Abstract
Objective
A role for thrombin in the pathogenesis of atherosclerosis has been suggested through clinical and experimental studies revealing a critical link between the coagulation system and inflammation. Although approved drugs for inhibition of thrombin and thrombin-related signaling have demonstrated efficacy, their clinical application to this end may be limited due to significant potential for bleeding side effects. Thus, we sought to implement a plaque-localizing nanoparticle-based approach to interdict thrombin-induced inflammation and hypercoagulability in atherosclerosis.
Approach and Results
We deployed a novel magnetic resonance spectroscopic method to quantify the severity of endothelial damage for correlation with traditional metrics of vessel procoagulant activity after dye-laser injury in fat-fed ApoE-null mice. We demonstrate that a one-month course of treatment with anti-thrombin nanoparticles carrying the potent thrombin inhibitor D-phenylalanyl-L-prolyl-L-arginyl chloromethylketone (PPACK-NP): 1) reduces the expression and secretion of proinflammatory and procoagulant molecules, 2) diminishes plaque procoagulant activity without the need for systemic anticoagulation, 3) rapidly restores disrupted vascular endothelial barriers, and 4) retards plaque progression in lesion prone areas.
Conclusions
These observations illustrate the role of thrombin as a pleiotropic atherogenic molecule under conditions of hypercholesterolemia, and suggest the utility of its inhibition with locally acting anti-thrombin nanoparticle therapeutics as a rapid-acting anti-inflammatory strategy in atherosclerosis to reduce thrombotic risk.
Keywords: Nanoparticles, Thrombin Inhibitors, Atherosclerosis, Endothelium, Inflammation
Introduction
Atherosclerosis, the leading cause of death in the Western world, is essentially a disease of inflammation from its inception through the evolution of vulnerable atheromas that eventually break down to induce focally occlusive thrombosis with consequential tissue death in subtended vascular beds.1 A direct link exists between inflammation and coagulation in atherosclerosis by way of the serine protease thrombin that plays a central role in both clot formation and inflammatory molecular signaling events that may instigate and potentiate plaque development.2–4 Thrombin’s vascular activity is mediated primarily by a family of G-protein coupled receptors known as protease-activated receptors (PARs).5 Activation of PAR-1 by thrombin initiates a signaling cascade that promotes proinflammatory, vasomotor, and cellular proliferative effects in various cell types, including endothelial cells, smooth muscle cells, and macrophages, among others. Thrombin signaling promotes the synthesis and release of procoagulant factors such as tissue factor, which establishes the conditions for repeated cycles of endothelial disruption, coagulation, inflammation, and plaque expansion.6
Given the abundance of thrombin in atherosclerotic plaques and its recognized contribution to plaque inflammation and hypercoagulability7,8, we sought to investigate the hypothesis that focal inhibition of plaque thrombin to abrogate its inflammatory signaling actions would both attenuate plaque procoagulant activity and facilitate restoration of naturally anticoagulant endothelial vascular barriers. Furthermore, we sought to elucidate direct relationships between thrombin inhibition, regulation of inflammatory signaling, recovery of endothelial barriers, and reduction in thrombotic risk with the use of clinically translatable functional methods for quantifying vascular barrier integrity. Although clinically approved anti-thrombotic pharmaceuticals have been evaluated in experimental primary and clinical secondary prevention trials9, no information exists as to their ability to directly attenuate focal plaque thrombotic propensity or to improve vascular barrier integrity, which could serve to deter acute vascular syndromes.
