Abstract
A low complexity diagnostic test that rapidly and reliably detects HIV infection in infants at the point of care could facilitate early treatment, improving outcomes. However, many infant HIV diagnostics can only be performed in laboratory settings. Recombinase polymerase amplification (RPA) is an isothermal amplification technology that can rapidly amplify proviral DNA from multiple subtypes of HIV-1 in under twenty minutes without complex equipment. In this study we added reverse transcription (RT) to RPA to allow detection of both HIV-1 RNA and DNA. We show that this RT-RPA HIV-1 assay has a limit of detection of 10 to 30 copies of an exact sequence matched DNA or RNA, respectively. In addition, at 100 copies of RNA or DNA, the assay detected 171 of 175 (97.7 %) sequence variants that represent all the major subtypes and recombinant forms of HIV-1 Groups M and O. This data suggests that the application of RT-RPA for the combined detection of HIV-1 viral RNA and proviral DNA may prove a highly sensitive tool for rapid and accurate diagnosis of infant HIV.
Keywords: Human immunodeficiency virus, Diagnostic, Infant HIV, Reverse transcription recombinase polymerase amplification, Point of care
1. Introduction
Without antiretroviral treatment (ART), it is estimated that over 50% of HIV-infected children die before age two, therefore early diagnosis and immediate treatment of HIV-1 in young infants remains a public health priority (Obimbo et al., 2009;Newell et al., 2004). While HIV can effectively be diagnosed via serologic testing in adults and children older than 18 months (World Health Organization, 2010), the presence of transplacentally acquired maternal antibodies that target HIV-1 make serologic testing unreliable in young infants (Ciaranello et al., 2011). Consequently, diagnosis of HIV-1 infection in young infants currently relies on immunoassays that detect the viral capsid antigen p24 via immunoassays (Palomba et al., 1992;Patton et al., 2008), or most commonly, the amplification and detection of HIV-1 DNA using PCR-based assays with a whole blood specimen or a dried blood spot (Panteleeff et al., 1999;Paterlini et al., 1990). However, these assays are not adequate for use at the point of care and require sophisticated laboratory facilities as well as significant specimen and results tracking logistics (Essajee et al., 2015). Improving outcomes for HIV-infected infants requires a highly sensitive and specific diagnostic that is simple to use, robust, does not require complex laboratory equipment and can be reliably performed at the point of care.
In hospital or clinic-based settings where laboratory equipment is available, PCR-based assays are typically used for HIV diagnosis in infants. Most rely solely on detecting HIV-1 DNA, however, there is evidence that use of RT-PCR to detect HIV-1 RNA improves sensitivity (Lambert et al., 2003;Young et al., 2000;Obaro et al., 2005;Karchava et al., 2006;Swanson et al., 2005). A number of studies have measured HIV-1 RNA levels in infants from birth up to 18 months of age (Obimbo et al., 2009;Ciaranello et al., 2011;Mutasa et al., 2012;Lambert et al., 2003;Young et al., 2000) and suggest that it can take as little as two weeks following infection for HIV-1 RNA to be detected, with an exponential increase thereafter (Butto et al., 2010). The median viral load has been reported from 4.1 × 105 copies/mL at birth (Young et al., 2000); 1.59 – 3.7 × 106 copies/mL at 6 – 8 weeks (Mutasa et al., 2012;Young et al., 2000;Richardson et al., 2003); 0.6 – 1.6 × 106 copies/mL at 6 months (Ciaranello et al., 2011;Young et al., 2000) and 6.0 × 105 copies/mL at 18 months (Ciaranello et al., 2011). Elevated levels of HIV-1 viremia in infants may be due to their increased number of CD4+ T cells, the primary target cells for HIV-1 replication, as compared to adults or immune systems with slower and weaker responses to viral infection (Richardson et al., 2003). We propose that a highly sensitive and specific assay that detects both HIV-1 RNA and DNA in whole blood will improve sensitivity compared to a diagnostic that detects only DNA.
A reoccurring theme with PCR-based diagnostics for early infant diagnosis (EID) of HIV infection is that they require skilled staff and a dedicated laboratory with complex equipment and reagents (Kiyaga et al., 2013), which are predominately available in urban or resource-rich settings. HIV testing of infants in low resource settings often requires specimen transport to central laboratories for testing. This typically results in large delays in reporting of test results to caregivers, delaying treatment initiation. While some logistical challenges can be addressed to improve turnaround time for infant HIV diagnosis (Finocchario-Kessler et al., 2014;Ghadrshenas et al., 2013;Kiyaga et al., 2013), an alternative solution is to simplify the diagnostics to enable testing at the point of care.
Our group, as well as a number of others, have shown that recombinase polymerase amplification (RPA) can be used for sensitive and specific detection of pathogen DNA or RNA in twenty minutes or less (Boyle et al., 2014;Boyle et al., 2013;Piepenburg et al., 2006;Rohrman and Richards-Kortum, 2012;Euler et al., 2012). RPA is an isothermal amplification method that utilizes a recombinase and a single stranded DNA binding protein to facilitate the insertion and stabilization of oligonucleotide primers into their complement in a double-stranded DNA molecule for subsequent amplification using a strand displacing DNA polymerase (Piepenburg et al., 2006). The use of opposing primers facilitates the exponential amplification of a defined region of DNA in a manner similar to PCR. RPA probes allow detection of amplification in real time via fluorescence, or by end point analysis using an immunochromatographic strip (ICS) (Boyle et al., 2013). In addition, RNA can be detected by RPA if reverse transcription (RT) of RNA into a complementary DNA (cDNA) is accompanied by RPA in an RT-RPA reaction (Rappolee et al., 1989;Euler et al., 2012).
The principle advantages of using RPA as a potential diagnostic in low resource settings (LRS) include rapid reaction time and the fact that amplification occurs over a broad range of temperatures (25 °C to 42 °C) allowing ambient temperature or body heat to be used to incubate RPA reactions (Boyle et al., 2014;Piepenburg et al., 2006;Lillis et al., 2014;Crannell et al., 2014a). Most pertinently for the detection of pathogens with high genetic diversity, such as HIV-1, RPA assays can accommodate some target sequence variation (Boyle et al., 2013;Euler et al., 2012). In this study we improved upon our previously described HIV-1 RPA assay (Boyle et al., 2013) by altering the primer and probe sequences and adding an RT step to allow for detection of both HIV-1 DNA and RNA. We assessed the performance of this new RT-RPA HIV-1 assay on a large panel of 175 highly diverse HIV-1 sequence variants including subtypes in groups M and O. Ultimately we aim to integrate this assay with a simple sample preparation method to create a high performance, yet easy to use, rapid HIV-1 diagnostic test that can reliably detect HIV-1 infection in infants at the point of care.
