Abstract
Objectives
Autoantibodies reactive with Ro52 (tripartite motif (TRIM) containing protein 21 [TRIM21]) are detected in 70% of primary Sjögren’s syndrome (pSjS) patients. TRIM21 belongs to a 34 member C-IV family of TRIM proteins. Although autoantibodies against other TRIM proteins within the C-IV family have been detected in the sera of pSjS patients, their clinical relevance remains unclear. This study investigates the frequency of anti-TRIM38 in pSjS patients and evaluates its association with different clinical measures of the disease.
Methods
Serum samples from pSjS patients (n=235) and controls (n=50) were analyzed for reactivity to in vitro transcribed and translated 35S-Met-TRIM38 protein. The association of anti-TRIM38 with different laboratory and clinical measures of pSjS were evaluated. Reactivity of anti-TRIM38 with different structural domains of TRIM38 was analyzed. Affinity purified anti-TRIM38 antibodies were used to immunoprecipitate TRIM21.
Results
TRIM38 reactive autoantibodies were detected in the sera of 24/235 pSjS patients and in 2/50 controls. Anti-TRIM38 positivity was significantly associated with the presence of anti-Ro60, anti-Ro52, anti-La, rheumatoid factor and hypergammaglobulinemia. Clinically, anti-TRIM38 was associated with significantly higher ocular surface staining scores, lower Schirmer’s test scores and minor labial salivary gland biopsy focus scores of ≥3.0. Anti-TRIM38 antibodies mainly recognized the Cortactin-binding protein-2 (CortBP-2, amino acids 128–238) and the B30.2/SPRY (amino acids 268–465) domains on TRIM38. Affinity purified antibodies to TRIM38-CortBP2 and TRIM38-B30.2/SPRY domains reacted with TRIM21.
Conclusion
Our data demonstrate that anti-TRIM38 specificity arising in a subset of pSjS patients is associated with higher severity of pSjS.
Primary Sjögren’s syndrome (pSjS) is a chronic autoimmune disorder affecting the exocrine salivary and lacrimal glands (1). The glandular dysfunction in this disorder leads to reduced fluid secretion that manifests as the dry mouth and dry eye symptoms. Another characteristic feature of pSjS is the presence of circulating autoantibodies reactive with multiple cellular proteins such as Ro/SSA and La/SSB. Amongst the two Ro/SSA antigens, Ro60 and Ro52, autoantibodies reactive with Ro52 are present in almost 70% of pSjS patients and they are associated with a higher severity of disease (2). Our recent work in an experimental mouse model demonstrates that in conjunction with innate immunity activation, anti-Ro52 can directly induce salivary gland dysfunction (3).
Apart from pSjS, anti-Ro52 are also present in patients with systemic lupus erythematosus (SLE), dermatomyositis, polymyositis, systemic sclerosis, primary biliary cirrhosis, autoimmune hepatitis type I and mothers of children with congenital heart block (CHB) (4). How immune responses to Ro52 exert pathogenic effects in such a diverse group of autoimmune disorders, involving different organ systems is not clear.
Ro52 belongs to a large family of tripartite motif (TRIM) containing proteins and is denoted as TRIM21 (5). In humans, almost 70 or more TRIM proteins have been described (6). Structurally, the TRIM proteins share a highly conserved N-terminal, comprised of the RING, B-box and Coiled-coil domains. Based on the C-terminal domain sequence, the TRIM proteins have been classified into 11 different families. TRIM21 belongs to the C-IV family with 34 member proteins.
Considering the significant structural and sequence similarities between different C-IV family TRIM proteins, we hypothesized that autoantibodies reactive with other TRIM proteins are present in patients with autoimmune disease and these autoantibodies influence the diverse clinical presentations attributed to anti-TRIM21. Indeed, autoantibody responses against SS-56/TRIM68 were detected in SjS and SLE patients, and they were associated with visceral complications in SLE (7). Since recombinant Ro60 and TRIM21 did not competitively inhibit the binding of antibodies to TRIM68, it was concluded that anti-TRIM68 is a distinct autoantibody specificity. In another study with a limited number of samples (n=8), sera from anti-TRIM21 positive pSjS patients reacted with RNF15 protein, which is now known as TRIM38 (8). Since TRIM21 and TRIM38 share a 40% sequence homology, it has been suggested that the autoantibodies might recognize both cross-reactive and specific B cell epitopes on these proteins. However, to date, this has not been experimentally tested and the clinical relevance of anti-TRIM38 autoantibody in pSjS has not been explored.