To those ends, we have reported recently that atherosclerotic endothelial damage can be quantified nondestructively with the in vivo use of semipermeant perfluorocarbon-core nanoparticles (PFC-NP) that passively diffuse beyond disrupted endothelial barriers in plaques, allowing both fluorine magnetic resonance imaging and quantification of PFC-NP deposition with fluorine magnetic resonance spectroscopy (19F-MRI and 19F-MRS, respectively).10 Using this method, we have delineated the temporal progression of endothelial barrier disruption in ApoE-null mice as a consequence of a prolonged high fat diet and demonstrated that barrier damage was related directly to the propensity for thrombotic occlusion in the dye-laser vessel injury model. Moreover, dietary management by restoration of a normal chow diet simultaneously recovered vascular barrier integrity and rapidly reduced plaque hypercoagulability within 1–2 months.11 Although these nanoparticles previously have been conjugated to selected antithrombin agents to serve as potent and safe antagonists of thrombosis in acute clotting events12,13, their therapeutic potential for chronic control of inflammatory signaling through thrombin inhibition is untested in atherosclerosis.
Accordingly, we hypothesize that the reported capability of PFC-NP to localize to atherosclerotic plaques manifesting disrupted barriers and be retained for prolonged periods10,11 would set the stage for the formulation and focal deposition of a reservoir of anti-thrombin nanoparticles in plaques (Fig. 1A) to exert prolonged surveillance against and rapid inactivation of any locally generated thrombin. The present work was designed to examine the efficacy and safety of nanoparticle-based strategies for focal inhibition of serine proteases, namely thrombin (Fig. 1A) in atherosclerosis. The test agent used for this study is a perfluorocarbon nanoparticle carrying the potent thrombin inhibitor PPACK, which has been previously demonstrated to be efficacious in limiting the growth of acute thrombi in mouse models of carotid artery thrombosis, with limited bleeding side effects.12 Furthermore, PPACK itself is a highly potent inhibitor of thrombin with several orders of magnitude increased affinity for thrombin over other serine proteases. By delivering doses of antithrombin nanoparticles over the course of several weeks, we aim to define the role of focal thrombin inhibition in mediating inflammatory signaling events in vitro and in vivo how that might play a mechanistic role in diminishing vascular endothelial barrier damage and thrombotic risk. The observed efficacy of the approach indicates a significant direct contribution of thrombin signaling to the evolution of atherosclerosis and the emergence of thrombotic risk, and implicates it as a key contributor to endothelial damage in this model.
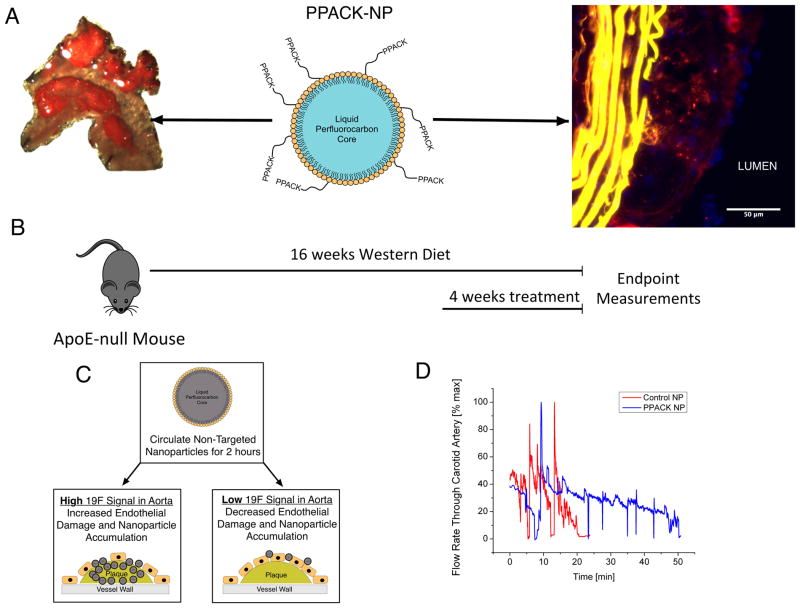
Figure 1.
