Abstract
Objectives
AIDS is caused by CD4+ T-cell depletion. While combination antiretroviral therapy can restore blood T-cell numbers, the clonal diversity of the reconstituting cells, critical for immunocompetence, is not well defined.
Methods
We performed an extensive analysis of parameters of thymic function in HIV-1 infected (n=39) and control (n=28) subjects ranging from 13 to 23 years of age. CD4+ T-cells including naïve (CD27+ CD45RA+) and recent thymic emigrant (RTE) (CD31+/CD45RA+) cells, were quantified by flow cytometry. Deep sequencing was used to examine T cell receptor (TCR) sequence diversity in sorted RTE CD4+ T-cells.
Results
Infected subjects had reduced CD4+ T-cell levels with predominant depletion of the memory subset and preservation of naïve cells. RTE CD4+ T-cell levels were normal in most infected individuals, and enhanced thymopoiesis was indicated by higher proportions of CD4+ T-cells containing TCR recombination excision circles. Memory CD4+ T-cell depletion was highly associated with CD8+ T-cell activation in HIV-1-infected persons and plasma IL-7 levels were correlated with naïve CD4+ T-cells, suggesting activation-driven loss and compensatory enhancement of thymopoiesis. Deep sequencing of CD4+ T-cell receptor sequences in well-compensated infected persons demonstrated supranormal diversity, providing additional evidence of enhanced thymic output.
Conclusions
Despite up to two decades of infection, many individuals have remarkable thymic reserve to compensate for ongoing CD4+ T cell loss, although there is ongoing viral replication and immune activation despite cART. The longer-term sustainability of this physiology remains to be determined.
INTRODUCTION
The hallmark of Human Immunodeficiency Virus Type 1 (HIV-1)-induced immunosuppression leading to acquired immunodeficiency syndrome is CD4+ T-cell depletion, which may be caused by direct cytopathic effects of infection, immune clearance of infected cells, persistent immune activation, and likely other factors.[1] In particular, immune activation is highly associated with the ongoing loss of CD4+ T-cells and believed to be the cause of increased T-cell turnover during chronic infection. The precise mechanisms for this inappropriate inflammatory state are unclear, but ongoing viral replication can be a major contributor even in persons with undetectable viremia. [1-4]
Peripheral blood CD4+ T-cell concentration is a widely used clinical predictor of the immunological status of an infected individual, with a level of less than 200/μL generally considered to reflect sharply increased risk for opportunistic infections that define AIDS.[1] However, this simple quantitative assessment does not precisely reflect immunocompetence. For example, recurrent bacterial pneumonias, malignancies, and AIDS-defining illnesses such as active cytomegalovirus infection and Pneumocystis pneumonia may occur at higher CD4+ T-cell levels in children, adolescents, and adults.[5-7] It is very likely that the clonal diversity of the CD4+ T-cell population and therefore breadth of pathogen recognition is also important.[8]
Effective antiretroviral therapy (ART) suppresses HIV-1 replication, reduces immune activation, and increases peripheral blood CD4+ T-cell concentrations.[9, 10] However, the extent to which normalization of clonal T-cell diversity occurs is less well documented. In HIV-1-infected adults, the rise in CD4+ T-cell levels seen after institution of ART is characterized by an initial rapid rise that is likely due to redistribution of total body memory CD4+ T-cells, followed by a slower and more prolonged increase in naïve CD4+ T-cells. [9, 11] By contrast, HIV-1-infected children demonstrate an early and sustained increase in naïve CD4+ T-cells [12-16] that likely reflects greater baseline thymic function than adults, who tend to have age-related involution of thymic epithelial tissue and attrition of thymic function.[17]
Supporting this concept, we previously demonstrated that adolescents and young adult survivors of perinatal HIV-1 infection on ART have markers of thymopoiesis that are comparable to uninfected age-matched controls, including concentrations of peripheral blood naïve CD4+ T-cells and T-cell receptor recombination excision circles (TREC) that reflect recent thymic emigrants.[18] Others have demonstrated that T-cell receptor CDR3 distribution perturbations are rapidly reduced in some children and adolescents during ART [19] suggesting that some degree of normalization of the TCR repertoire is possible. However, these measurements have not excluded qualitative abnormalities in thymopoiesis that might result from the known impact of HIV-1 on the architecture of both the thymus and secondary lymphoid tissues.[13-15, 20-22]
Thus it is unclear if CD4+ T-cell clonal diversity is maintained in conjunction with recovered total CD4+ T-cell numbers on ART, particularly in individuals who were infected before immunologic maturity. To address this uncertainty, we assess immune reactivity to HIV-1, thymopoiesis and CD4+ T-cell diversity in a cohort of long term survivors of perinatal HIV-1 infection. These data address key questions as to whether infection early in life (during immunologic development), in conjunction with chronic infection (spanning more than 13 years), limit CD4+ T-cell reconstitution.
METHODS
Study approval
Healthy control and HIV-1-infected study volunteers were enrolled under protocols approved by institutional review boards of the University of California Los Angeles and Children's Hospital Los Angeles. Written informed consent was received from all participants prior to inclusion in the study.
Cohort and preparation of peripheral blood mononuclear cells
All study participants were enrolled from 2003 to 2006. Individuals with known hepatitis B or C infections were excluded. Twenty control subjects and 20 HIV-1-infected subjects were described in previous reports.[18, 23] Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density centrifugation gradient, washed twice with phosphate buffered saline, and viably cryopreserved. Fresh umbilical cord blood was obtained from the UCLA CFAR Virology Core. For quantitative spectratyping and pyrosequencing studies, CD3+CD4+CD31+CD45RA+ T-cells were purified from cryopreserved PBMC by fluorescence-activated cell sorting (FACSAria II using FACSDiva Version 6.1, Becton Dickinson). CD4+ T-cells from cord blood samples were isolated by negative selection (human CD4+ T-cell enrichment mixture, RosetteSep, StemCell Technologies).