In the present study, using serum samples from a pSjS patient population, we have determined the frequency of anti-TRIM38 and evaluated their association with different clinical measures of pSjS.
Material and Methods
Patients
All procedures were approved by the Oklahoma Medical Research Foundation (OMRF) Institutional Review Board. Serum samples from 235 pSjS patients and 50 verified controls from the Oklahoma Sjögren’s Syndrome Center of Research Translation (OSSCORT) were used for autoantibody analysis. The pSjS classification was based on the American European Consensus Group (AECG) criteria (9). Clinical laboratory data for age, anti-Ro60, anti-Ro52, anti-La, rheumatoid factor (RF), hypocomplementemia, hypergammaglobulinemia, ocular surface staining/van Bijsterveld (vB) scores, Schirmer’s test (mm/5 min), minor salivary gland biopsy, and whole unstimulated saliva flow (WUSF, ml/15 min) were obtained from the OSSCORT database. As described by Vitali et al (9), the most abnormal score from either eye was used for the analysis of dry eye (lowest score in the case of the Schirmer’s test and the highest for the vB score). The patient cohort and data collection methods have been described in detail previously (10). Serum samples from SLE patients used as positive controls were obtained from the OMRF Autoimmune Disease Institute Biorepository Core.
Autoantibody Analysis
35S-Methionine labeled full length TRIM38 protein (NM_006355, GeneCopoeia, Rockville, MD, USA) with no extraneous tags and TRIM21-GST protein (BC010861, DNASU/PSI:Biology-MR, Tempe, AZ, USA) were generated by using the TNT® Quick coupled transcription/translation system (Promega, Madison, WI, USA). Anti-TRIM38 reactivity was analyzed by a quantitative immunoprecipitation assay as described previously for anti-Ro52 (3). Antibody reactivity to TRIM38 was normalized against the positive control (set at 100 antibody units/ml). Mean + 2SD antibody units in the verified control sera (n=50) were used as the cut-off for anti-TRIM38 positivity.
Characterization of TRIM38 reactive antibodies
Different structural domains of TRIM21 and TRIM38 were PCR cloned in to the pT7-IRES Myc-N vector (TaKaRa Clontech, Mountain View, CA, USA) and then used for generating the in vitro transcribed, translated, and 35S-Met labeled proteins. Quantitative immunoprecipitation assay was used for the analysis of antibody reactivity. The amino acid boundaries for different domains of TRIM38 were: 16–129 (RING and B-box together), 133–238 (CortBP2) and 274–455 (B30.2/SPRY), and for TRIM21 they were 16–123 (RING and B-box together), 128–238 (Coiled coil) and 268–465 (B30.2/SPRY) (supplemental figure 1).
To analyze antibody cross reactivity between TRIM38 and TRIM21, the full length human TRIM38 cDNA, CortBP2 domain and B30.2/SPRY domain were PCR cloned into the pMAL-c5E vector (New England BioLabs, Ipswich, MA) to generate respective maltose-binding protein (MBP) fusion proteins. These recombinant proteins were purified under native conditions and coupled to the Amino Link coupling resin (Thermo Scientific, Grand Island, NY, USA) by following manufacturer’s instructions. TRIM38 reactive antibodies from pSjS patient sera were affinity purified and used to immunoprecipitate TRIM38 and TRIM21.
Statistical Methods
To compare differences in autoantibody levels between pSjS patients and controls, the Mann-Whitney test was used. Fisher’s exact test was performed to determine the association of TRIM38 positivity with different clinical laboratory parameters in pSjS patients. A p Value<0.05 at 95% confidence interval was considered significant. Graph Pad Prism software was used to perform these tests. Multiple regression analysis was performed using generalized linear models (GLMs) via the stats package in R. Simple linear regression was used to select significant covariates (α = 0.05) for inclusion in the multiple regression models. Each model was generated using backwards selection from a saturated model including covariates for age, disease duration, hypergammaglobulinemia, hypocomplementemia, RF, and presence of autoantibodies to La, Ro52, and Ro60. With the exception of age, all covariates retained in the final models had significant main effects.
