Abstract
DNA catalysts (deoxyribozymes) for a variety of reactions have been identified by in vitro selection. However, for certain reactions this identification has not been achieved. One important example is DNA-catalyzed amide hydrolysis, for which a previous selection experiment instead led to DNA-catalyzed DNA phosphodiester hydrolysis. Subsequent efforts in which the selection strategy deliberately avoided phosphodiester hydrolysis led to DNA-catalyzed ester and aromatic amide hydrolysis, but aliphatic amide hydrolysis has been elusive. In the present study, we show that including modified nucleotides that bear protein-like functional groups (any one of primary amino, carboxyl, or primary hydroxyl) enables identification of amide-hydrolyzing deoxyribozymes. In one case, the same deoxyribozyme sequence without the modifications still retains substantial catalytic activity. Overall, these findings establish the utility of introducing protein-like functional groups into deoxyribozymes for identifying new catalytic function. The results also suggest the longer-term feasibility of deoxyribozymes as artificial proteases.
The discovery of natural RNA catalysts (ribozymes) in the early 1980s revealed that proteins are not the only biomolecules that have catalytic function.1 In the laboratory, in vitro selection methodology can be used to identify both artificial RNA aptamers (sequences with binding capabilities) and ribozymes.2 Although nature does not appear to use DNA as a catalyst, artificial single-stranded DNA aptamers3 and deoxyribozymes4 can be found by in vitro selection.5 The binding and catalytic abilities of RNA and DNA are similar, despite the absence of 2′-hydroxyl groups in DNA.6 Moreover, DNA enjoys many practical advantages relative to RNA in cost, stability, and ease of synthesis.
A growing variety of reactions have been catalyzed by DNA. These reactions include challenging transformations such as phosphoserine hydrolysis, whose uncatalyzed half-life of ~1010 years is reduced to ~1 h by a deoxyribozyme.7 Nevertheless, some DNA-catalyzed reactions have been elusive. In a previous study, we sought DNA-catalyzed amide hydrolysis, but the selection process instead provided deoxyribozymes that catalyze DNA phosphodiester bond hydrolysis.8 We subsequently improved the selection methodology to avoid phosphodiester hydrolysis; although we found DNA-catalyzed ester and aromatic amide (anilide) hydrolysis, no deoxyribozymes emerged for aliphatic amide hydrolysis.9 We then performed numerous additional selections seeking DNA-catalyzed aliphatic amide cleavage using water and also various nitrogen-based nucleophiles (e.g., amines and hydrazides), but no activity was observed (data not shown). Joyce and co-workers evolved a group I intron ribozyme to catalyze amide cleavage by a guanosine nucleophile,10 but their finding is difficult to expand to amide hydrolysis.
RNA and DNA lack many important functional groups found in protein enzymes, such as primary amino and carboxyl groups characteristic of lysine and aspartate/glutamate. The secondary 2′-hydroxyl group found in RNA is sterically encumbered and may not contribute to catalysis as well as the primary hydroxyl of serine. Such considerations have led others to evaluate the inclusion of protein-like functional groups on deoxyribozymes. This has been done extensively in the context of improving DNA-catalyzed RNA cleavage by transesterification (attack of the 2′-hydroxyl group on the neighboring phosphodiester linkage), particularly to reduce or remove the divalent metal ion requirement.11 However, to date there are no reports of including protein-like functional groups for the purpose of expanding DNA catalysis to entirely new reactions, especially those for which unmodified DNA is not successful as a catalyst. Modified nucleotides have been reported for inclusion in RNA and DNA aptamers, and in many cases substantial enhancement of binding ability is observed.12
Considering the challenge evident in identifying deoxyribozymes for amide hydrolysis using standard unmodified DNA, we decided to evaluate experimentally the contributions of protein-like functional groups in this context. In random-sequence DNA pools for in vitro selection, we replaced all instances of the standard DNA nucleotide thymidine (dT) with one of the three modified DNA nucleotides of Figure 1A, thereby preparing all deoxyribozyme candidates with multiple occurrences of the particular modification. Upon performing in vitro selection, we were pleased to find deoxyribozymes that, for the first time, hydrolyze aliphatic amide bonds. These results establish that introducing protein-like functionality into DNA enables identification of important new catalytic function.