Clinical laboratory tests
Complete blood counts and plasma HIV-1 RNA measurements were obtained through the Children's Hospital Los Angeles and UCLA clinical laboratories.
Volumetric tomography of thymic tissue
Non-contrast helical computed tomography (CT) studies of the chest were performed with 3-mm collimation extending from the thoracic inlet to the lung bases, using previously described methods.[18] All female participants had negative pregnancy tests confirmed prior to imaging. Volumetric CT scans were discontinued after 49 scans (29 HIV-1-infected and 20 uninfected controls) because an interim analysis indicated futility to detect statistically significant differences with the initially planned sample size.
T-cell immunophenotyping by flow cytometry
Whole blood T-cell staining and flow cytometry was performed as described previously, with naive CD4+ T-cells defined as CD4+CD45RA+CD27+.[18] CD45RA−CD4+ T-cells were defined as memory cells (combined central and effector subsets). Staining was also performed to quantify the CD45RA+CD31+ subset of CD4+ T-cells (recent thymic emigrants) [24] and the CD38+HLA-DR+ subset of CD8+ T-cells (activated).[25] Quantitation of naïve, recent thymic emigrants, and memory CD4+ T-cells was not performed in one control subject and the percentage of CD38+HLA-DR+ CD8+ T-cells was not determined for two other control subjects. Due to the absence of a complete blood count, the concentrations (cells/μL) of T-cell subsets of one HIV-1-infected individual are absent from panels of Figures 1 and 2.
Figure 1. Clinical and immune parameters of study participants.
The HIV-1-infected participants included 39 persons, of whom 18 had plasma viremia <50 HIV-1 RNA copies/mL (uVL group, 11 male and 7 female) and 21 had plasma viremia ≥ 50 HIV-1 RNA copies/mL (dVL group, 11 male and 10 female), who were compared to a control group of healthy uninfected persons (10 male, 18 female) of similar ages (A). Most of the infected persons had had symptomatic disease in the past (B). Evaluated parameters included thymic volume (C), concentrations of blood CD4+ T-cells (D) and their characteristics (E-H), blood CD8+ T-cell concentrations (I) and the ratio of CD4+ to CD8+ T-cells (J), and CD8+ T-cell activation (K) and HIV-1 targeting (L). Filled circles represent uninfected control subjects; unfilled circles, represent uVL participants with plasma HIV-1 RNA <50 copies/ml; open triangles represent dVL individuals with plasma HIV-1 RNA 50 to<400 copies/ml. Statistically significant results (p<0.05), as determined by Mann-Whitney U Tests, are indicated. Bars indicate median values.
Figure 2. Relationship of immune activation to memory CD4+ T-cell loss, and resulting homeostatic proliferation of CD4+ T-cells.
A. Systemic immune activation, as reflected by CD8+ T-cell co-expression of activation markers, is plotted against blood levels of memory CD4+ T-cells (CD45RA−, including central and effector memory subsets). B. The relationship of blood levels of naïve CD4+ T-cells to plasma levels of the homeostatic cytokine IL-7 is plotted. Linear regression regression line and its associated p value is indicated for relationship between naïve T-cells and plasma IL-7 concentrations of HIV infected subjects. In both panels, filled circles represent uninfected control subjects, unfilled circles, represent uVL participants, and open triangles represent dVL individuals.
Detection of HIV-1-specific CD8+ T-cell responses against HIV-1 by interferon (IFN)-γ ELISpot analysis
Peripheral blood HIV-1 specific CD8+ T-cell responses in HIV-1 infected individuals with plasma HIV-1 levels of ≤400 RNA copies/mL at study entry were quantified by IFN-γ ELIspot analysis, as previously described.[26] In brief, purified CD8+ T-cells were screened against 53 pools of overlapping peptides spanning the total HIV-1 clade B consensus sequence proteome (NIH AIDS Reference and Reagent Repository) to determine the frequency of spot-forming cells (SFC) per added CD8+ T-cells. The frequency of HIV-1-specific SFC per volume of peripheral blood was calculated by multiplying the frequency of SFC in CD8+ T-cells and the number of CD8+ T-cells per volume of blood.
Peripheral blood TREC analyses
Cellular DNA was prepared from PBMCs and signal joint T-cell receptor recombination excision circles (TREC) were quantified by real time PCR as previously described [27-29], and reported as TREC/million cells. TREC were measured using isolated CD4+ T-cells (Rosette-Sep beads, StemCell Technologies, Vancouver, Canada) for most participants. The number of TREC+ CD4+ T-cells per volume of peripheral blood was calculated by multiplying the frequency of TREC in isolated CD4+T-cells and the concentration of CD4+T-cells per volume of blood.
HLA and CCR5 genetic analyses
Using PBMC DNA, HLA typing was performed by the clinical laboratory at the UCLA Immunogenetics Center, and PCR was used to determine if the Δ32 deletion was present at the CCR5 locus using oligonucleotide primers described by others. [30]
Quantitation of TCR BV family RNA transcripts
From 3 to 15 million cryopreserved PBMC from HIV-1-infected or control subjects were stained and sorted to purify CD31+CD45RA+CD4+ T-cells, yielding 230,000 to 700,000 cells per individual. RNA was isolated from purified lymphocytes (RNeasy MiniKit, Qiagen, Valencia CA.), and reverse-transcribed to cDNA using random primers (High Capacity Reverse Transcription Kit, Applied Biosystems, Carlsbad, CA). Quantitative spectratyping (QS) was used to examine BV family usage as described previously. [31] In brief, RT-PCR was employed to determine the relative concentration of each BV gene family (IMGT nomenclature (http://www.imgt.org)), and capillary electrophoretic size resolution of each family yielded a profile of TCR sequence size distribution within each family.