Results
Anti-TRIM38 positivity is associated with higher severity of pSjS
Serum samples from 235 pSjS patients and 50 controls were analyzed for the presence of anti-TRIM38 autoantibodies by using a quantitative immunoprecipitation assay (figure 1, top panel). Based on this method, anti-TRIM38 autoantibodies were detected in 24/235 (10.21%) of pSjS patients and in 2/50 (4%) of controls.
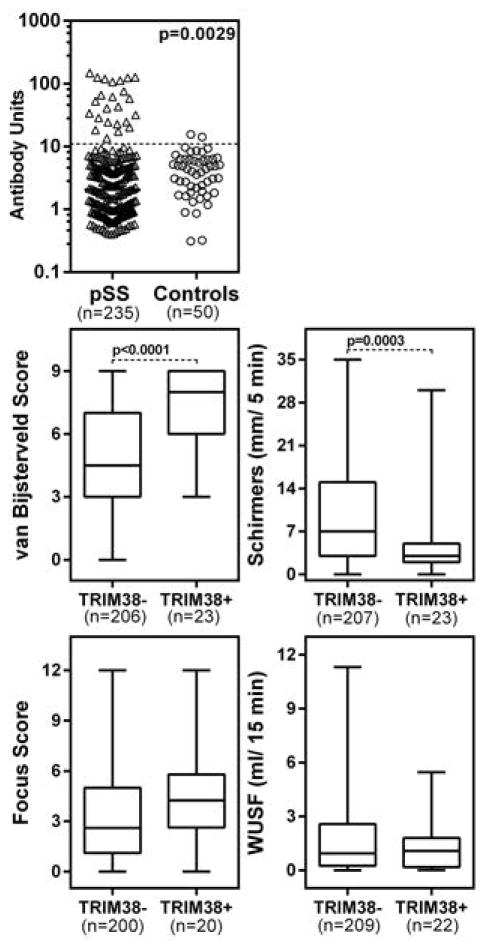
Figure 1. Anti-TRIM38 autoantibody levels in pSjS patients and their association with clinical measures of dry eye and dry mouth.
Top panel: Sera from 235 pSjS patients and 50 verified controls were tested for reactivity with in vitro transcribed, translated, and 35S-Met labeled TRIM38. Data are shown as antibody units based on a positive control pool from anti-TRIM38 positive lupus patients. The dotted line shows the cut-off for positivity, based on Mean+2SD of verified control sera. All samples above the cut-off were considered positive. Based on the reactivity with TRIM38, the pSjS population was split into 2 groups, anti-TRIM38+ (n=24) and anti-TRIM38− (n=211) and clinical measurements for dry eye (middle panel: vB score, Schirmer’s test scores) and dry mouth (bottom panel: minor labial salivary gland focus score and WUSF) were compared between these 2 groups. Statistical significance was determined by the Mann-Whitney test.
To determine the clinical significance of anti-TRIM38, the association of anti-TRIM38 positivity with different clinical measurements for dry eye (ocular surface staining and Schirmer’s test scores) and dry mouth (WUSF and minor salivary gland biopsy scores) were evaluated. The anti-TRIM38 positive patients showed significantly higher vB scores and lower Schirmer’s test scores (figure 1, middle panel). The anti-TRIM38 positive patients showed a higher trend of minor labial salivary gland focus scores, however, the differences were statistically not significant (figure 1, bottom panel). A further analysis of the association between anti-TRIM38 positivity with focus scores of ≥3.0, which would be representative of severe sialoadenitis was carried out. The proportion of pSjS patients with focus scores ≥3.0 was significantly higher in anti-TRIM38 positive patients in comparison with anti-TRIM38 negative patients (0.75 versus 0.465, p=0.0185). The difference in WUSF values between anti-TRIM38 positive and negative patients was statistically not significant.
To determine whether anti-TRIM38 positivity was associated with other autoantibody specificities, the OSSCORT database was analyzed for anti-Ro52, anti-Ro60, anti-La, RF, hyper IgG, hyper IgM, hyper IgA and hypocomplementemia (Table 1). The presence of anti-TRIM38 was significantly associated with anti-Ro52 (p<0.0001), anti-Ro60 (p=0.0001), anti-La (p<0.0001), RF (p<0.0001), hyper IgG (p=0.0007) and hyper IgA (p=0.0201). Anti-TRIM38 positivity was not associated with age (at the time of inclusion in the study), hyper IgM, and hypocomplementemia.