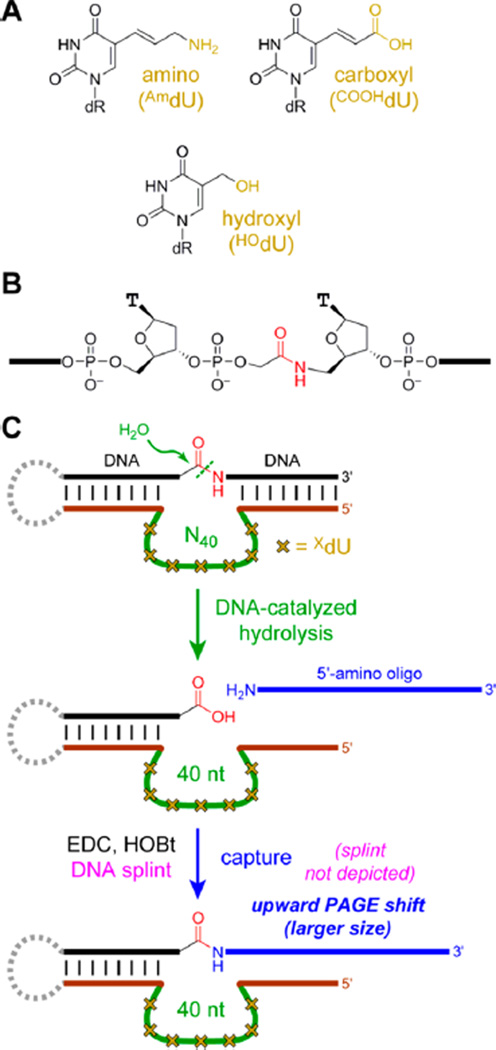
Figure 1.
In vitro selection using modified DNA to achieve amide hydrolysis. (A) Modified XdUTP nucleosides used in this study (dR = deoxyribose). (B) Amide substrate. (C) Key steps of in vitro selection, in which DNA-catalyzed amide hydrolysis is followed by DNA-splinted capture of the revealed carboxylic acid with a 5′-amino oligonucleotide. The DNA modifications in the initially random (N40) region are denoted schematically by yellow crosses. Not depicted is PCR after the capture step to form the reverse complement of the captured DNA sequences (without modified XdU), followed by primer extension (with modified XdUTP) to synthesize the DNA pool for the next round, now enriched in catalytically active sequences. See SI text and Figure S1 for selection details.
Three modified DNA nucleotides were investigated, with protein-like functional groups of primary amino, carboxyl, and primary hydroxyl attached to the 5-position of thymidine, thereby formally creating 5-substituted 2′-deoxyuridine derivatives AmdU, COOHdU, and HOdU (Figure 1A). The cleavage substrate presents a simple aliphatic amide bond covalently anchored between two DNA oligonucleotide strands (Figure 1B). In the selection process (Figure 1C), the substrate binds via Watson–Crick base pairs to fixed DNA sequences that flank an initially random N40 DNA pool (40 random nucleotides). These fixed DNA sequences also enable PCR at the end of each selection round. A close variant of this amide substrate was not cleaved by unmodified DNA in our previous selection experiments.9
Our standard in vitro selection strategy was adjusted as required for incorporating the nucleotide modifications; see Supporting Information. To prepare the N40 pool, the appropriately modified (XdU) nucleoside phosphoramidite was used in solid-phase oligonucleotide synthesis. The amide hydrolysis step was performed in 70 mM HEPES, pH 7.5, 1 mM ZnCl2, 20 mM MnCl2, 40 mM MgCl2, and 150 mM NaCl at 37 °C for 14 h, where each of Zn2+, Mn2+, and Mg2+ have been useful cofactors for other deoxyribozymes. A “capture” step5d was included as a specific selection pressure for amide hydrolysis rather than DNA phosphodiester cleavage by either direct hydrolysis or deglycosylation and subsequent elimination reactions. Following each selection round, the new deoxyribozyme pool, enriched in catalytically active sequences, was synthesized by primer extension from the reverse complement template using the modified XdUTP and KOD XL DNA polymerase.