Deep sequencing of TCR coding sequences
The cDNA (6.5 to 15 μg) generated for spectratyping was PCR-amplified (Phusion High-Fidelity DNA Polymerase, New England BioLabs) for 35 cycles under the following conditions: initial denaturation 98°C (30sec), denaturation 98°C (10sec), annealing 62°C (30sec), extension 72°C (15sec) and final extension (5 min). The PCR products were then purified (PureLink PCR Purification Kit, Invitrogen) and further amplified using nested PCR (Phusion High-Fidelity DNA Polymerase) for 35 cycles under the following conditions: initial denaturation 98°C (30sec), denaturation 98°C (10sec), annealing 62°C (30sec), extension 72°C (15sec) and final extension (5 min). These PCR products were then separated in 2% agarose gels and cDNA from the appropriate bands was purified (QIA Gel Extraction Kit, Qiagen). Pyrosequencing of the nested PCR purified products using 454 FLX Titanium chemistry was performed according to the manufacturer's protocols (Roche Applied Science). The primers used for the 3 BV families were the same as those used in QS analysis [31], but additionally tagged with multiplex identifier (MID) and primer key sequences (Supplemental Table 1). To check that diversity within the samples was retained during PCR amplification with the modified primers, the following control experiments were performed. First, three rounds of PCR amplification were performed on an aliquot of an umbilical cord blood DNA sample, and spectratyping was performed after each round, showing that the TCR genes of the third round of amplification remained Gaussian in size distribution (Supplemental Figure 1). Second, the PCR products from the third round of amplification were cloned and sequenced, showing polyclonality of TCRs in all cases (Supplemental Table 2), thus demonstrating no evidence of biased amplification.
Statistical analyses
Clinical parameters analyzed as continuous variables were compared using two-tailed Mann Whitney U Test (except for the comparison of HIV ELISpot responses). Categorical variables were compared using Fisher's exact test. Pyrosequencing (454, Roche) of TCRs in nine samples of sorted CD31+CD45RA+CD4+ T-cells (from three HIV-1-infected participants receiving ART with suppressed viremia (uVL), three uninfected controls, and three umbilical cord blood (CB) Supplemental Table 3) yielded between 32,000 and 198,000 TCR sequences per sample. To compare the diversity of TCR sequences in these individuals, we examined TCR sequences in 3 specific BV families: BV03, BV19 and BV29 (IMGT nomenclature). These families were selected because they represented about 5% of total BV families in the CD31+CD4+ T-cells in HIV infected individuals, control study participants, and cord blood specimens that were selected. Two samples with lower yields (control BN02 and infected subject CB13 with 33,000 and 32,000 sequences respectively) were excluded from analyses that are especially sensitive to sample size.
Pyrosequencing is typically associated with significant sequencing errors [32, 33], but this will alter comparisons of diversity estimates if the error statistics do not differ across samples. Differences in the diversity of TCR coding sequences were evaluated by methods commonly employed in ecologic studies including Shannon index of diversity, sample size-corrected Shannon index, rarefaction curves, and analysis of the fraction of singleton species (that occur only once in the sample). We also used a histogram shape estimation technique using an “unseen estimator,” which uses the observed distribution of species in a sample to estimate the total number of unique species missed in sampling, as well as the full species distribution.[34]
As an additional control, sequences were also clustered with two different algorithms, as described by others.[35] Finally, the clusters were translated into stop codon-free amino acid sequences with verified BV and Jβ flanking regions.
RESULTS
Cohort characteristics
The study participants included 39 persons who were infected with HIV-1 as infants (22 male and 17 female) and an uninfected control group of 28 individuals (10 male and 18 female), ranging from 13.3 to 23.0 and 13.1 to 22.9 years of age respectively at the time of study (Figure 1A). Most infections (85%) were from mother to child transmission (including one by breastfeeding from a mother who acquired infection post-partum by blood transfusion), and the remainder (15%) were from blood transfusions in 1982 and 1983, including twin brothers who were described extensively in an earlier report [23]. Among the infected individuals, none had the CCR5 Δ32 mutation; one and three respectively had HLA-B*27 and HLA-B*57 genotypes associated with slower disease progression [36], and one and none respectively had HLA-B*3502 and HLA-B*3503 associated with accelerated disease progression. All infected participants were receiving combination antiretroviral therapy (ART) at the time of study; 18 had plasma viremia <50 HIV-1 RNA copies/mL (uVL group, 11 male and 7 female) and 21 had plasma viremia ≥ 50 HIV-1 RNA copies/mL (dVL group, 11 male and 10 female). Most (77%) of the HIV-1 infected individuals had clinical or laboratory evidence of immunodeficiency (CDC class B or C) in the past (Figure 1B), although imaging revealed relatively normal thymic size overall (Figure 1C). Thus, the infected individuals represented a group of long term survivors of whom most had sustained clinically significant immunodeficiency due to HIV-1 infection at some point.