Table 1.
Demographics and clinical laboratory data in anti-TRIM38 positive and negative pSjS patients
| Total patients | anti-TRIM38 + | anti-TRIM38 − | p Value | |
|---|---|---|---|---|
| Age Years (mean ± SD) | 56.35±13.67 | 56.67±10.79 | 56.32±13.98 | 0.9269* |
| Females | 213/235 (90.63) | 22/24 (91.66) | 191/211 (90.52) | 1 |
| anti-Ro52 | 125/215 (58.13) | 23/24 (95.83) | 102/191 (53.40) | <0.0001 |
| anti-Ro60 | 130/232 (56.03) | 22/24 (91.66) | 108/208 (51.92) | 0.0001 |
| anti-La | 92/234 (39.31) | 20/24 (83.33) | 71/210 (33.80) | <0.0001 |
| RF | 86/234 (36.75) | 21/24 (87.5) | 65/210 (30.95) | <0.0001 |
| Hyper IgG | 71/234 (30.34) | 15/24 (62.5) | 56/154 (26.66) | 0.0007 |
| Hyper IgM | 39/234 (16.66) | 5/24 (20.83) | 34/210 (16.19) | 0.5653 |
| Hyper IgA | 42/234 (17.94) | 9/24 (37.5) | 33/210 (15.71) | 0.0201 |
| Hypocomplementemia | 143/234 (61.11) | 17/24 (70.83) | 126/210 (60.00) | 0.3794 |
All fractions indicate positive patients out of the number of patients on whom the data was available in the OSSCORT database.
The numbers in parenthesis indicate % positive.
Statistical significance determined by Two-tailed Fisher’s exact test and a p Value <0.05 was considered significant.
Mann-Whitney test.
Anti-TRIM38 antibodies recognize 2 structural domains of TRIM38
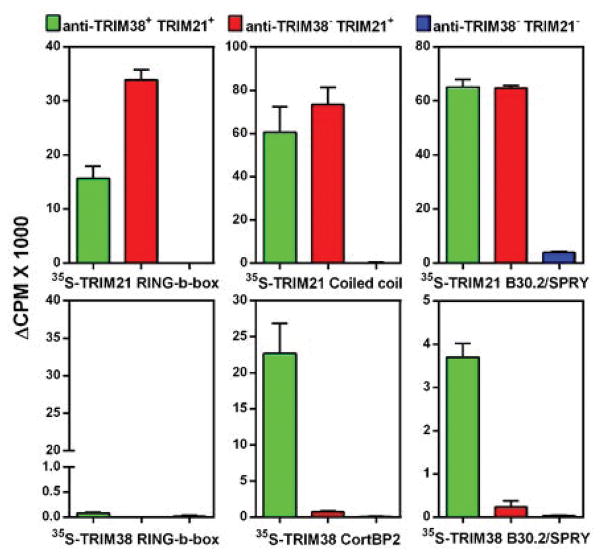
Different structural domains (RING, B-box, Coiled coil, B30.2/SPRY) on TRIM21 have been shown to harbor multiple B cell epitopes (summarized in supplemental figure 2). Thus, 4 structural domains of TRIM38 (RING and B-box together, CortBP2 and B30.2/SPRY) were generated by in vitro transcription and translation and their reactivity with anti-TRIM38 positive sera assessed. Anti-TRIM38 immunoprecipitated the CortBP2 domain and the B30.2/SPRY domain but not the RING-B-box domains (figure 2). In contrast, all domains of TRIM21 were reactive with antibodies from anti-TRIM21 positive sera. Such reactivity was not observed in sera from anti-TRIM38 and anti-TRIM21 negative pSjS patients. Although anti-TRIM38 antibodies affinity purified with the whole recombinant MBP-TRIM-38 only immunoprecipitated TRIM38 (supplemental figure 3A), affinity purified anti-TRIM38-CortBP2 and anti-TRIM38-B30.2/SPRY domain antibodies immunoprecipitated both TRIM38 and TRIM21 (supplemental figure 3B).
Figure 2. Characterization of antibody reactivity with different structural domains of TRIM21 and TRIM38.