(A) Schematic of PPACK-NP (center panel). Nanoparticles consist of a perfluorocarbon core surrounded by a stabilizing phospholipid monolayer to which PPACK is covalently conjugated. In this work, PPACK-NP inhibits the inflammatory effects of both surface-bound and intraplaque thrombin. Fluorescent microscopy image (right panel) of mouse atherosclerotic plaque at 3 months of cholesterol feeding demonstrates intraplaque accumulation of PPACK-NP (red). Sudan IV staining of the aortic arch of cholesterol fed ApoE mice demonstrates ample plaque deposition serving as a target for PPACK-NP therapy (left panel) (B) Feeding and dosing schedule for ApoE-null mice. During the 4-week treatment period, mice received i.v. treatments three times per week. (C) Schematic representation of 19F-MRS-based detection of endothelial barrier disruption. Non-targeted CE-NP are administered and circulated for 2 hours. Aortas are removed and 19F signal is measured. High 19F signal corresponds to increased endothelial barrier disruption and increased nanoparticle accumulation, where as low 19F signal corresponds to decreased endothelial barrier disruption and diminished nanoparticle accumulation. (D) Representative kinetics of photochemically-induced thrombus formation in ApoE-null mice treated with either saline, control NP or PPACK-NP.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Anti-thrombin Nanoparticles Reduce Vascular Procoagulant Activity in vivo
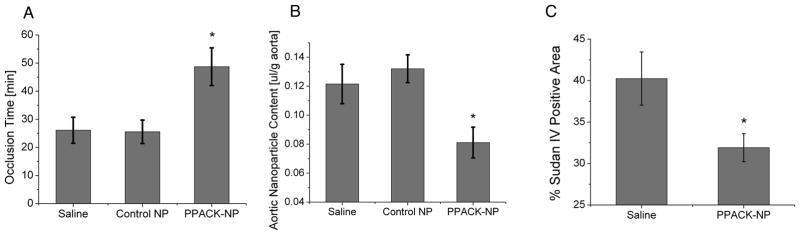
To characterize the effects of PPACK-NP on reducing procoagulant activity, groups of ApoE-null mice were fed a Western diet for 3 months followed by continuation of the diet with treatment (saline, control NP, and PPACK-NP) for 1 additional month. At the conclusion of the treatment/feeding period, mice were subjected to a previously validated11,14,15 model for measuring thrombotic risk using photochemical injury of the carotid artery that yields a quantitative metric of coagulant activity (vessel occlusion time) with good dynamic range and monotonic responsiveness to therapeutic agents that affect clotting. Prior to performing the vessel injury, mice were maintained on their diets for 2–3 additional days without further treatment to allow washout of any residual anti-thrombin nanoparticles. After 1 month of PPACK-NP treatment, carotid occlusion times increased significantly over control groups (Fig. 2A), with occlusion times reaching 48.71 ± 6.7 min (N=7) compared to saline treatment (26.11 ± 4.63 min, N=9, p=0.005) and control NP treatment (25.55 ± 4.17 min, N=9, p=0.004). We note that the occlusion times in the 1-month PPACK-NP treated group approximated those of previously reported fat-fed ApoE null mice after 2 months off diet, indicating that the PPACK NP therapy may more rapidly attenuate vessel procoagulant activity than does dietary management in this model, despite maintenance of the high fat diet in the PPACK NP treated group.11
Figure 2.
(A) PPACK-NP treatment (rightmost bar) significantly increases time to occlusion of the carotid artery by 46% over saline (p=0.005) and control NP (p=0.004) treatments. (B) Plaque permeability is reduced with PPACK-NP treatment by 33% compared to saline (p=0.023) and control NP treatments (p=0.014). (C) Sudan IV staining of the aortic arch of saline and PPACK-NP treated mice demonstrates a 20.69% decrease in gross plaque deposition with PPACK-NP treatment vs. saline treatment (p=0.03) as quantified with ImageJ.