Many long term survivors of perinatal HIV-1 infection have relatively normal total and naïve CD4+ T-cell concentrations on ART, despite generally depressed levels of memory CD4+ T-cells
At enrollment, peripheral blood CD4+ T-cell levels were lower overall in the HIV-1-infected persons versus uninfected controls (mean 514 versus 686 cells/μL blood respectively), although the uVL group had levels similar to the controls (mean 601 versus 686 CD4+ T-cells/μL respectively, Figure 1D). Examining the CD4+ T-cell population phenotypically, both HIV-1-infected groups exhibited significant depletion of the memory (CD45 RA−) subset (Figure 1E). By contrast, the naïve (CD45RA+/CD27+) subset was relatively normal to elevated in the uVL group and slightly reduced in the dVL group (neither statistically significantly different) (Figure 1F). More detailed analysis of the naïve CD4+ T-cell population suggested overall normal levels of recent thymic emigrants (CD45RA+/CD31+)[24] in the uVL group and normal to reduced levels in the dVL group compared to controls (no statistically significant differences, Figure 1G). Furthermore, the frequency of the total CD4+ T-cell population with T-cell receptor excision circles (TRECs) was elevated in both uVL and dVL groups compared to controls (statistically significantly only for the uVL group, Figure 1H), suggesting higher percentages of cells produced in the thymus (versus peripheral homeostatic proliferation). As a whole, these data demonstrate that these long term survivors of perinatal HIV-1 infection had depleted levels of memory CD4+ T-cells, but generally exhibited quantitative restoration of naïve T-cell populations via increased thymic output on ART.
Despite suppression of viremia by treatment, perinatally-infected individuals have evidence of ongoing HIV-1-driven immune activation
Compared to controls, both groups of HIV-1-infected subjects had significantly elevated blood CD8+ T-cells levels (Figure 1I). Examining the ratio of CD4+ to CD8+ T-cells, it was apparent that the relative increase of CD8+ T-cells and decrease of CD4+ T-cells was especially marked in the dVL group (Figure 1J), suggesting an association between abnormality in the CD8+ and CD4+ T-cell compartments. Additionally, CD8+ T-cell activation (CD38+/HLA-DR+) was increased in both infected groups versus the control group, significantly greater in the dVL versus uVL group (Figure 1K). Finally, screening of participants with <400 HIV-1 RNA copies/mL plasma (12 from the uVL group, 5 from the dVL group) for CD8+ T-cell responses against the whole HIV-1 proteome [26] (Figure 1L) revealed persisting responses (predominately targeting Gag and Nef proteins similarly to infected older adults [37])(not shown) in most persons despite undetectable or low viremia (between 50 and 400 copies/mL). Lower blood levels of memory CD4+ T-cells were seen in infected individuals with higher levels of CD8+ T-cell activation (Figure 2A), and there was a significant inverse correlation between the number of naïve CD4+ T-cells and the plasma concentration of IL-7 in the HIV-1 infected group (Figure 2B); no such correlation was seen in the uninfected control group. These results suggest persistent generalized immune activation was present and was associated with ongoing loss of memory CD4+ T-cells and secondary enhanced homeostatic proliferation of naïve CD4+ T-cells in addition to the enhanced thymic output suggested by the data above.
The long term survivors of perinatally HIV-1 infection exhibit increased CD4+ T-cell receptor diversity and breadth
To evaluate thymopoiesis more qualitatively, we examined the TCR repertoire of the CD31+ subset of CD4+ T-cells, which represent thymic emigrants and their early progeny because CD31 is lost after a few cycles of homeostatic proliferation. Quantitative spectratyping analysis [23, 38] revealed Gaussian distributions of TCR size populations for three control umbilical cord blood samples, as expected for unperturbed native populations [31] (Supplemental Figure 2, Panel A). TCR repertoires of control subjects and uVL individuals also showed generally Gaussian distributions (data not shown), suggesting grossly diverse TCR production.
To better define the diversity of TCR production, we performed deep sequencing analysis of TCR families BV03, BV19 and BV29 (IMGT nomenclature), selected for having relatively consistent representation of ~5% of total BV families in CD31+CD4+ T-cells from representative uVL and control study participants and 3 cord blood specimens ((Supplemental Table 3, Supplemental Table 4 and Supplemental Figure 2, Panel B). The Shannon entropy index (S) for raw sequences (initially excluding BN02 (an uninfected control)) and cord blood CB13, which had insufficient sampling), ranged from 14.3 to 16.3 for CB, 11.3 to 14.1 for controls, and 13.6 to 14.1 uVL. The Shannon index of the estimated histogram from filtered sequences ranged from 16.8 to 17.2 for CB, 13.2 to 13.5 for uninfected controls, and 13.8 to 14.1 for uVL (Table), indicating that TCR diversity was greatest in CB and least in controls (p<0.01). The two samples initially excluded (control BN02 and cord blood CB13) were also consistent with this pattern (Table).
Table.
Numbers of TCR sequences obtained by deep sequencing and Shannon Diversity Indexes (parentheses).
| uVL | Uninfected | Cord Blood | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AP04 | AP22 | CP04 | CN13 | CN02 | BN02 | CB12 | CB11 | CB13 | |
| BV03 | 27537 (10.79) | 32125 (12.62) | 37942 (11.84) | 112979 (11.71) | 54912 (11.23) | 25396 (9.63) | 47801 (15.06) | 82367 (15.95) | 10987 (14.23) |
| BV19 | 25337 (12.27) | 13015 (12.81) | 23497 (11.69) | 72688 (12.99) | 30868 (11.02) | 4734 (10.46) | 10868 (14.01) | 73392 (15,00) | 11325 (16.86) |
| BV29 | 38031 (14.07) | 3377 (14.23) | 24832 (14.45) | 12789 (14.08) | 33727 (12.92) | 2883 (11.77) | 29607 (16.81) | 19827 (14.66) | 9627 (15.59) |
| Overall | 90905 (14.11) | 48517 (13.78) | 86271 (14.14) | 198456 (13.50) | 119507 (13.23) | 33013 (10.79) | 88276 (17.15) | 175586 (16.82) | 31939 (16.86) |
As sample sizes could have biased estimates of Shannon index even after simple corrections, we also analyzed the TCR repertoire using rarefaction curves plotting the number of unique species found in random subsamples of the total sequence population of all three BV families (Figure 3), which revealed the same relative pattern of TCR diversity being highest in CB, intermediate in uVL subjects, and least in control subjects (p = 0.018). These differences also held true for BV families considered individually (p = 0.041, Supplemental Figure 3).