2. Materials and methods
2.1. RPA and RT-RPA Amplification and Detection
RPA reactions were supplied by TwistDx Ltd. (Cambridge, United Kingdom) in the TwistAmp Exo (probe cleavage via exonuclease III) and TwistAmp Nfo (probe cleavage via endonuclease IV) format for real time or end point detection of RPA amplicons respectively. Oligonucleotide primers were purchased from Integrated DNA Technologies (Coralville, USA) and oligonucleotide probes from Biosearch Technologies (Novato, USA). Our original HIV pol RPA primers and probe sequences were previously described (Boyle et al., 2013). The Twist Alpha HIV-1 assay used novel primers and probe designed by our team at TwistDx. All TwistAmp reaction mixtures were prepared in a final volume of 50 µL per the TwistAmp protocols as previously described (Boyle et al., 2013). Reaction incubation and detection via real time fluorescence measurement was performed in a Twista™ real time reactor at 39 °C for 20 minutes, with a brief mixing step after 4 – 5 minutes carried out by inverting reactions 3 times. Positivity was scored as a double or greater level of fluorescence from the baseline value read at 4 minutes. For RT-RPA, a range of commercially available reverse transcriptases were screened for compatibility with RT-RPA by adding 2–5 units to each reaction. The best performing reverse transcriptase, OmniScript RT (Qiagen Valencia, USA) was subsequently selected to be used in all subsequent RT-RPA reactions. The final reaction volume and incubation conditions were otherwise unchanged for RT-RPA assays. The TwistAmp Nfo assays were prepared and incubated in an identical manner, except that the probe was labeled at the 5’ end with FAM and the reverse primer was labeled with biotin at the 5’ end to allow for immunochromatographic strip detection of hapten labelled RPA products. Immunochromatographic strips to detect Nfo-based RPA reactions were purchased from Milenia Biotech (Gießen, Germany) and Ustar Biotechnologies (Hangzhou, China). After incubation, RPA reaction tubes were immediately placed on ice and 5 µL of 0.5 M EDTA added to each tube to terminate amplification. Prior to immunochromatographic strip detection, 2 µL of each stopped reaction was diluted by mixing in 98 µL PBS/0.1% Tween buffer (flow buffer) and 10 µL of this solution applied to the capture pad of the strip. The strip was then placed in 100 µL of flow buffer and left to develop for 5 minutes. Positive results were scored with the development of a stripe on the test line and at the control line while negative results displayed only the control line. Tests that did not develop any lines were repeated.
2.2. Preparation of DNA and RNA
Exact sequence match HIV-1 proviral target DNA was extracted from the ACH-2 cell line that contains a single full-length integrated copy of subtype B HIV-1 Bru (subtype B; GenBank accession number K02013.1) using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, USA) (Clouse et al., 1989). The DNA was prepared and quantified as previously described (Boyle et al., 2013). To test for amplification of diverse HIV DNA sequences, plasmid clones of sequence variants of the HIV-1 pol gene derived from 56 HIV-1 strains of the International Reference Panel from the NIH AIDS Research and Reference Reagent Program and from an existing library of subtype A, C and D primary isolates derived by short-term co-culture from the Overbaugh laboratory were prepared as described previously (Boyle et al., 2013). Plasmid DNAs were purified using QIAprep Spin Miniprep Kit (Qiagen, Valencia, USA) according to the manufacturer’s instructions, and concentrations of the purified plasmids were first quantified by spectrophotometry. Plasmid dilutions were then further quantified by real-time PCR to create samples with specific HIV-1 copy numbers as described in (Rousseau et al., 2003). To examine RNA amplification, a panel of 104 culture supernatants of HIV-1 strains that span common subtypes, circulating recombinant forms (CRFs) and unique recombinant forms (URFs) within HIV-1 group M and also group O was obtained from the External Quality Assurance Program Oversight Laboratory (EQAPOL; Duke University, USA) (Sanchez et al., 2014). The genome sequence of each isolate is publically available in GenBank (Benson et al., 2013) and in addition the viral load of each culture supernate was quantified via the COBAS AmpliPrep/COBAS TaqMan 48 HIV-1 Test Version 2.0 (Roche Diagnostics, Pleasanton, CA, USA) (Sanchez et al., 2014). The total RNA from 140 µL aliquots of each supernate was extracted using the QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer’s instructions and eluted in 60 µL of elution buffer. The viral copy number in each eluate was estimated based on the initial sample input volume and expected RNA recovery. Based on a Qiagen extraction efficiency estimate of 90%, a series of eight HIV-1 dilutions ranging from 500 to 0.1 HIV-1 RNA copies per reaction was prepared with 10 mM Tris (pH8.0) supplemented with human genomic DNA at 1ng/µL. To improve accuracy of quantitation, the HIV-1 RNA in each dilution of all variants except the group O isolates, was then quantified by qRT-PCR with the method described by Rouet et al. using Superscript® III one- step RT-PCR system (Life Technologies, Carlsbad, CA, USA) (Rouet et al., 2007). A specificity panel comprising of blood borne viruses and skin associated microflora was constructed from isolates purchased from the National Institute for Biological Standards and Control (NIBSC; Potters Bar, UK) or kindly gifted by Drs. G. Cangelosi and J.S. Meschke (University of Washington, Seattle, USA). Genomic material was extracted from each isolate using QIA Miniprep Kit or QIAamp Viral RNA Mini Kit and then quantified via a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, MA, USA).