Anti-thrombin Nanoparticles Restore Vascular Barrier Integrity in vivo
We tested the ability of PPACK-NP treatment to restore functional endothelial barriers, which concomitantly might be expected to reduce plaque procoagulant activity as previously reported.11 Mice were injected with a dose of CE-NP for MR spectroscopy that were allowed to circulate for 2 hours prior to sacrifice, at which time plaque saturation occurs.10 Following this circulation time, the entire length of the aorta (from the aortic root to the bifurcation) was removed for ex vivo 19F-MRS measurements at 11.7T. Figure 2B illustrates the beneficial effects of PPACK-NP treatment for 1 month on plaque endothelial permeability according to the decreased deposition of CE-NP (0.084 ± 0.009 μl/g aorta, N=7) compared to saline (0.122 ± 0.011 μl/g aorta, N=8, p=0.023) and control NP (0.132 ± 0.013 μl/g aorta, N=10, p=0.014). Using paired samples, we observed an inverse correlation (R = −0.56, Suppl. Fig. I) between plaque permeability and vessel procoagulant activity (p=0.004), consistent with our previous observation that thrombotic risk tracks with plaque permeability to PFC-NP.
A subset of mice was allocated for measurements of plaque extent in the aortic arch by conventional Sudan IV staining and computer assisted planimetry.16 Overall, PPACK-NP treatment resulted in a 20.69% decrease in aortic arch plaque extent (Fig. 2C): 40.24 ± 3.21% plaque area for saline-treated mice (N=5) and 31.91 ± 1.69% plaque area for PPACK-NP treated mice (N=7, p=0.03).
Anti-thrombin Nanoparticles Attenuate Inflammatory Signaling Molecules
PAR-1 responses
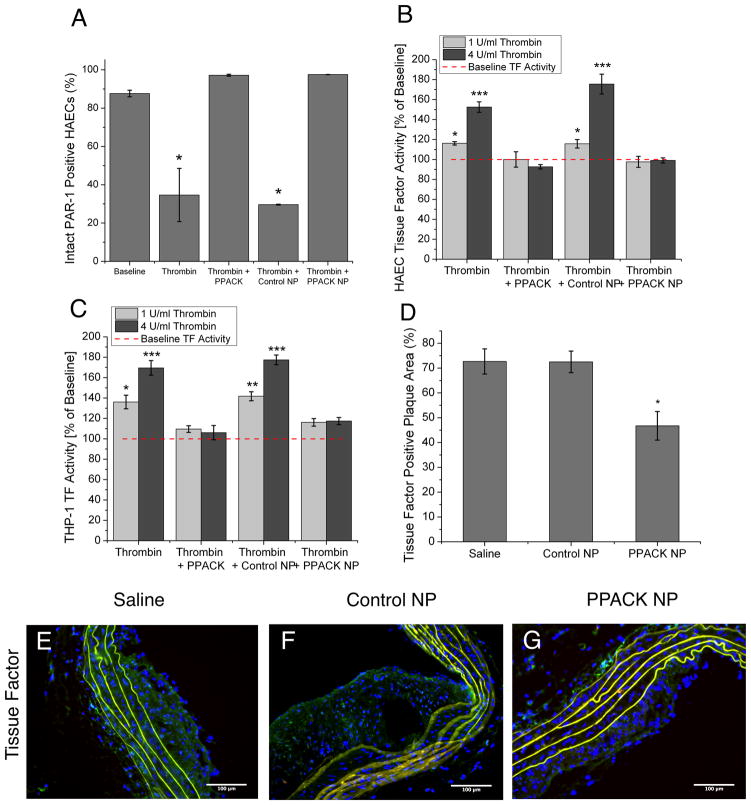
To delineate molecular signaling events responsible for the beneficial effects of PPACK-NP, cell culture studies were used to quantify the responses of activated endothelial and monocytic cell lines to thrombin inhibition. First, as thrombin’s effect on cell types is mediated primarily by cleavage of the PAR-1 receptor on cell surfaces5, flow cytometry was used to determine the percentage of intact PAR-1 receptors that was left after treatment with thrombin in the various treatment groups. PPACK-NP treatment completely prevented thrombin cleavage of PAR-1 receptors on human aortic endothelial cells (HAECs), compared to thrombin or thrombin/control NP groups, which manifested significantly decreased PAR-1 expression (N=3 for both groups, p=0.019 and p=0.000005, respectively) as compared to baseline (Fig. 3A).