Figure 3. Rarefaction analysis of TCR species in CD31+ naïve CD4+ T-cells.
Rarefaction curves are plotted for TCR sequences isolated from each sample, indicating that diversity is greater in the uVL subjects compared to uninfected control subjects (considering the number of species at x=9809, p=0.02).
As a third approach to confirming the diversity comparisons between groups, we also performed analyses using random subsamples 24,000 sequences (corresponding to the smallest sampling size, obtained for CB13) from each individual sequence set. The number of discrete sequences, Shannon index, and fraction of singletons (number of sequences observed only once divided by the total number of observed sequences) were assessed (Supplemental Table 5). Again, all parameters revealed the pattern of highest TCR diversity in CB followed by uVL, both greater than uninfected control persons (p<0.01). The HIV-1-infected individuals had overall more species and a higher fraction of singleton sequences. Additional analyses examining the abundance of rare and common sequences (Supplemental Figures 4 and 5) corroborated these results, supporting the overall conclusion that the recent thymic emigrant CD4+ T-cells of uVL subjects had a broader TCR repertoire than uninfected subjects.
DISCUSSION
Immune reconstitution following initiation of antiretroviral therapy of HIV-1 infection clearly differs between children and adults. In children, expansion of naïve T-cell populations begins soon after initiation of ART, whereas redistribution and expansion of memory T-cell populations initially predominate in adults, in whom increases in naïve CD4+ T-cells are typically seen only months after therapy [9, 11-15, 39]. To investigate the nature of these differences, we performed an extensive survey of thymic function markers and found evidence of robust thymopoiesis in long-term survivors of perinatal infection (>13 years) receiving ART, compared to healthy controls of similar age. The majority in the HIV-1 infected group had evidence of abundant thymic tissue and active thymopoiesis, with naïve CD4+ T-cell levels and markers (TREC) suggesting elevated production compared to uninfected persons. This was further supported by deep sequencing of TCRs in the naïve CD4+ T-cell population, which demonstrated not only preserved but enhanced diversity in these long term survivors of perinatal infection versus uninfected persons. These findings are consistent with previous studies of immune reconstitution during ART [12-16], and observations that “thymic rebound” (expansion of histologically normal thymic tissue occurring after illness, stress, and cancer chemotherapy) is more common in children than adults, likely underlying age-dependent recovery of lymphocyte populations after cancer chemotherapy[40]. Of note a recent report demonstrated that restoration of naïve T-cell populations may be impaired in adult individuals with advanced HIV-1 infection, possibly due to loss of the normal stromal fibroblastic reticular cell (FRC) network in lymphoid tissue [41]. Given the long average duration of infection (~17 years) and histories of AIDS-defining illness in more than half of our study participants, this underscores the likely importance of age in HIV-1-induced damage to secondary lymphoid tissues and/or its reversal during ART.
As a whole, the data presented above suggest a model in which HIV-1 replication (and/or secondary immune dysregulation) drives loss of memory CD4+ T-cells, leading to compensatory supranormal thymic output of naïve CD4+ T-cells (Figure 4) in these youths. Evidence of ongoing immune activation and replication is provided by the high fraction of CD38+ HLA-DR+ CD8+ T-cells (Figure 1K) [25] and the persistence of CD8+ T-cell responses to HIV (Figure 1L) which indicate ongoing HIV antigen production. We note that the persistence of broad antiviral responses to HIV during cART has previously been observed in other perinatally-infected persons and contrasts with the situation in older adults, in whom complete decay of these responses is common. [37, 42-45]
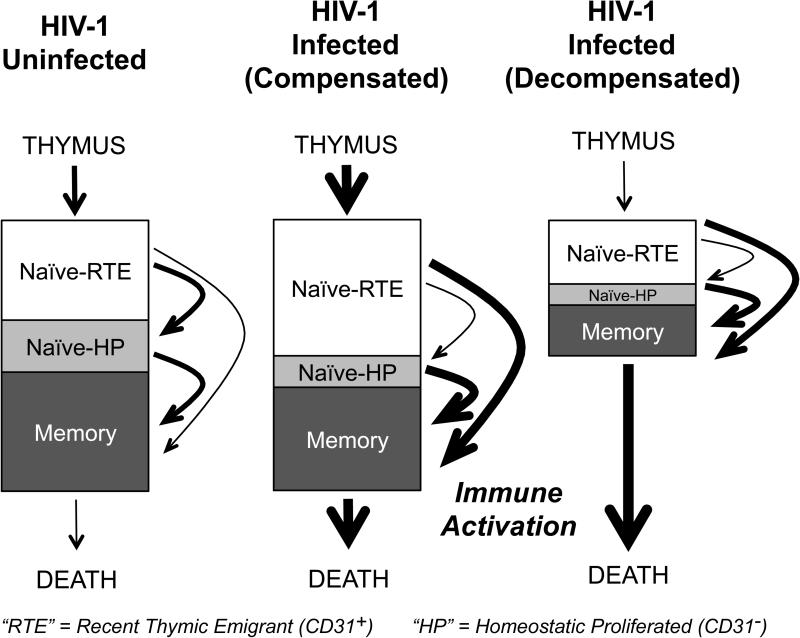
Figure 4. Schematic model of CD4+ T-cell homeostasis in long term survivors of perinatal HIV-1-infection.