3. Results
3.1. Novel RPA primers and probe detect HIV-1 DNA with increased sensitivity
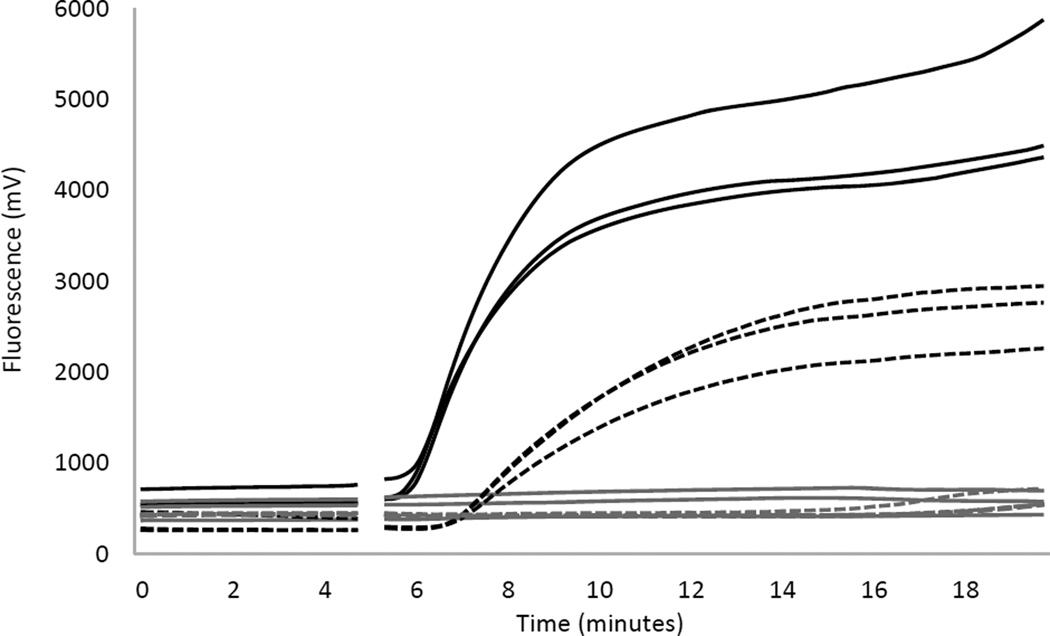
Previously we described the development of the “pol RPA HIV-1 assay” that utilized primers and a probe that target the pol gene and allowed amplification of 98.6% of 72 HIV-1 DNA variants tested, including subtypes A, B, C, D, CRF-AE and CRF-AG (Boyle et al., 2013). In an effort to ensure detection of additional subtypes (including other CRFs as well as group O subtypes) and to improve amplification efficiency and detection signal, a new set of primers and probe designed by TwistDx that target a different conserved region of pol were tested (unpublished data). We refer to this new HIV-1 RPA assay as the “Twist Alpha HIV-1 RPA assay”. We directly compared the ability of the new Twist Alpha HIV-1 RPA assay and the previously described pol RPA HIV-1 assay to amplify 100 copies of exact sequence matched HIV-1 DNA (ACH-2) with real time fluorescence detection (Figure 1). Our original pol RPA HIV-1 assay had a time to detection of 7 minutes and a final fluorescence intensity of 2000 – 3000 units. The new Twist Alpha HIV-1 RPA assay had an accelerated time to detection of 5.5 minutes, more rapid increase in fluorescence intensity representing more efficient amplification, and higher final fluorescence intensities of ≥4000 units.
Figure 1. Comparison of HIV-1 RPA assays using real time fluorescence detection.
Real time fluorescence amplification curves using the previously published pol HIV-1 RPA assay (dashed lines) (Boyle et al., 2013) and new Twist Alpha HIV-1 RPA assay (solid lines) to amplify 100 copies of sequenced matched HIV-1 DNA (black) or negative controls (grey). Data is shown for triplicate amplifications under each reaction condition. The gaps at 5 minutes reflects the mix step where reagent tubes were removed from the device, mixed and reinserted to complete amplification.
We next evaluated the sensitivity of both assays to determine the limit of detection (LOD) using 100, 40, 20, 10, 5 and 1 copies of exact sequence matched ACH-2 HIV-1 DNA (Table 1). Eight replicate reactions at each copy number were assessed via real time fluorescence detection of Exo probes and end point detection of Nfo probes using immunochromatographic strips (ICS). Both RPA assays detected sequence matched HIV-1 DNA in all 8/8 (100%) replicates of 100, 40 and 20 copies tested using real time or endpoint detection. When <20 HIV-1 DNA copies were tested, the Twist Alpha HIV-1 RPA assay was more consistent than the original pol assay. While the Twist Alpha assay detected amplification in >87.5% of the 10 and 5 copy reactions, the original pol RPA assay was less consistent at these lower copies, detecting only between 2/8 (25%) and 7/8 (87.5%) reactions with 10 and 5 copies (Table 1). In reactions with only 1 copy, the Twist Alpha assay amplified between 62.5% and 75% of reactions tested with either real time or end point detection, respectively, while the original pol assay detected only 12.5% of all one copy reactions tested.
Table 1.
A comparison of the analytical sensitivity of the original pol and Twist Alpha HIV-1 RPA assays using either real time detection of RPA (Exo) and ICS-based endpoint detection of RPA amplicons (Nfo).
| HIV DNA copies per reaction |
Exo format | Nfo format | ||||||
|---|---|---|---|---|---|---|---|---|
| pol | Twist Alpha | pol | Twist Alpha | |||||
| +ve/Total | % | +ve/Total | % | +ve/Total | % | +ve/Total | % | |
| 100 | 8/8 | 100 | 8/8 | 100 | 8/8 | 100 | 8/8 | 100 |
| 40 | 8/8 | 100 | 8/8 | 100 | 8/8 | 100 | 8/8 | 100 |
| 20 | 8/8 | 100 | 8/8 | 100 | 8/8 | 100 | 8/8 | 100 |
| 10 | 2/8 | 25 | 7/8 | 87.5 | 7/8 | 87.5 | 8/8 | 100 |
| 5 | 4/8 | 50 | 7/8 | 87.5 | 5/8 | 75 | 8/8 | 100 |
| 1 | 1/8 | 12.5 | 5/8 | 62.5 | 1/8 | 12.5 | 6/8 | 75 |
| 0 | 0/8 | 0 | 0/8 | 0 | 0/8 | 0 | 0/8 | 0 |
3.2. Reverse Transcriptase added to RPA allows the detection of HIV-1 RNA
To determine whether HIV-1 RNA could be detected with RPA, we tested 15 different reverse transcriptase (RT) enzymes for their ability to synthesize complementary DNA from HIV-1 RNA for subsequent amplification by RPA. The performance of each RT was independently assessed by adding the RT to Twist Alpha HIV-1 RPA reagents, and tested to amplify 2000 copies of exact sequence matched HIV-1 RNA (Supplemental Table 1). All RT enzymes were compatible with the RPA reagents and HIV RNA was successfully amplified by this RT-RPA method. Because OmniScript RT has an optimum temperature range of 37°C to 42°C that is compatible with RPA incubation temperatures in addition to the fastest time to detection (Table S1), it was chosen for use in all further experiments.