Reactivity of sera from 3 different groups of pSjS patients (anti-TRIM38+ anti-TRIM21+, anti-TRIM38− anti-TRIM21+, anti-TRIM38− anti-TRIM21−) against different structural domains of TRIM21 (top panel) and TRIM38 (bottom panel) were evaluated in a quantitative immunoprecipitation assay. The data are presented as mean ΔCPM (CPM with antibody-CPM without antibody) + SD from 2 independent experiments.
Discussion
Using serum samples from a well characterized cohort of pSjS patients, this study shows that autoantibodies targeting the TRIM38 protein were present in 10.21% (24/235) of pSjS patients and their presence was associated with overall higher severity of disease.
Amongst the 4 structural domains present on TRIM38, anti-TRIM38 recognized the CortBP2 domain and the C-terminal B30.2/SPRY domain. Antibody reactivity to the N-terminal domains (RING and B-box expressed together), was not observed. This reactivity pattern was distinct from anti-TRIM21, which recognized all structural domains of TRIM21 (figure 2). The Coiled coil and the B30.2/SPRY domains of TRIM21 harbor multiple B cell epitopes (supplemental figure 2). Since the corresponding regions on TRIM38 show considerable homology, antibodies from anti-TRIM38 positive sera were affinity purified and their reactivity with whole TRIM21 was evaluated. Antibodies affinity purified using TRIM38 domains CortBP2 and B30.2/SPRY, immunoprecipitated both TRIM38 and TRIM21, indicating cross-reactivity. However, antibodies affinity purified using whole TRIM38 only reacted with TRIM38. They did not immunoprecipitate TRIM21. The discrepancy in the reactivity observed between the 2 populations of affinity purified anti-TRIM38 antibodies might be due to the different conformations of recombinant MBP-fusion proteins employed for affinity purification. Thus, the TRIM21 and TRIM38 cross reactive antibodies are readily purified when individual domains are employed for making the affinity columns, and not when the whole MBP-TRIM38 is used. Regardless of the recombinant protein used as affinity matrix, the affinity purified antibodies recognize the in vitro transcribed and translated TRIM38, made by using the mammalian translational machinery. Overall these data suggest that TRIM38 reactive antibodies in pSjS patients consist of both TRIM 21 cross-reactive as well as TRIM38 specific antibodies.
A previous study characterizing immune responses against Ro52 had reported that autoantibodies to TRIM38 were present in 5/8 anti-TRIM21 positive pSjS patients (8). The difference in the incidence of anti-TRIM38 reactivity between this report and our study might be due to the differences in patient cohorts (UK versus USA) or due to the fortuitous selection of serum samples. Clearly this discrepancy can only be addressed by screening additional pSjS samples in the UK as well as in other patient cohorts. Regardless, considered together, these studies establish that TRIM38 is a target for autoantibodies in pSjS.
In pSjS, the presence of anti-TRIM21 has been linked to higher disease severity (11, 12). Our data demonstrates that anti-TRIM38 positivity was significantly associated with the presence of autoantibodies to Ro52, Ro60, and La, RF and hypergammaglobulinemia. Amongst the anti-TRIM38 positive patients, 80% were also positive for anti-Ro52, anti-Ro60, anti-La, and RF. In contrast, only 19% of anti-TRIM38 negative patients, had the simultaneous presence of these autoantibodies.
Clinically, with respect to the salivary gland disease, the differences in WUSF in anti-TRIM38 positive and anti-TRIM38 negative patients were statistically not significant. However, anti-TRIM38 reactivity was significantly associated with severe sialoadenitis (focus score >3.0). Anti-TRIM38 positive patients had significantly higher vB scores and lower Schirmer’s test scores. After adjusting for age, anti-Ro60, and RF in a multiple regression analysis, the anti-TRIM38 positivity by itself was still significantly associated (p=0.021) with higher vB scores (supplemental Table 1). The presence of RF, anti-Ro60 and age by themselves were also significantly associated with higher vB score. Interestingly, age was the only significant factor influencing Schirmer’s test score in our model.