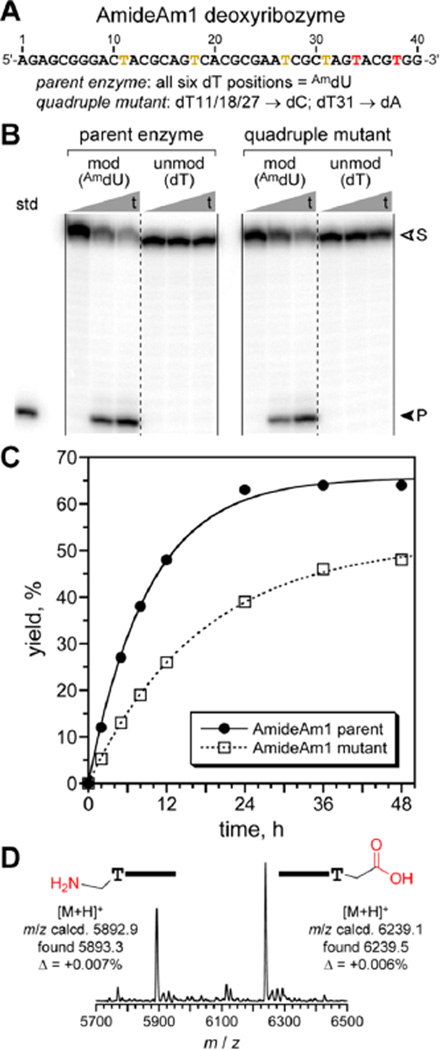
For deoxyribozymes incorporating the AmdU modification, after 8 rounds, 16% capture yield for the DNA pool was observed, compared with ~55% capture yield for a hydrolyzed reaction standard (see Figure S2A for selection progression). Individual deoxyribozymes were cloned from the round 8 pool. A single sequence family was found, with a predominant sequence designated AmideAm1 and numerous variants that have only one or two nucleotide differences (Figure 2A; see all sequences in Figure S3A). Of the 40 nucleotides in AmideAm1’s initially random region, only six are AmdU. Moreover, in the sequence alignment, four of these six positions are mutated in a catalytically active variant, suggesting that just two of the six AmdU nucleotides (at positions 34 and 38 of the initially random 40 nucleotides) may be catalytically relevant. Both AmideAm1 and its quadruple mutant in which only positions 34 and 38 are retained as AmdU were assayed for cleaving the amide substrate (Figure 2B). With the amide substrate and the same incubation conditions as in the selection process, the parent AmideAm1 (six AmdU) had amide hydrolysis kobs 0.11 h−1 and 64% yield at 48 h, while the quadruple mutant (two AmdU) had kobs 0.06 h−1 and slightly lower 49% yield at 48 h (Figure 2C). MALDI mass spectrometry confirmed amide hydrolysis (Figure 2D). Neither deoxyribozyme had any detectable cleavage activity for the analogous all-DNA substrate lacking an amide bond (data not shown).
Figure 2.
AmdU-modified AmideAm1 deoxyribozyme for amide hydrolysis. (A) Sequence of the initially random (N40) region of AmideAm1. The four yellow T positions are AmdU in the parent sequence but mutatable as indicated. (B) PAGE assay (t = 0, 12, 48 h). (C) Kinetic plots. kobs, h−1 (n = 3): parent 0.11 ± 0.01 and quadruple mutant 0.057 ± 0.007. Because kbkgd < 10−4 h−1 (i.e., <0.5% in 48 h), the rate enhancement kobs/kbkgd is >103. (D) Mass spectrometry analysis of AmideAm1 product.