To determine the LOD of an RT-RPA Twist Alpha HIV-1 assay. Replicates of 8 reactions were performed at each HIV-1 RNA copy level input: 1000, 300, 200, 100, 30, 25, 10 and 1 copies. All reactions with ≥30 copies of HIV-1 RNA were positive, regardless of the detection format used. Below 30 copies per reaction, the proportion of positive reactions gradually decreased relative to copy number, with RT-RPA using Nfo endpoint detection appearing more sensitive for these low copy reactions than the RT-RPA using Exo for real time fluorescence detection (Table 2). Both RT-RPA assay formats were able to detect a single copy of HIV 1 RNA in 1/8 (12.5%) reactions; however, the fact that detection was not consistent in the reactions with <30 copies suggests a LOD of 30 copies of HIV-1 RNA. LOD
Table 2.
A comparison of the LOD of RT-RPA for the detection of HIV-1 RNA using fluorescence and ICS detection formats.
| Estimated copy number | RT Twist Alpha Exo | RT Twist Alpha Nfo | ||
|---|---|---|---|---|
| +ve/Total | % | +ve/Total | % | |
| 1000 | 8/8 | 100 | 8/8 | 100 |
| 300 | 8/8 | 100 | 8/8 | 100 |
| 200 | 8/8 | 100 | 8/8 | 100 |
| 100 | 8/8 | 100 | 8/8 | 100 |
| 30 | 8/8 | 100 | 8/8 | 100 |
| 25 | 6/8 | 75 | 7/8 | 87.5 |
| 10 | 1/8 | 12.5 | 6/8 | 75 |
| 1 | 1/8 | 12.5 | 1/8 | 12.5 |
| 0 | 0/8 | 0 | 0/8 | 0 |
3.3. Specificity of the Twist Alpha HIV-1 RT-RPA assay
In order to determine the specificity of the Twist Alpha HIV-1 RT-RPA assay, 18 samples of genomic RNA or DNAs derived from other pathogens or commensal microflora were used (Table S2). The equivalent of ~50,000 genome copies of each pathogen was added to the Twist Alpha HIV-1 assay to determine whether the assay cross reacts with non-HIV specimens. None of the 18 samples from the specificity panel, including SIVMne (Henderson et al., 1988), produced a false positive result with the Twist Alpha HIV-1 RT-RPA assay (Table S2).
3.4. The Twist Alpha HIV-1 RT-RPA assay can detect diverse HIV-1 subtypes
We previously described that our original pol HIV-1 RPA assay could tolerate up to 9 mismatches in the target sequences from a large panel of diverse HIV-1 subtype variants (Boyle et al., 2013). Here we performed a similar assessment of the Twist Alpha HIV-1 RT-RPA assay on RNA extracted from HIV-1 variants from the Duke EQAPOL Panel (reference Duke EQAPOL). Initial testing included one representative variant of each of subtypes A, B, C, D, F, and G from group M as well as a group O isolate. These 7 variants were tested in triplicate at 10-fold serial dilutions of RNA copies ranging from an estimated ~2 ×106 down to 20 copies. The Twist Alpha HIV-1 RT-RPA assay was able to detect all replicates of these diverse variants tested between 2×106 and ~200 copies of RNA (Table 3). At the lowest copy input tested, which differed by variant between 20 and 67 copies of HIV-1 RNA, at least one replicate from each subtype was positive (Table 3).
Table 3.
RT-RPA detection of RNA from HIV-1 subtypes A, B, C, D, F, G and group O.
| Subtype/group | EQAPOL identifier |
NCBI Accession number |
Total number of sequence mismatches |
Estimated copy number1 |
Actual Copy number 2 |
# of positives/3 |
|---|---|---|---|---|---|---|
| A | DEMA105TZ001 | JX140650 | 0 | 1,000,000 | 580400 | 3/3 (100%) |
| 100,000 | 56390 | 3/3 (100%) | ||||
| 10,000 | 12850 | 3/3 (100%) | ||||
| 5,000 | 7602 | 3/3 (100%) | ||||
| 1,000 | 840 | 3/3 (100%) | ||||
| 500 | 290 | 3/3 (100%) | ||||
| 100 | 38 | 3/3 (100%) | ||||
| B | DEMB09CN002 | KC596066 | 6 | 1,000,000 | 2979000 | 3/3 (100%) |
| 100,000 | 109500 | 3/3 (100%) | ||||
| 10,000 | 19700 | 3/3 (100%) | ||||
| 5,000 | 10950 | 3/3 (100%) | ||||
| 1,000 | 1059 | 3/3 (100%) | ||||
| 500 | 380 | 3/3 (100%) | ||||
| 100 | 67 | 2/3 (67%) | ||||
| C | DEMC07AO001 | JX140662 | 3 | 1,000,000 | 1095000 | 3/3 (100%) |
| 100,000 | 75300 | 3/3 (100%) | ||||
| 10,000 | 9038 | 3/3 (100%) | ||||
| 5,000 | 5054 | 3/3 (100%) | ||||
| 1,000 | 539 | 3/3 (100%) | ||||
| 500 | 256 | 3/3 (100%) | ||||
| 100 | 20 | 3/3 (100%) | ||||
| D | DEMD07UG007 | KF716503 | 5 | 1,000,000 | 1007000 | 3/3 (100%) |
| 100,000 | 81330 | 3/3 (100%) | ||||
| 10,000 | 14460 | 3/3 (100%) | ||||
| 5,000 | 5977 | 3/3 (100%) | ||||
| 1,000 | 673 | 3/3 (100%) | ||||
| 500 | 285 | 3/3 (100%) | ||||
| 100 | 23 | 1/3 (33%) | ||||
| F | DEMF110ES001 | JX140671 | 4 | 1,000,000 | 953800 | 3/3 (100%) |
| 100,000 | 85140 | 3/3 (100%) | ||||
| 10,000 | 10800 | 3/3 (100%) | ||||
| 5,000 | 4985 | 3/3 (100%) | ||||
| 1,000 | 488 | 3/3 (100%) | ||||
| 500 | 229 | 3/3 (100%) | ||||
| 100 | 33 | 3/3 (100%) | ||||
| G | DEMG05ES001 | JX140674 | 5 | 1,000,000 | 947600 | 3/3 (100%) |
| 100,000 | 96670 | 3/3 (100%) | ||||
| 10,000 | 11620 | 3/3 (100%) | ||||
| 5,000 | 6540 | 3/3 (100%) | ||||
| 1,000 | 730 | 3/3 (100%) | ||||
| 500 | 355 | 3/3 (100%) | ||||
| 100 | 33 | 3/3 (100%) | ||||
| Group O3 | DEOXXDE004 | KF859742 | 15 | 1,000,000 | N/A | 3/3 (100%) |
| 100,000 | N/A | 3/3 (100%) | ||||
| 10,000 | N/A | 3/3 (100%) | ||||
| 5,000 | N/A | 3/3 (100%) | ||||
| 1,000 | N/A | 3/3 (100%) | ||||
| 500 | N/A | 3/3 (100%) | ||||
| 100 | N/A | 2/3 (67%) | ||||
| NTC | Water | - | - | 0 | 0 | 0/21 (0%) |
Estimated copy number based on viral load data provided by EQAPOL.