Box depicts the partitioning of CD4+ T cells into three discrete populations following emigration from the thymus: recent thymic emigrant CD31+ T cells (Naïve-RTE) that have not undergone peripheral expansion, naïve T-cells that have undergone homeostatic proliferation (CD27+ CD31−), and memory (CD45RA−) cells. In the infected persons receiving ART, loss of memory (and possibly naïve) CD4+ T-cells is associated with enhanced thymopoiesis (thicker bold lines) and possibly less homeostatic proliferation in the naïve subset. The naïve T cell population is retained. In some individuals, this compensatory results in resulting in an increased fraction of Naïve RTE cells with relatively increased TREC content, and enrichment of TCR diversity.
The Naïve CD4+ T-cell population is composed of both CD31+ and CD31− CD45RA+ cells and homeostatic proliferation is thought to transform the former into the latter, resulting in a decrease in the concentration of TREC in CD4+ T-cells. [24, 46] Within the naïve CD4+ T-cell population, the population size of the recent thymic emigrant (CD31+ CD45RA+) CD4+ T-cells (Naïve RTE) is maintained in most individuals studied (Figure 1G), suggesting increased thymic output occurs to replace loss of naïve (CD27+CD45RA+) cells that differentiate into memory CD4+ T-cells. The hypothesis that supranormal thymopoiesis occurs is also supported by our observation of enrichment of TREC in CD4+ T cells of uVL subjects (Figure 1H) and the enhanced diversity of TCRs in the CD31+ CD4+ T-cell population observed by pyrosequencing.
TCR breadth is likely an important clinical factor, as severe AIDS-defining illnesses may occur years after blood CD4+ T-cell concentrations have “reconstituted” to seemingly safe levels in both children and adults receiving ART. [5-7] Our detailed analysis of TCR repertoire substantiates and extends prior studies of HIV-1-infected adults in whom ART does not generally restore CD4+ T-cell numbers to normal or fully normalize skewing of the TCR repertoire, as assessed by various tools ranging from relatively indirect to more precise measures of diversity such as CDR3 size distributions (“spectratyping” or “immunoscoping”), DNA hybridization kinetics (“Amplicot”), multiplex amplification of V-J segments, and CDR3 sequencing,[8, 23, 47-50] and one study in children/adolescents indicating that perturbations in TCR diversity of naïve cells begin to resolve within several months of therapy.[19] Because HIV-1 infection is typically associated with disrupted thymic architecture, involution of the thymic cortical epithelial space, and fibrosis of the peripheral lymph nodes that are required for expansion of thymic emigrants,[41, 51-53] the novel finding of substantially increased TCR breadth in the recent thymic emigrant CD4+ T-cell compartment of our HIV-1-infected subjects was surprising. Supporting this observation, recent trials of administering recombinant human IL-7 to infected persons on ART have demonstrated enhanced naïve CD4+ T-cell production accompanied by indirect measures of increased TCR diversity.[54]
Our study has several limitations, including its cross-sectional nature and the ~2.3 year average age difference between the groups of HIV-1-infected and control individuals. These concerns are mitigated by evidence that thymic architecture and function change little over this short age span [39, 51, 55, 56]. Moreover, we found no evidence of a correlation between age and the number of CD31+ T cells in any of the three groups studied (Controls, uVL or dVL individuals) (data not shown), consistent with earlier reports indicating that CD31+ T cells decrease less than 50% between 20 and 60 years of age.[24, 46] We also observed stability of thymopoiesis parameters over 1 to 3 years in our cohort (TREC, number and fraction of blood CD31+CD4+ T-cells, manuscript in preparation). Our assessment of TCR repertoire was limited to three BV families representing about 5% of naïve CD4+ T-cells, and may not reflect the total functional repertoire, although there is no reason to suspect BV family-specific differences. Despite these limitations, the composite data support the sanguine view that thymic function and naïve T-cell homeostasis may be restored by prolonged ART in adolescent and young adult survivors of perinatal infection.
Overall, our study suggests that thymic function is resilient in most persons, even~17 years after HIV-1 infection that occurred when immunologically immature. Despite prior clinically significant immunosuppression (including AIDS defining illness and conditions indicative of moderate immune deficiency), ART appears to allow recovery of an apparently adequate TCR repertoire in many survivors of perinatal infection who have reached young adulthood, which is encouraging in light of numerous studies showing damaging effects of HIV-1 on the thymus. This appears to differ from persons infected as adults, and it is unclear whether the difference is simply due to better age-related regenerative potential and immunologic reserve, or perhaps a difference in viral persistence or reservoirs specific to infection when immunologically immature. While the data are hopeful that long term survivors of perinatal infection are well compensated immunologically, there remain questions about whether the supraphysiologic TCR repertoire could in fact reflect an abnormality such as reduced stringency in thymic T-cell negative selection, and whether heightened thymic output will remain sustainable over longer periods of time if persistent HIV-1-driven memory CD4+ T-cell loss continues. Indeed there appeared to be some persons in our cohort with low memory and naïve CD4+ T-cell levels. Additional studies will be needed to determine the extent to which normalization of TCR diversity in and other T-cell parameters is indicative of true restoration of normal immune function in the setting of prolonged HIV-1 infection, and to examine the impact of detectable HIV replication and residual HIV-specific immune responses on these processes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Theodore Moore and Judith Currier for their service as members of a Data and Safety Monitoring Committee. HLA typing was performed by UCLA Immunogenetics Center.