For the experiments of Figure 2, each 74 nt AmideAm1 deoxyribozyme was prepared by primer extension from the corresponding reverse complement template, as was done for the selection pool during each round. Because of this approach, not only did the initially random 40 nucleotides incorporate AmdU in place of unmodified dT, but also the fixed-sequence 3′-segment (binding arm) included AmdU. We established that the 3′-binding arm did not require any of its six AmdU nucleotides by preparing AmideAm1 via splint ligation using a truncated primer extension product and an unmodified 3′-segment (Figure S4). We then prepared several versions of AmideAm1 by solid-phase DNA synthesis, using the AmdU phosphoramidite specifically for one or both of positions 34 and 38 of the initially random region; all other nucleotides that were originally AmdU in the sequence identified by selection were replaced by unmodified dT. We could not replace any AmdU with dT in AmideAm1 when prepared by primer extension rather than solid-phase synthesis, because primer extension does not allow site-specific replacements. Assays using the AmideAm1 variants prepared by solidphase synthesis revealed that AmdU at both positions 34 and 38 are required for high catalytic activity, although reduced activity (~10% in 48 h; kobs decreased by ~3-fold) was still observed with either of the single-AmdU variants (Figure S5). Therefore, as few as one of the initially random 40 nucleotides of AmideAm1 must be AmdU to achieve amide hydrolysis, whereas 39 of the remaining 40 nucleotides can be standard unmodified DNA.
For the COOHdU modification, the capture yield was 7% at round 11 (Figure S2B), and two unique sequences were identified upon cloning, AmideCa1 and AmideCa2 (Figure S3B). These sequences, respectively, included 7 and 3 COOHdU nucleotides in the initially random region. Assays revealed kobs 0.3–0.4 h−1 and 10–17% yield at 48 h for amide hydrolysis (Figure S6). Using variants prepared by splint ligation, we found that amide hydrolysis did not need COOHdU modifications in the fixed-sequence 3′-binding arm; yields were slightly higher (16–24%) when modifications were omitted (Figure S6). Amide hydrolysis was not observed when these deoxyribozymes were prepared without any COOHdU modifications (Figure S6).
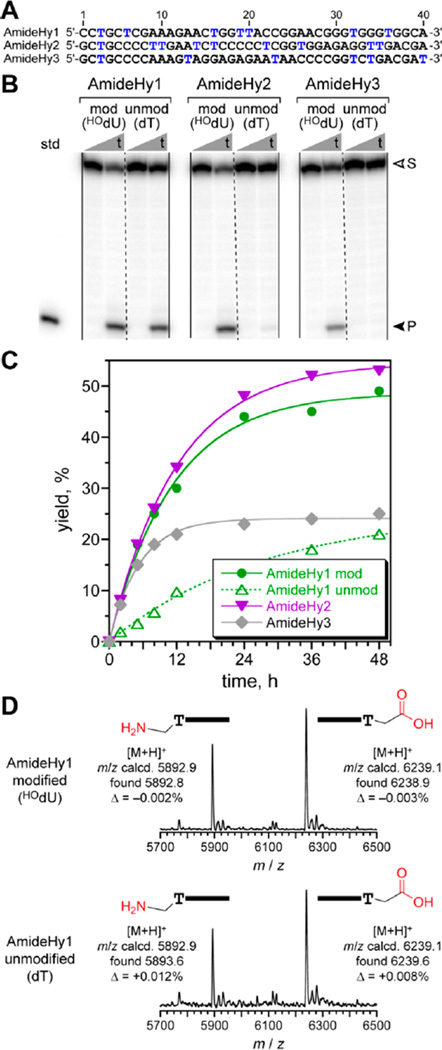
Finally, for the HOdU modification, capture yield of 16% was observed at round 14, at which point cloning was performed (Figure S2C). Three unique sequences were identified and designated AmideHy1, AmideHy2, and AmideHy3 (Figure 3A; see full sequences in Figure S3C). In the initially random regions of these three sequences were 7, 9, and 6 HOdU modifications, respectively. The three HOdU deoxyribozymes had kobs 0.1–0.2 h−1 and yield up to 53% at 48 h for amide hydrolysis (Figure 3B,C), as confirmed by MALDI mass spectrometry (Figure 3D). As for AmdU and COOHdU, HOdU modifications were not required in the 3′-binding arm (Figure S7).