Actual copy numbers after RNA purification were determined using qRT-PCR.
The RT-PCR assay used does not amplify from Group O RNAs.
N/A, not applicable.
The number of nucleotide mismatches between sequences of each of these HIV-1 variants compared to the primer and probe sequences used in the Twist Alpha assay was determined (Table 3) and found to range from 0 for the subtype A isolate to 15 for the group O isolate. Interestingly, 100% of replicates tested at the lowest copy number input had a positive result for the isolates in which the number of mismatches ranged from 0 to 5 (Table 3). Isolates with 5 or more mismatches (variants of subtype B, D and group O) were positive in only 2/3 (67%) or 1/3 (33%) at the lowest copy number tested. The fact that the Twist Alpha HIV-1 RT-RPA assay was able to amplify the isolate O variant with 15 mismatches across the target sequences, was noteworthy suggesting that this RT-RPA assay is very tolerant to sequence variation across highly divergent HIV-1 subtypes. However, analysis of the fluorescent detection data from the group O isolate when compared to other sequence variants tested that had fewer mismatches revealed that the time to amplification was increased, and final fluorescent signal was decreased (Figure S1). These results suggest that significant sequence variation in RPA primer and/or probe binding sites may have an impact on RT-RPA signal strength from reactions, but that qualitative test results on samples with low target copy numbers may be possible, even with highly diverse HIV variants. To assess the ability to use RT-RPA to quantitate HIV RNA levels, the median time to detection was plotted against HIV RNA copy number for each subtype independently (Figure S2). The time to detection of similar copy numbers of HIV-1 RNA between subtypes was significantly variable, suggesting that using this assay for quantitative viral load analysis may be problematic.
To further test the ability of the Twist Alpha HIV-1 RPA primers and probe to detect diverse subtype variants, viral genomic RNA or DNA from 175 HIV-1 isolates were tested at 100 copies in triplicate: 104 HIV-1 RNAs from the Duke EQApol panel (Sanchez et al., 2014), 56 DNAs from the NIH International Panel of HIV-1 Viruses (Brown et al., 2005), and 15 genomic DNAs derived from primary isolates (Table S3), representing isolates of subtypes A, B, C, D, F, and G, plus circulating and unique recombinant forms, as well as group O (Boyle et al., 2013;Sanchez et al., 2014). Of the 175 isolates tested 171 isolates amplified in 3/3 (100%) replicates at 100 copies of HIV-1 RNA or DNA. One other isolate, a subtype C strain (AYF13417), amplified in 2/3 (66.6%) replicates. Only 3 isolates did not amplify at 100 copy input: Two subtype A strains (Q1842 and KF716474), and one subtype B strain (KC473827). The presence of HIV-1 nucleic acid was verified in all four by qRT-PCR, and all were tested again at 1000 copy input. At the 1000 copy input level, 3 of the 4 isolates amplified (data not shown). Therefore, from a total of 175 variant HIV-1 pol RNA or DNA sequences screened with the Twist Alpha HIV assay, HIV-1 was detected in 171/175 (97.7 %) at 100 copies per reaction, with 174/175 (99.4%) at 1000 copies per reaction. RT-RPA was performed on both Q1842 (subtype A) and AY713417 (subtype C) using forward primers that had an exact sequence match to demonstrate that the Twist Alpha assay performance was compromised by the sequence diversity in the target region accessed by the forward primer (data not shown). Interestingly, even with exact sequence match primers, the remaining subtype A isolate (KF716474) was not detected by the RT-RPA even at 1000 copies suggesting that the probe does not bind to its complement in the RPA amplicon. This is surprising since there are only 2 mismatches in the probe sequence as compared to a group O isolate that had 7 mismatches and yet was still detected at 100 copies.
It has been reported that target specific RPA primers with mismatches at their 3' end can prevent amplification with RPA (Daher et al., 2015). Within the 174 sequences that amplified there were 16 (9.1%) that had a single mismatch within the first three bases at the 3’ end of the forward primer and one isolate, KF716479, had mismatches at both positions 1 and 3 and yet still amplified 100 copies of RNA. With the reverse primer, the conservation of the 3’ end was greater with only 3/175 (1.7%) with sequence variation in the first three bases. Interestingly, the sequences that did not amplify in all replicates were not those that had variation in the 3’ end of their primers. The variance of the subtype A strains that did not amplify (Q1842 and KF716474) had an exact sequence match to the reverse primer, while the probe sequences had mismatches at base 45 and 47, and there were 3 mismatches to the forward primers including at positions 4 and 6 from the 3’ end for Q1842 and KF716474, respectively.