Figure 3.
(A) Flow cytometry for intact PAR-1 receptors following thrombin stimulation in the presence of each treatment group demonstrates inhibition of thrombin-mediated cleavage of PAR-1 with PPACK-NP treatment. *p<0.05 (B) PPACK-NP inhibit expression of surface tissue factor on HAECs and (C) THP-1 cells in response to stimulation with different concentrations of thrombin (light gray bars, 1U/ml; dark gray bars, 4U/ml). **p<0.005, ***p<0.0005 (D) diminished tissue factor expression in PPACK-NP treated-mice (p=0.027 and 0.023 vs saline and control NP, respectively) as quantified by ImageJ in tissue sections stained for tissue factor (green) in ApoE-null mice treated with (E) saline, (F) control NP, and (G) PPACK-NP.
Tissue Factor responses
Because PPACK-NPs successfully prevented PAR-1 activation, we tested the downstream signaling effects of PAR-1 activation related to inflammation and coagulation. Expression of tissue factor on the surface of HAECs and THP-1 monocytes in response to thrombin stimulation was assayed using a functional assay of measuring FXa generation as a result of the presence of TF/FVIIa complexes. PPACK-NP prevented thrombin-induced TF expression on the surface of both HAECs (Fig. 3B) and THP-1 monocytes (Fig. 3C), with no significant increase over baseline TF levels at both concentrations of thrombin utilized (1 U/ml and 4 U/ml). Whole excised aortic arch segments exhibited a marked reduction in TF-positive plaque area after PPACK-NP treatment: 46.75 ± 5.74% (N=3) in PPACK treated mice (Fig. 3D), versus 72.69 ± 5.06% (N=3, p=0.027) for saline treatment and 72.52 ± 4.34% (N=3, p=0.023) for control NP treatment as quantified in immunofluorescent staining using ImageJ (Fig. 3E–G).
NFkB responses
Because thrombin is known to stimulate NFkB transcriptional regulation of a panoply of inflammatory genes through PAR-1 signaling, we delineated the effect of PPACK-NP on the inhibition of NF-kB activation in HAEC (Fig. 4A–E) and THP-1 cells (Fig. 4F–J). Thrombin cleavage of PAR-1 results in the activation of Gαq and dissociation of the Gβγ complex, which subsequently results in the parallel activation of PKCσ and PI3-kinase/Akt pathways. These parallel pathways then converge to stimulate IKK, which results in the binding of the p65 homodimer to IκB and subsequent phosphorylation and degradation of IκB. Activation of the PKCσ pathway results in activation of p38 which in turn, phosphorylates p65 to induce the nuclear translocation and transcriptional activity of the p65.17
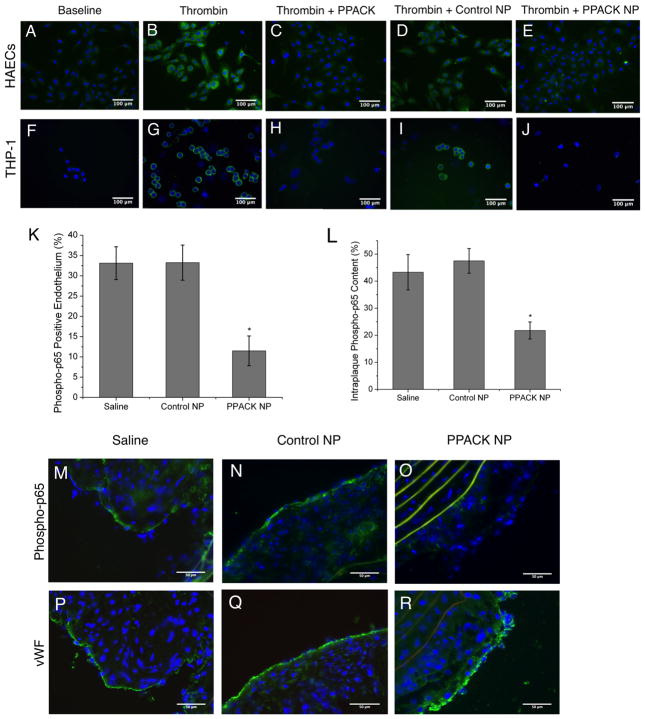
Figure 4.