Figure 3.
HOdU-modified deoxyribozymes for amide hydrolysis. (A) Sequence of the initially random (N40) regions of AmideHy1, 2, and 3. Each blue T is HOdU in the deoxyribozyme. (B) PAGE assay (t = 0, 48 h). (C) Kinetic plots. kobs, h−1 (n = 3): AmideHy1 mod 0.089 ± 0.011 and unmod 0.034 ± 0.003; AmideHy2 0.084 ±0.004; AmideHy3 0.17 ± 0.02. (D) Mass spectrometry analysis of products (similar for AmideHy2,3; not shown).
When synthesized without any modified HOdU nucleotides, AmideHy1 was unexpectedly found to retain substantial catalytic activity (Figure 3B,C). Separately, AmideHy2 showed a trace of amide hydrolysis when evaluated without HOdU modifications (1.2% in 48 h, n = 4; Figure 3B). These observations are surprising in the context of our prior experiments, in which unmodified DNA was never observed to hydrolyze amide bonds.6,9 One possible explanation is that the α-hydroxyacetic acid linker to the amide bond in the this substrate (Figure 1B) is sufficiently activating to enable hydrolytic cleavage, whereas the linker in our previous report was less activating.9 To test this, we performed new selections using unmodified N40 DNA and various peptide substrates linked via α-hydroxyacetic acid, and in all cases no activity was observed (data not shown), indicating that linker activation is not responsible for the amide hydrolysis. Another hypothesis, not yet evaluated empirically, is that any catalytically relevant HOdU 5-(hydroxymethyl) group can be replaced by a combination of the dT 5-methyl group and a water molecule, efficiently for AmideHy1 and much less well for AmideHy2; this would be analogous to abasic and other rescue experiments for ribozymes13 and similar rescue studies for protein mutants.14 Further experiments, likely involving high-resolution structural characterization,15 are needed to explain the role of HOdU modifications in DNA-catalyzed amide hydrolysis.
Others have found that modified RNA-cleaving DNA catalysts can have reduced dependence on divalent metal ions,11 although such an outcome was not our own goal. We investigated the metal ion dependence of our new deoxyribozymes (Figure S8). All of the deoxyribozymes require Zn2+ for full activity, in most cases additionally requiring Mn2+, Mg2+, or both. All of the deoxyribozymes have reduced activity below pH 7.5, with little or no activity at pH 7.0 (data not shown).
In parallel with the identification of AmdU-, HOdU-, and COOHdU-modified deoxyribozymes for amide hydrolysis, from the same selections we also identified several sequence-unrelated deoxyribozymes that catalyze a different cleavage reaction in which a nucleoside appears to be oxidatively excised via a radical-based mechanism (data not shown). These deoxyribozymes will be described elsewhere.
