Surg. doi: 10.1097/TA.0000000000000692. Epub 2015 Sep 2. [DOI] [PubMed] [Google Scholar]