Immunocytochemistry for phosphorylated p65 demonstrates inhibition of NF-κB activation in (A–E) HAECs and (F–J) THP-1 cells with PPACK-NP treatment. ImageJ quantification of (K) endothelial and (L) intraplaque phospho-p65 staining on (M–O) ApoE-null mouse plaques demonstrate significantly less endothelial and macrophage phospho-p65 (green). Elastin autofluorescence is depicted in yellow. (P–R) Von Willebrand factor staining was conducted on neighboring sections to rule out loss of endothelial phospho-p65 staining due to missing endothelium in this case.
Cell cultures were stained for phosphorylated p65 following six hours of thrombin stimulation and treatment. Treatment with PPACK-NP resulted in little to no observable positive staining for intracellular phospho-p65 and preservation of IkB protein (Suppl. Fig. II) compared to thrombin and thrombin/control NP treatment groups. The preservation of IkB indicates that this cytoplasmic regulatory component of p65/p50 retains control of the preexisting cytoplasmic stores of p65 thereby preventing subsequent p65 phosphorylation and translocation to the nucleus.
Next we quantified pp65 in the endothelium (Fig. 4K) and intraplaque regions (Fig. 4L), by staining sections of the excised aortic arch for phosphorylated NF-kB p65 (pp65, Fig. 4M–O), where increased phosphorylation of p65 indicates increased NF-kB activity. After PPACK-NP treatment, aortic plaques exhibited significantly decreased endothelial pp65 (11.49 ± 3.66%, N=3) compared to saline (33.11 ± 4.05%, N=3, p=0.017) and control NP treatments (33.25 ± 4.33%, N=3, p=0.019). Decreased pp65 also was observed in the same regions as plaque macrophages after PPACK-NP treatment (21.78 ± 3.15%, N=3) compared to saline (43.31 ± 6.55%, N=3) and control NP treatments (47.51 ± 4.59%, N=3). To rule out loss of endothelial pp65 staining due to missing endothelium, selected neighboring slide sections where pp65 was noted to be reduced were stained for vWF, indicating that endothelium was present, thus confirming the specificity for NFkB downregulation by PPACK-NP in endothelium in vivo (Fig. 4P–R).
Systemic coagulation and inflammation markers
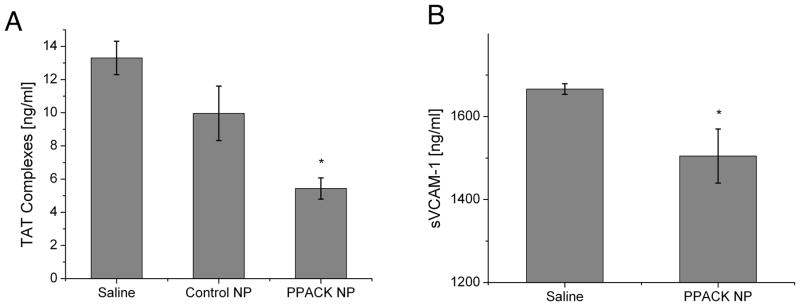
Thrombin-antithrombin (TAT) complexes are correlative systemic harbingers of procoagulant activity.18 PPACK-NP treatment significantly reduced serum thrombin-antithrombin (TAT) complexes (5.43 ± 0.64 ng/ml, N=5; p=0.0001 vs. saline, p=0.032 vs control NP) (Fig. 5A) versus saline (13.32 ± 1.01 ng/ml, N=6) and control NP (9.96 ± 1.64 ng/ml, N=5, p=NS vs. saline).
Figure 5.