FUNDING
This work was supported by NIH RO1 AI 51996 (PK) and the Eugene Cota-Robles Fellowship (CAS). Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA-16042 and AI-28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
Footnotes
AUTHORSHIP CONTRIBUTIONS
CRA-S helped design and carried out pyrosequencing studies and manuscript preparation. PK, MB, and JC were responsible for the organization of the cohorts, subject recruitment and specimen collection, interactions with institutional review boards, and manuscript preparation. PK also supervised all technical aspects of the study, apart from ELIspot and spectratyping. OY collaborated in design and implementation of the pyrosequencing studies and manuscript preparation, supervised spectratyping and ELISpot analysis. PC and DC performed flow cytometry and spectratyping studies. MIB supervised and interpreted CT scan analysis, blinded to subject status. NJ and PL helped analyze the pyrosequencing data.
CONFLICT OF INTEREST DISCLOSURES
CRA-S owns Roche stock.
REFERENCES
- 1.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 2.Frenkel LM, Wang Y, Learn GH, McKernan JL, Ellis GM, Mohan KM, et al. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J Virol. 2003;77:5721–5730. doi: 10.1128/JVI.77.10.5721-5730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 4.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komanduri KV, Donahoe SM, Moretto WJ, Schmidt DK, Gillespie G, Ogg GS, et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279:459–470. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 6.Zoufaly A, an der Heiden M, Kollan C, Bogner JR, Fatkenheuer G, Wasmuth JC, et al. Clinical outcome of HIV-infected patients with discordant virological and immunological response to antiretroviral therapy. J Infect Dis. 2011;203:364–371. doi: 10.1093/jinfdis/jiq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krogstad P, Patel K, Karalius B, Hazra R, MJ A, Oleske J, et al. Incomplete Immune Reconstitution Despite Virological Suppression in HIV-1 Infected Children and Adolescents. AIDS. 2015;29:11. doi: 10.1097/QAD.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum PD, Young JJ, Schmidt D, Zhang Q, Hoh R, Busch M, et al. Blood T-cell receptor diversity decreases during the course of HIV infection, but the potential for a diverse repertoire persists. Blood. 2012;119:3469–3477. doi: 10.1182/blood-2011-11-395384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 10.Giorgi JV, Majchrowicz MA, Johnson TD, Hultin P, Matud J, Detels R. Immunologic effects of combined protease inhibitor and reverse transcriptase inhibitor therapy in previously treated chronic HIV-1 infection. Aids. 1998;12:1833–1844. doi: 10.1097/00002030-199814000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Pakker NG, Notermans DW, de Boer RJ, Roos MT, de Wolf F, Hill A, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 12.Jankelevich S, Mueller BU, Mackall CL, Smith S, Zwerski S, Wood LV, et al. Long-term virologic and immunologic responses in human immunodeficiency virus type 1-infected children treated with indinavir, zidovudine, and lamivudine. J Infect Dis. 2001;183:1116–1120. doi: 10.1086/319274. [DOI] [PubMed] [Google Scholar]
- 13.Resino S, Bellon JM, Gurbindo D, Leon JA, Munoz-Fernandez MA. Recovery of T-cell subsets after antiretroviral therapy in HIV-infected children. Eur J Clin Invest. 2003;33:619–627. doi: 10.1046/j.1365-2362.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 14.Resino S, Seoane E, Perez A, Ruiz-Mateos E, Leal M, Munoz-Fernandez MA. Different profiles of immune reconstitution in children and adults with HIV-infection after highly active antiretroviral therapy. BMC Infect Dis. 2006;6:112. doi: 10.1186/1471-2334-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibb DM, Newberry A, Klein N, de Rossi A, Grosch-Woerner I, Babiker A. Immune repopulation after HAART in previously untreated HIV-1-infected children. Paediatric European Network for Treatment of AIDS (PENTA) Steering Committee. Lancet. 2000;355:1331–1332. doi: 10.1016/s0140-6736(00)02117-6. [DOI] [PubMed] [Google Scholar]
- 16.Sleasman JW, Nelson RP, Goodenow MM, Wilfret D, Hutson A, Baseler M, et al. Immunoreconstitution after ritonavir therapy in children with human immunodeficiency virus infection involves multiple lymphocyte lineages. J Pediatr. 1999;134:597–606. doi: 10.1016/s0022-3476(99)70247-7. [DOI] [PubMed] [Google Scholar]
- 17.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 18.Lee JC, Boechat MI, Belzer M, Church JA, De Ville J, Nielsen K, et al. Thymic volume, T-cell populations, and parameters of thymopoiesis in adolescent and adult survivors of HIV infection acquired in infancy. Aids. 2006;20:667–674. doi: 10.1097/01.aids.0000216366.46195.81. [DOI] [PubMed] [Google Scholar]
- 19.Yin L, Kou ZC, Rodriguez C, Hou W, Goodenow MM, Sleasman JW. Antiretroviral therapy restores diversity in the T-cell receptor Vbeta repertoire of CD4 T-cell subpopulations among human immunodeficiency virus type 1-infected children and adolescents. Clin Vaccine Immunol. 2009;16:1293–1301. doi: 10.1128/CVI.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohler T, Walcher J, Holzl-Wenig G, Geiss M, Buchholz B, Linde R, et al. Early effects of antiretroviral combination therapy on activation, apoptosis and regeneration of T cells in HIV-1-infected children and adolescents. Aids. 1999;13:779–789. doi: 10.1097/00002030-199905070-00006. [DOI] [PubMed] [Google Scholar]