In summary, including modified DNA nucleotides that have protein-like functional groups enables DNA-catalyzed amide hydrolysis. DNA with modified nucleotides is still “DNA” from the important practical viewpoint of in vitro selection. In particular, because modified nucleotides are tolerated during PCR using an appropriate DNA polymerase, the in vitro selection process is still fundamentally possible. The value of the catalytic activity enabled by inclusion of the modifications justifies the greater synthetic complexity of modified DNA. Assessing the structural and mechanistic contributions of the modifications to the DNA catalysis will be part of future efforts. The finding that an unmodified DNA catalyst can have substantial amide hydrolysis activity is intriguing and also requires further investigation. The present study used a model substrate in which the amide bond to be hydrolyzed was located between two DNA oligonucleotide binding arms. Our ongoing efforts focus on expanding this DNA catalysis to discrete peptide substrates, including the important practical objective of peptide sequence selectivity.16 The long-term goal of these efforts is to establish DNA-catalyzed amide hydrolysis using peptide and protein substrates, which is likely to have valuable applications in molecular biology, chemical biology, and proteomics.17
Supplementary Material
Acknowledgments
This work was supported by a grant to S.K.S. from the National Institutes of Health (No. R01GM065966). B.M.B. was partially supported by NIH T32GM070421. We thank Yun Xie for technical assistance during the early stage of this work.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b12647.
Experimental details and additional data (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Cell. 1982;31:147. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]; (b) Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. Cell. 1983;35:849. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 2.(a) Tuerk C, Gold L. Science. 1990;249:505. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]; (b) Ellington AD, Szostak JW. Nature. 1990;346:818. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]; (c) Robertson DL, Joyce GF. Nature. 1990;344:467. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]; (d) Joyce GF. Annu. Rev. Biochem. 2004;73:791. doi: 10.1146/annurev.biochem.73.011303.073717. [DOI] [PubMed] [Google Scholar]; (e) Joyce GF. Angew. Chem., Int. Ed. 2007;46:6420. doi: 10.1002/anie.200701369. [DOI] [PubMed] [Google Scholar]
- 3.Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ. Nature. 1992;355:564. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- 4.Breaker RR, Joyce GF. Chem. Biol. 1994;1:223. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mayer G. Angew. Chem., Int. Ed. 2009;48:2672. doi: 10.1002/anie.200804643. [DOI] [PubMed] [Google Scholar]; (b) Schlosser K, Li Y. Chem. Biol. 2009;16:311. doi: 10.1016/j.chembiol.2009.01.008. [DOI] [PubMed] [Google Scholar]; (c) Silverman SK. Angew. Chem., Int. Ed. 2010;49:7180. doi: 10.1002/anie.200906345. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Silverman SK. Acc. Chem. Res. 2015;48:1369. doi: 10.1021/acs.accounts.5b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hollenstein M. Molecules. 2015;20:20777. doi: 10.3390/molecules201119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Chandra M, Silverman SK. J. Am. Chem. Soc. 2008;130:2936. doi: 10.1021/ja7111965. [DOI] [PubMed] [Google Scholar]; (b) McKeague M, et al. J. Mol. Evol. 2015;81:150. doi: 10.1007/s00239-015-9708-6. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekar J, Silverman SK. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5315. doi: 10.1073/pnas.1221946110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra M, Sachdeva A, Silverman SK. Nat. Chem. Biol. 2009;5:718. doi: 10.1038/nchembio.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandsen BM, Hesser AR, Castner MA, Chandra M, Silverman SK. J. Am. Chem. Soc. 2013;135:16014. doi: 10.1021/ja4077233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai X, De Mesmaeker A, Joyce GF. Science. 1995;267:237. doi: 10.1126/science.7809628. Correction: Science1996, 272, 18. [DOI] [PubMed] [Google Scholar]