4. Discussion
In this study we describe the development and assessment of the Twist Alpha HIV-1 RT-RPA assay, a rapid and highly sensitive RT-RPA assay for the detection of both HIV-1 RNA and DNA. This assay is an improvement on our previously described assay, showing both an increase in sensitivity and a more rapid reaction time to detection without compromising specificity (Boyle et al., 2013). The addition of an RT enzyme enabled the detection of both HIV-1 RNA and DNA without negatively affecting assay performance. Levels of proviral DNA in HIV infected infants are reported to be between 1–382 copies per µL of blood (Beck et al., 2001), while higher median levels of HIV-1 RNA in blood have been described, ranging from 4.1 × 105 copies/µL at birth to 6.0 × 105 copies/µL at 18 months (Ciaranello et al., 2011;Mutasa et al., 2012;Young et al., 2000), suggesting the ability to detect both HIV-1 RNA in addition to DNA will substantially increase sensitivity. Ideal infant diagnostics typically rely on small blood volumes from heel pricks to minimize the impact on infants, and thus highly sensitive diagnostics are required. In addition, it has been observed that excessive amounts of host-derived genomic DNA can inhibit RPA, and thus small blood volumes are ideal for RPA-based assays (Rohrman and Richards-Kortum, 2012). A simple, low complexity method to lyse and release HIV-1 RNA and DNA from small volumes of whole blood that is compatible with RPA is currently in development.
An RT-RPA assay targeting HIV-1 RNA has not been previously described. Similar to RPA assays that only detect DNA, the Twist Alpha HIV-1 RT-RPA assay is rapid and typically reaches peak fluorescent signal within 10 minutes. The LOD of the assay when exact sequence match DNA or RNA templates were used was determined to be 10 or 30 copies, respectively. In addition to having high sensitivity with exact match target sequences, it is critical to ensure that the Twist Alpha HIV-1 assay has similarly high sensitivity to a broad range of sequence variants, ensuring global coverage of the significant genetic diversity exhibited by HIV-1. To investigate this we screened 175 isolates selected from the common group M subtypes: A, B, C, D, F, G, and related CRFs and URFs, in addition to some group O isolates. Of these, 102 of 104 (98.1%) RNAs and 69/71 (97.2%) DNA sequences were detected by the Twist Alpha, with an overall assay sensitivity of 97.7%. The assay was able to detect as low as 23–100 copies of DNA or RNA from each subtype, including group O which had the greatest sequence diversity within the primer/probe binding sites. Of the 4 sequences that failed to amplify at the 100 copy level, 3 were amplified when 1000 copies were tested resulting in 174/175 (99.4%) of isolates detected at higher copy number. Furthermore, we confirmed that the Twist Alpha HIV-1 assay was 100% specific, with no false positives detected when challenged with a high copy number of genomic material from other unrelated microorganisms, including SIV, which shares 70 % sequence similarity (30 polymorphisms) in the full target sequence.
Interestingly, an increased number of mismatches with primers and probe did not necessarily result in reduced sensitivity. In particular the group O isolates had significant mismatches to the assay primer/probe sequences with a total of 13–15 polymorphisms (6–7 of which were in the reverse primer) and yet were reproducibly detected at the 100 copy level. In contrast, the sequence analysis of the isolates that did not efficiently amplify had relatively fewer mismatches (range 5–6), most of which were not in the 3’ end of the primer binding sites (Daher et al., 2014). As others have observed, we did note that mismatches at the 3’ end of a primer prevented amplification. During initial primer optimization, a single base change at the 3’ terminal base of the forward primer binding site was present in multiple subtype D variants and caused failure to amplify (data not shown). As a result, in our final assay design we removed the terminal nucleotide at the 3’ end of the forward primer, which permitted detection of all subtypes as demonstrated. Sequence variation within the primer binding sites did appear to have an impact on time to detection and the final signal intensity across subtypes. This could have implications for ongoing efforts to use RPA for quantitative assays (Crannell et al., 2015;Crannell et al., 2014b). While we observed some linearity within dilution series for each HIV-1 subtype, quantitation across subtypes appeared less reliable (Figure S2). Therefore, the current version of our Twist Alpha HIV-1 assay is not yet suitable for real time RT-RPA to quantify HIV viral loads. It is possible that the inclusion of oligonucleotides of different sequence in the assay formulation (e.g. ambiguous bases) to account for different target pathogen variants may improve the overall inclusivity of the assay and improve the sensitivity of otherwise problematic strains.
There are a wide range of factors that must be fully addressed in order to create an effective diagnostic for early infant diagnosis of HIV-1 infection in low resource settings. Sample collection, processing, amplification and detection must be performed in a short period of time, and ideally the test would be simple, non-instrumented and have minimal hands on requirements. High sensitivity and specificity for HIV-1 is critical, and the ability to detect both HIV-1 RNA and DNA would improve sensitivity of early detection of HIV infection in infants, as they typically have high viral loads at the time of testing (Ciaranello et al., 2011;Mutasa et al., 2012;Young et al., 2000). We have demonstrated that the Twist Alpha HIV-1 RT-RPA assay described here can detect very low copies of both HIV-1 DNA and RNA with high specificity across a large number of sequence variants representing the global distribution of HIV-1 groups M and O. We are currently finalizing and validating our sample preparation approach which will be integrated with the Twist Alpha HIV-1 RT-RPA assay along with a simple battery-operated incubator and a sealed amplicon cassette (Lillis et al., 2014), to create a practical tool for EID in resource limited settings.
5. Conclusions
In this study, we report on the performance of a new RT-RPA HIV-1 assay design that shows a significant improvement upon our original assay. The addition of reverse transcriptase to the assay has demonstrated that RPA is a highly sensitive isothermal amplification method that permits the detection of very low copy numbers of both HIV-1 proviral DNA and genomic viral RNA. Both RT-RPA amplicon detection formats are highly sensitive and offer low to medium test throughput with an LOD of only 10 copies target sequence. The assay is highly specific to HIV-1 and yet is able to detect different HIV-1 groups, subtypes and variant forms therein, many of which have considerable sequence diversity to the primers and oligonucleotide probe used in the assay. A very important consideration for a point of care diagnostic tool is the time to result that needs limited or no mains electrical power. We demonstrate that RT-RPA assay results can be interpreted in under 20 minutes, which is significantly quicker than other nucleic acid amplification strategies described to date. By requiring an incubation temperature of only 39°C, RT-RPA reactions do not require significant power for optimal incubation conditions and so is amendable for use with simple heaters or battery powered devices. The preparation of test samples is not addressed in this work but by using HIV-1 RNA as a target, the target copy number typically very high in infected infants. HIV-1 RNA preparation is simpler than purifying and extracting proviral DNA from peripheral blood mononuclear cells and so a method to rapidly extract HIV-1 RNA from infected whole blood is in development. The end goal is for the integration of rapid sample preparation of HIV-1 RNA with the Twist Alpha HIV-1 RT-RPA assay that will create a simple and rapid, yet high performance assay that can detect HIV-1 in infected infants outside of traditional laboratory settings.