Dry eye is a multifactorial disease involving tears and the ocular surface (13). At present the mechanisms responsible for the higher severity of ocular surface staining scores in TRIM38 positive pSjS patients are not known. Whether anti-TRIM38 contributes towards ocular surface damage or is a consequence of heightened autoimmune response is not known. TRIM38 is an E3 ubiquitin ligase and it has been shown to regulate inflammatory responses triggered by TNFα and IL-1B, as well as the IFN-β responses induced via TLR3 engagement (14, 15). Using an experimental mouse model system, we have recently demonstrated that mouse TRIM21 reactive antibodies penetrate live salivary gland cells (in vitro) (3). Thus, the possibility that anti-TRIM38 penetrate live cells and cause dysregulated inflammatory response exists. Clearly, future experiments measuring anti-TRIM38 in tears and saliva might provide clues regarding the participation of this and other autoantibody specifities in contributing towards ocular surface damage.
In summary, our study clearly establishes that TRIM38 is a valid target for autoantibodies in pSjS and anti-TRIM38 positivity is associated with higher severity of pSjS.
Supplementary Material
The amino acid boundaries for structural domains of TRIM21 are based on information from UniProt (http://www.uniprot.org/uniprot/P19474#structure). For TRIM38, they are based on information from UniProt (http://www.uniprot.org/uniprot/O00635#structure) and NCBI (http://www.ncbi.nlm.nih.gov/protein/O00635.1). CortBP2: Cortactin-binding protein-2.
Sequence alignment was performed by using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Identical amino acids are denoted by dots and homologous substitutions are marked by “+”. Dashes indicate spacing inserted for alignment. The B cell epitope boundaries are color coded and are based on data summarized by Wahren-Herlenius et al Immunology Today, 20:234, 1999, and by Routsias and Tzioufas, J Autoimmunity, 35:256, 2010. For example in B cell epitope 230–239, amino acid at positions 230 (S) and 239 (S) are highlighted in red. Following epitopes are highlighted in this figure: 14–54, 55–125, 125–196, 136–227, 138–340, 169–291, 190–245, 197–232, 216–292, 230–239, and 277–292. TRIM21 domains (RING, B-box, Coiled coil and B30.2/SPRY) are depicted on the right. Note that several epitopes have been mapped in the Coiled coil domain of TRIM21.
Left Panel: Reactivity of affinity purified anti-TRIM38 antibodies with TRIM38 and TRIM21. Right Panel: Reactivity of affinity purified anti-TRIM38 B30.2/SPRY and CortBP2 domain antibodies with TRIM38 and TRIM21. Data are shown as mean ΔCPM (ΔCPM= CPM for sample - CPM for beads only).
Supplemental Table 1. Multiple regression analysis to assess the effects of anti-TRIM38 on clinical measures of dry eye.
Acknowledgments
Funding Support: Oklahoma Medical Research Foundation, National Institutes of Health.
The authors thank all individuals who participated in the study and the clinical staff and OSSCORT personnel. The lupus samples were kindly provided by the OMRF Autoimmune Disease Institute Biorepository Core. This study was supported by funding from OMRF (USD), and grants from the National Institutes of Health, P50AR060804 (KLS); U54GM104938, P30AR053483, P30GM103510, U19AI082714 (JJ).
Footnotes
Disclosure: None of the authors have any conflicts of interest.