- 21.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang OO, Church J, Kitchen CM, Kilpatrick R, Ali A, Geng Y, et al. Genetic and stochastic influences on the interaction of human immunodeficiency virus type 1 and cytotoxic T lymphocytes in identical twins. J Virol. 2005;79:15368–15375. doi: 10.1128/JVI.79.24.15368-15375.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 26.Ibarrondo FJ, Anton PA, Fuerst M, Ng HL, Wong JT, Matud J, et al. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–4297. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halnon NJ, Jamieson B, Plunkett M, Kitchen CM, Pham T, Krogstad P. Thymic function and impaired maintenance of peripheral T cell populations in children with congenital heart disease and surgical thymectomy. Pediatr Res. 2005;57:42–48. doi: 10.1203/01.PDR.0000147735.19342.DE. [DOI] [PubMed] [Google Scholar]
- 28.Pham T, Belzer M, Church JA, Kitchen C, Wilson CM, Douglas SD, et al. Assessment of thymic activity in human immunodeficiency virus-negative and -positive adolescents by real-time PCR quantitation of T-cell receptor rearrangement excision circles. Clin Diagn Lab Immunol. 2003;10:323–328. doi: 10.1128/CDLI.10.2.323-328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiff A, Krogstad P, Moore S, Shaham B, Parkman R, Kitchen C, et al. Study of thymic size and function in children and adolescents with treatment refractory systemic sclerosis eligible for immunoablative therapy. Clin Immunol. 2009;133:295–302. doi: 10.1016/j.clim.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Misrahi M, Teglas JP, N'Go N, Burgard M, Mayaux MJ, Rouzioux C, et al. CCR5 chemokine receptor variant in HIV-1 mother-to-child transmission and disease progression in children. French Pediatric HIV Infection Study Group. JAMA. 1998;279:277–280. doi: 10.1001/jama.279.4.277. [DOI] [PubMed] [Google Scholar]
- 31.Balamurugan A, Ng HL, Yang OO. Rapid T cell receptor delineation reveals clonal expansion limitation of the magnitude of the HIV-1-specific CD8+ T cell response. J Immunol. 2010;185:5935–5942. doi: 10.4049/jimmunol.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huse SM, Huber JA, Morrison HG, Sogin ML, Welch DM. Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol. 2007;8:R143. doi: 10.1186/gb-2007-8-7-r143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo C, Tsementzi D, Kyrpides N, Read T, Konstantinidis KT. Direct comparisons of Illumina vs. Roche 454 sequencing technologies on the same microbial community DNA sample. PLoS One. 2012;7:e30087. doi: 10.1371/journal.pone.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valiant G, Valiant P. Estimating the Unseen:Improved Estimators for Entropy and other Properties. Advances in Neural Information Processing Systems. 2013:R143. [Google Scholar]
- 35.Niu B, Fu L, Sun S, Li W. Artificial and natural duplicates in pyrosequencing reads of metagenomic data. BMC Bioinformatics. 2010;11:187. doi: 10.1186/1471-2105-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrington M, O'Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 37.Frahm N, Korber BT, Adams CM, Szinger JJ, Draenert R, Addo MM, et al. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol. 2004;78:2187–2200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balamurugan A, Lewis MJ, Kitchen CM, Robertson MN, Shiver JW, Daar ES, et al. Primary human immunodeficiency virus type 1 (HIV-1) infection during HIV-1 Gag vaccination. J Virol. 2008;82:2784–2791. doi: 10.1128/JVI.01720-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris JM, Hazenberg MD, Poulin JF, Higuera-Alhino D, Schmidt D, Gotway M, et al. Multiparameter evaluation of human thymic function: interpretations and caveats. Clin Immunol. 2005;115:138–146. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 41.Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ching N, Yang OO, Deville JG, Nielsen-Saines K, Ank BJ, Sim MS, et al. Pediatric HIV- 1-specific cytotoxic T-lymphocyte responses suggesting ongoing viral replication despite combination antiretroviral therapy. Pediatr Res. 2007;61:692–697. doi: 10.1203/pdr.0b013e31805365ef. [DOI] [PubMed] [Google Scholar]
- 43.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegel HM, DeFalcon E, Ogg GS, Larsson M, Beadle TJ, Tao P, et al. Changes in frequency of HIV-1-specific cytotoxic T cell precursors and circulating effectors after combination antiretroviral therapy in children. J Infect Dis. 1999;180:359–368. doi: 10.1086/314867. [DOI] [PubMed] [Google Scholar]
- 46.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baum PD, McCune JM. Direct measurement of T-cell receptor repertoire diversity with AmpliCot. Nat Methods. 2006;3:895–901. doi: 10.1038/NMETH949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Killian MS, Monteiro J, Matud J, Hultin LE, Hausner MA, Yang OO, et al. Persistent alterations in the T-cell repertoires of HIV-1-infected and at-risk uninfected men. Aids. 2004;18:161–170. doi: 10.1097/00002030-200401230-00004. [DOI] [PubMed] [Google Scholar]
- 49.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci U S A. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connors M, Kovacs JA, Krevat S, Gea-Banacloche JC, Sneller MC, Flanigan M, et al. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat Med. 1997;3:533–540. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 51.Haynes BF, Hale LP. Thymic function, aging, and AIDS. Hosp Pract (Minneap) 1999;34:59–60. 63–55, 69–70. doi: 10.3810/hp.1999.03.134. passim. [DOI] [PubMed] [Google Scholar]
- 52.Ho Tsong Fang R, Colantonio AD, Uittenbogaart CH. The role of the thymus in HIV infection: a 10 year perspective. Aids. 2008;22:171–184. doi: 10.1097/QAD.0b013e3282f2589b. [DOI] [PubMed] [Google Scholar]
- 53.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo- controlled, multicenter study. Clin Infect Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 56.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.