Supplementary Material
Highlights.
RT RPA can detect low copies of HIV-1 proviral DNA and genomic RNA.
Result scoring via fluorescence or visual detection have similar sensitivity.
An HIV-1 assay that can detect variant target sequences in groups M and O.
The test result is available in 20 minutes.
Acknowledgments
The research reported in this publication was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS under award number R01AI097038. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to thank the NIH AIDS Reagent Repository for supplying the HIV-1 International Reference Panel and also the Duke University External Quality Assurance Program Oversight Laboratory (EQAPOL) for the supply of the reference panel of quantified HIV-1 culture supernates. We thank Dr Ana Sanchez (Duke University) for her assistance with technical enquiries around the EQAPOL panel. We thank Drs. JS Meschke and G Cangelosi from the University of Washington for the kind gift of viral and bacterial gDNAs. We also thank Dr G. Domingo (PATH) for critical edits and comments during the drafting of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Mathew Parker and Olaf Piepenburg are employees of TwistDx Ltd. All other authors report no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version
Reference List
- 1.Beck IA, Drennan KD, Melvin AJ, Mohan KM, Herz AM, Alarcon J, Piscoya J, Velazquez C, Frenkel LM. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J. Clin. Microbiol. 2001;39:29. doi: 10.1128/JCM.39.1.29-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2013;41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle DS, Lehman DA, Lillis L, Peterson D, Singhal M, Armes N, Parker M, Piepenburg O, Overbaugh J. Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. MBio. 2013;4 doi: 10.1128/mBio.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle DS, McNerney R, Teng LH, Leader BT, Perez-Osorio AC, Meyer JC, O'Sullivan DM, Brooks DG, Piepenburg O, Forrest MS. Rapid detection of Mycobacterium tuberculosis by recombinase polymerase amplification. PLoS One. 2014;9:e103091. doi: 10.1371/journal.pone.0103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown BK, Darden JM, Tovanabutra S, Oblander T, Frost J, Sanders-Buell E, de Souza MS, Birx DL, McCutchan FE, Polonis VR. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J. Virol. 2005;79:6089. doi: 10.1128/JVI.79.10.6089-6101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butto S, Suligoi B, Fanales-Belasio E, Raimondo M. Laboratory diagnostics for HIV infection. Ann. Ist. Super. Sanita. 2010;46:24. doi: 10.4415/ANN_10_01_04. [DOI] [PubMed] [Google Scholar]
- 7.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC. Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clouse KA, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci AS, Folks TM. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J. Immunol. 1989;142:431. [PubMed] [Google Scholar]
- 9.Crannell ZA, Rohrman B, Richards-Kortum R. Equipment-free incubation of recombinase polymerase amplification reactions using body heat. PLoS One. 2014a;9:e112146. doi: 10.1371/journal.pone.0112146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crannell ZA, Rohrman B, Richards-Kortum R. Quantification of HIV-1 DNA using real-time recombinase polymerase amplification. Anal. Chem. 2014b;86:5615. doi: 10.1021/ac5011298. [DOI] [PubMed] [Google Scholar]
- 11.Crannell ZA, Rohrman B, Richards-Kortum R. Development of a quantitative recombinase polymerase amplification assay with an internal positive control. J. Vis. Exp. 2015 doi: 10.3791/52620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daher RK, Stewart G, Boissinot M, Boudreau DK, Bergeron MG. Influence of sequence mismatches on the specificity of recombinase polymerase amplification technology. Mol. Cell Probes. 2014 doi: 10.1016/j.mcp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Daher RK, Stewart G, Boissinot M, Boudreau DK, Bergeron MG. Influence of sequence mismatches on the specificity of recombinase polymerase amplification technology. Mol. Cell Probes. 2015;29:116. doi: 10.1016/j.mcp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Essajee S, Vojnov L, Penazzato M, Jani I, Siberry GK, Fiscus SA, Markby J. Reducing mortality in HIV-infected infants and achieving the 90-90-90 target through innovative diagnosis approaches. J. Int. AIDS Soc. 2015;18:20299. doi: 10.7448/IAS.18.7.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Euler M, Wang Y, Nentwich O, Piepenburg O, Hufert FT, Weidmann M. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. J. Clin. Virol. 2012;54:308. doi: 10.1016/j.jcv.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Finocchario-Kessler S, Gautney BJ, Khamadi S, Okoth V, Goggin K, Spinler JK, Mwangi A, Kimanga D, Clark KF, Olungae HD, Preidis GA. If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS. 2014;28(Suppl 3):S313–S321. doi: 10.1097/QAD.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghadrshenas A, Ben AY, Chang J, Dale H, Sherman G, Vojnov L, Young P, Yogev R. Improved access to early infant diagnosis is a critical part of a child-centric prevention of mother-to child transmission agenda. AIDS. 2013;(27 Suppl 2):S197–S205. doi: 10.1097/QAD.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 18.Henderson LE, Benveniste RE, Sowder R, Copeland TD, Schultz AM, Oroszlan S. Molecular characterization of gag proteins from simian immunodeficiency virus (SIVMne) J. Virol. 1988;62:2587. doi: 10.1128/jvi.62.8.2587-2595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karchava M, Pulver W, Smith L, Philpott S, Sullivan TJ, Wethers J, Parker MM. Prevalence of drug-resistance mutations and non-subtype B strains among HIV-infected infants from New York State. J. Acquir. Immune. Defic. Syndr. 2006;42:614. doi: 10.1097/01.qai.0000225871.87456.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyaga C, Sendagire H, Joseph E, McConnell I, Grosz J, Narayan V, Esiru G, Elyanu P, Akol Z, Kirungi W, Musinguzi J, Opio A. Uganda's new national laboratory sample transport system: a successful model for improving access to diagnostic services for Early Infant HIV Diagnosis and other programs. PLoS One. 2013;8:e78609. doi: 10.1371/journal.pone.0078609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert JS, Harris DR, Stiehm ER, Moye J, Jr, Fowler MG, Meyer WA, III, Bethel J, Mofenson LM. Performance characteristics of HIV-1 culture and HIV-1 DNA and RNA amplification assays for early diagnosis of perinatal HIV-1 infection. J. Acquir. Immune. Defic. Syndr. 2003;34:512. doi: 10.1097/00126334-200312150-00011. [DOI] [PubMed] [Google Scholar]