References
- 1.Mavragani CP, Moutsopoulos HM. Sjögren’s syndrome. Annu Rev Pathol. 2014;9:273–85. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 2.Kyriakidis NC, Kapsogeorgou EK, Tzioufas AG. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J Autoimmun. 2014;51:67–74. doi: 10.1016/j.jaut.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Szczerba BM, Kaplonek P, Wolska N, Podsiadlowska A, Rybakowska PD, Dey P, et al. Interaction between innate immunity and Ro52-induced antibody causes Sjögren’s syndrome-like disorder in mice. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menéndez A, Gómez J, Escanlar E, Caminal-Montero L, Mozo L. Clinical associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies: Diagnostic utility of their separate detection. Autoimmunity. 2013;46:32–9. doi: 10.3109/08916934.2012.732131. [DOI] [PubMed] [Google Scholar]
- 5.Espinosa A, Zhou W, Ek M, Hedlund M, Brauner S, Popovic K, et al. The Sjogren’s syndrome-associated autoantigen Ro52 is an E3 ligase that regulates proliferation and cell death. J Immunol. 2006;176:6277–85. doi: 10.4049/jimmunol.176.10.6277. [DOI] [PubMed] [Google Scholar]
- 6.Meroni G. Genomics and evolution of the TRIM gene family. Adv Exp Med Biol. 2012;770:1–9. doi: 10.1007/978-1-4614-5398-7_1. [DOI] [PubMed] [Google Scholar]
- 7.Billaut-Mulot O, Cocude C, Kolesnitchenko V, Truong MJ, Chan EK, Hachula E, et al. SS-56, a novel cellular target of autoantibody responses in Sjögren syndrome and systemic lupus erythematosus. J Clin Invest. 2001;108:861–9. doi: 10.1172/JCI13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes DA, Ihrke G, Reinicke AT, Malcherek G, Towey M, Isenberg DA, et al. The 52 000 MW Ro/SS-A autoantigen in Sjögren’s syndrome/systemic lupus erythematosus (Ro52) is an interferon-gamma inducible tripartite motif protein associated with membrane proximal structures. Immunology. 2002;106:246–56. doi: 10.1046/j.1365-2567.2002.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitali C, Bombardieri S, Jonsson R, et al. European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen A, Ice JA, Li H, Grundahl K, Kelly JA, Radfar L, et al. Comparison of the American-European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann Rheum Dis. 2014;73:31–8. doi: 10.1136/annrheumdis-2013-203845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Retamozo S, Akasbi M, Brito-Zerón P, Bosch X, Bove A, Perez-de-Lis M, et al. Anti-Ro52 antibody testing influences the classification and clinical characterisation of primary Sjögren’s syndrome. Clin Exp Rheumatol. 2012;30:686–92. [PubMed] [Google Scholar]
- 12.Hu MM, Yang Q, Zhang J, Liu SM, Zhang Y, Lin H, et al. TRIM38 inhibits TNFα- and IL-1β-triggered NF-κB activation by mediating lysosome-dependent degradation of TAB2/3. Proc Natl Acad Sci U S A. 2014;111:1509–14. doi: 10.1073/pnas.1318227111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue Q, Zhou Z, Lei X, Liu X, He B, Wang J, Hung T. TRIM38 negatively regulates TLR3-mediated IFN-β signaling by targeting TRIF for degradation. PLoS One. 2012;7(10):e46825. doi: 10.1371/journal.pone.0046825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun. 2012;39:77–82. doi: 10.1016/j.jaut.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Tincani A, Andreoli L, Cavazzana I, Doria A, Favero M, Fenini MG, et al. Novel aspects of Sjögren’s syndrome in 2012. BMC Med. 2013;11:93. doi: 10.1186/1741-7015-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The amino acid boundaries for structural domains of TRIM21 are based on information from UniProt (http://www.uniprot.org/uniprot/P19474#structure). For TRIM38, they are based on information from UniProt (http://www.uniprot.org/uniprot/O00635#structure) and NCBI (http://www.ncbi.nlm.nih.gov/protein/O00635.1). CortBP2: Cortactin-binding protein-2.
Sequence alignment was performed by using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Identical amino acids are denoted by dots and homologous substitutions are marked by “+”. Dashes indicate spacing inserted for alignment. The B cell epitope boundaries are color coded and are based on data summarized by Wahren-Herlenius et al Immunology Today, 20:234, 1999, and by Routsias and Tzioufas, J Autoimmunity, 35:256, 2010. For example in B cell epitope 230–239, amino acid at positions 230 (S) and 239 (S) are highlighted in red. Following epitopes are highlighted in this figure: 14–54, 55–125, 125–196, 136–227, 138–340, 169–291, 190–245, 197–232, 216–292, 230–239, and 277–292. TRIM21 domains (RING, B-box, Coiled coil and B30.2/SPRY) are depicted on the right. Note that several epitopes have been mapped in the Coiled coil domain of TRIM21.
Left Panel: Reactivity of affinity purified anti-TRIM38 antibodies with TRIM38 and TRIM21. Right Panel: Reactivity of affinity purified anti-TRIM38 B30.2/SPRY and CortBP2 domain antibodies with TRIM38 and TRIM21. Data are shown as mean ΔCPM (ΔCPM= CPM for sample - CPM for beads only).
Supplemental Table 1. Multiple regression analysis to assess the effects of anti-TRIM38 on clinical measures of dry eye.