- 11.(a) Hollenstein M, Hipolito CJ, Lam CH, Perrin DM. ChemBioChem. 2009;10:1988. doi: 10.1002/cbic.200900314. [DOI] [PubMed] [Google Scholar]; (b) Sidorov AV, Grasby JA, Williams DM. Nucleic Acids. Res. 2004;32:1591. doi: 10.1093/nar/gkh326. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hollenstein M. Chimia. 2011;65:770. doi: 10.2533/chimia.2011.770. [DOI] [PubMed] [Google Scholar]
- 12.(a) Lin Y, Qiu Q, Gill SC, Jayasena SD. Nucleic Acids. Res. 1994;22:5229. doi: 10.1093/nar/22.24.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gold L, et al. PLoS ONE. 2010;5:e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kuwahara M, Sugimoto N. Molecules. 2010;15:5423. doi: 10.3390/molecules15085423. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Vaught JD, Bock C, Carter J, Fitzwater T, Otis M, Schneider D, Rolando J, Waugh S, Wilcox SK, Eaton BE. J. Am. Chem. Soc. 2010;132:4141. doi: 10.1021/ja908035g. [DOI] [PubMed] [Google Scholar]; (e) Davies DR, et al. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19971. doi: 10.1073/pnas.1213933109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hollenstein M. Chem. - Eur. J. 2012;18:13320. doi: 10.1002/chem.201201662. [DOI] [PubMed] [Google Scholar]; (g) Tolle F, Mayer G. Chem. Sci. 2013;4:60. [Google Scholar]; (h) Rohloff JC, Gelinas AD, Jarvis TC, Ochsner UA, Schneider DJ, Gold L, Janjic N. Mol. Ther.– Nucleic Acids. 2014;3:e201. doi: 10.1038/mtna.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Gupta S, et al. J. Biol. Chem. 2014;289:8706. doi: 10.1074/jbc.M113.532580. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Gelinas AD, et al. J. Biol. Chem. 2014;289:8720. doi: 10.1074/jbc.M113.532697. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Tolle F, Brandle GM, Matzner D, Mayer G. Angew. Chem., Int. Ed. 2015;54:10971. doi: 10.1002/anie.201503652. [DOI] [PubMed] [Google Scholar]; (l) Liu E, Lam CH, Perrin DM. Molecules. 2015;20:13591. doi: 10.3390/molecules200813591. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Temme JS, Krauss IJ. Curr. Protoc. Chem. Biol. 2015;7:73. doi: 10.1002/9780470559277.ch140233. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Diafa S, Hollenstein M. Molecules. 2015;20:16643. doi: 10.3390/molecules200916643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Peracchi A, Beigelman L, Usman N, Herschlag D. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11522. doi: 10.1073/pnas.93.21.11522. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Perrotta AT, Shih I, Been MD. Science. 1999;286:123. doi: 10.1126/science.286.5437.123. [DOI] [PubMed] [Google Scholar]; (c) Kuzmin YI, Da Costa CP, Fedor MJ. J. Mol. Biol. 2004;340:233. doi: 10.1016/j.jmb.2004.04.067. [DOI] [PubMed] [Google Scholar]
- 14.(a) Toney MD, Kirsch JF. Science. 1989;243:1485. doi: 10.1126/science.2538921. [DOI] [PubMed] [Google Scholar]; (b) Tu C, Silverman DN, Forsman C, Jonsson B-H, Lindskog S. Biochemistry. 1989;28:7913. doi: 10.1021/bi00445a054. [DOI] [PubMed] [Google Scholar]
- 15.Ponce-Salvatierra A, Wawrzyniak-Turek K, Steuerwald U, Höbartner C, Pena V. Nature. 2016;529:231. doi: 10.1038/nature16471. [DOI] [PubMed] [Google Scholar]
- 16.(a) Chu C, Wong O, Silverman SK. ChemBioChem. 2014;15:1905. doi: 10.1002/cbic.201402255. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Walsh SM, Konecki SN, Silverman SK. J. Mol. Evol. 2015;81:218. doi: 10.1007/s00239-015-9699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Yoo TH, Pogson M, Iverson BL, Georgiou G. ChemBioChem. 2012;13:649. doi: 10.1002/cbic.201100718. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wu C, Tran JC, Zamdborg L, Durbin KR, Li M, Ahlf DR, Early BP, Thomas PM, Sweedler JV, Kelleher NL. Nat. Methods. 2012;9:822. doi: 10.1038/nmeth.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.