- 22.Lillis L, Lehman D, Singhal MC, Cantera J, Singleton J, Labarre P, Toyama A, Piepenburg O, Parker M, Wood R, Overbaugh J, Boyle DS. Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA. PLoS One. 2014;9:e108189. doi: 10.1371/journal.pone.0108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutasa K, Ntozini R, Prendergast A, Iliff P, Rukobo S, Moulton LH, Ward BJ, Humphrey JH. Impact of six-week viral load on mortality in HIV-infected Zimbabwean infants. Pediatr. Infect Dis J. 2012;31:948. doi: 10.1097/INF.0b013e318266aac2. [DOI] [PubMed] [Google Scholar]
- 24.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 25.Obaro SK, Losikoff P, Harwell J, Pugatch D. Failure of serial human immunodeficiency virus type 1 DNA polymerase chain reactions to identify human immunodeficiency virus type 1 clade A/G. Pediatr. Infect Dis J. 2005;24:183. doi: 10.1097/01.inf.0000151040.57772.40. [DOI] [PubMed] [Google Scholar]
- 26.Obimbo EM, Wamalwa D, Richardson B, Mbori-Ngacha D, Overbaugh J, Emery S, Otieno P, Farquhar C, Bosire R, Payne BL, John-Stewart G. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J. Acquir. Immune. Defic. Syndr. 2009;51:209. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palomba E, Gay V, de MM, Fundaro C, Perugini L, Tovo PA. Early diagnosis of human immunodeficiency virus infection in infants by detection of free and complexed p24 antigen. J. Infect Dis. 1992;165:394. doi: 10.1093/infdis/165.2.394. [DOI] [PubMed] [Google Scholar]
- 28.Panteleeff DD, John G, Nduati R, Mbori-Ngacha D, Richardson B, Kreiss J, Overbaugh J. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J. Clin. Microbiol. 1999;37:350. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterlini P, Lallemant-Le CS, Lallemant M, M'Pele P, Dazza MC, Terre S, Moncany M, Jourdain G, Courgnaud V, N'Zingoula S. Polymerase chain reaction for studies of mother to child transmission of HIV1 in Africa. J. Med. Virol. 1990;30:53. doi: 10.1002/jmv.1890300112. [DOI] [PubMed] [Google Scholar]
- 30.Patton JC, Coovadia AH, Meyers TM, Sherman GG. Evaluation of the ultrasensitive human immunodeficiency virus type 1 (HIV-1) p24 antigen assay performed on dried blood spots for diagnosis of HIV-1 infection in infants. Clin. Vaccine Immunol. 2008;15:388. doi: 10.1128/CVI.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006;4:e204. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappolee DA, Wang A, Mark D, Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J. Cell Biochem. 1989;39:1. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- 33.Richardson BA, Mbori-Ngacha D, Lavreys L, John-Stewart GC, Nduati R, Panteleeff DD, Emery S, Kreiss JK, Overbaugh J. Comparison of human immunodeficiency virus type 1 viral loads in Kenyan women, men, and infants during primary and early infection. J. Virol. 2003;77:7120. doi: 10.1128/JVI.77.12.7120-7123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohrman BA, Richards-Kortum RR. A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab Chip. 2012;12:3082. doi: 10.1039/c2lc40423k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouet F, Chaix ML, Nerrienet E, Ngo-Giang-Huong N, Plantier JC, Burgard M, Peeters M, Damond F, Ekouevi DK, Msellati P, Ferradini L, Rukobo S, Marechal V, Schvachsa N, Wakrim L, Rafalimanana C, Rakotoambinina B, Viard JP, Seigneurin JM, Rouzioux C. Impact of HIV-1 genetic diversity on plasma HIV-1 RNA Quantification: usefulness of the Agence Nationale de Recherches sur le SIDA second-generation long terminal repeat-based real-time reverse transcriptase polymerase chain reaction test. J. Acquir. Immune. Defic. Syndr. 2007;45:380. doi: 10.1097/QAI.0b013e3180640cf5. [DOI] [PubMed] [Google Scholar]
- 36.Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J. Infect Dis. 2003;187:741. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez AM, DeMarco CT, Hora B, Keinonen S, Chen Y, Brinkley C, Stone M, Tobler L, Keating S, Schito M, Busch MP, Gao F, Denny TN. Development of a contemporary globally diverse HIV viral panel by the EQAPOL program. J. Immunol. Methods. 2014;409:117. doi: 10.1016/j.jim.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson P, de MC, Joshi Y, Golden A, Hodinka RL, Soriano V, Devare SG, Hackett J., Jr Impact of human immunodeficiency virus type 1 (HIV-1) genetic diversity on performance of four commercial viral load assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J. Clin. Microbiol. 2005;43:3860. doi: 10.1128/JCM.43.8.3860-3868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Geneva, Switzerland: World Health Organization; [1-1-2010]. Antiretroviral therapy for HIV infection in infants and children: Recommendations for a public health approach; pp. 1–206. [Google Scholar]
- 40.Young NL, Shaffer N, Chaowanachan T, Chotpitayasunondh T, Vanparapar N, Mock PA, Waranawat N, Chokephaibulkit K, Chuachoowong R, Wasinrapee P, Mastro TD, Simonds RJ. Early diagnosis of HIV-1-infected infants in Thailand using RNA and DNA PCR assays sensitive to non-B subtypes. J. Acquir. Immune. Defic. Syndr. 2000;24:401. doi: 10.1097/00126334-200008150-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.