(A) ELISA evaluation of thrombin-antithrombin complexes reveals a significant decrease in detected TAT-complexes following one month of PPACK-NP treatment. (B) ELISA analysis for detection of serum soluble VCAM-1 (sVCAM-1) demonstrated a decrease in detectable sVCAM-1 following one month of PPACK-NP treatment.
Because NF-kB is a known driver of endothelial adhesion molecules, we measured soluble VCAM-1 (sVCAM-1) levels as biomarkers of activated endothelium in atherosclerosis.19 ELISA analysis of sVCAM-1 (Fig. 5B) revealed a modest, but significant decrease in sVCAM-1 with PPACK-NP treatment (1504.88 ± 65.25 ng/ml, N=4) compared to saline treatment (1666.37 ± 12.78 ng/ml, N=5, p=0.029)
Macrophage responses
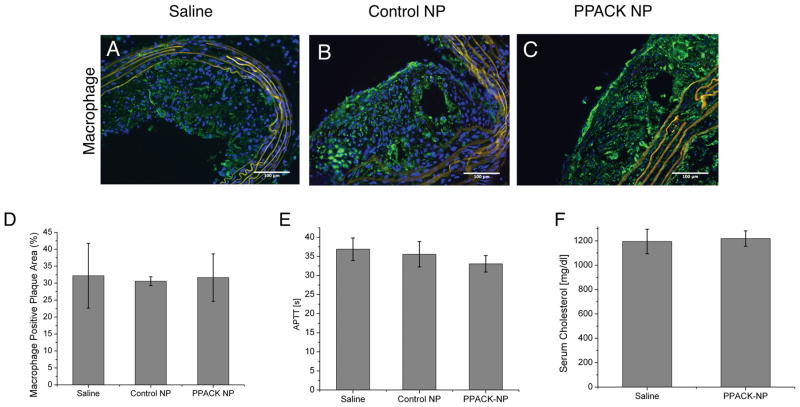
Excised aortic arch sections were stained for macrophages (Fig. 6A–C) and plaque macrophage content was quantified using ImageJ. We observed no significant difference between treatment groups in terms of overall plaque macrophage content (Fig. 6D).
Figure 6.
Immunofluorescent staining for macrophages in ApoE-null mouse plaques treated with (A) saline, (B) control NP, and (C) PPACK-NP revealed no significant difference in (D) detected plaque macrophages as quantified with ImageJ. (E) No significant difference in activated partial thromboplastin time (APTT) between treatment groups indicating no persisting systemic effect of PPACK-NP on coagulation 2–3 days after the penultimate treatment dose. (F) No significant difference was observed on serum cholesterol between treatment groups, indicating that therapeutic effects observed occurred without cholesterol lowering as a consequence of nanoparticle treatment.
Systemic responses to PPACK-NP
APTT measurements conducted on serum collected at the time of sacrifice indicated no persistent non-specific effects of PPACK-NP after the terminal treatment dose 2–3 days prior to sacrifice (Fig. 6E). Furthermore, no significant difference was observed in serum cholesterol following the PPACK-NP treatment regimen (Fig. 6F).
Pharmacokinetics
Quantitative 19F spectroscopy was used to estimate the clearance half-life of PPACK-NP in vivo. The half-life of the nanoparticles was determined by measuring the exponential decay of 19F signal intensity emanating regionally from the tail blood pool (Suppl. Fig. III) and fitting the data to a bi-exponential, two compartment model. PPACK-NP exhibited a mean clearance half-life (Suppl. Fig. IV) of 105.87 ± 23.38 min (N=3), compared to clearance half-lives for plain PFC-NP (181.3 ± 40.7 min, N=3) and PEGylated PFC-NP (240.16 ± 23.42 min, N=3), indicative of only modest effects on pharmacokinetics with selected particle surface modifications. The anticipated clearance mechanism for PFC-NP was the reticuloendothelial system as demonstrated by 19F-MR imaging of mice post-mortem, which depicted accumulation of nanoparticles in the liver and spleen following 2 hours of nanoparticle circulation prior to sacrifice (Suppl. Fig. V).
Discussion
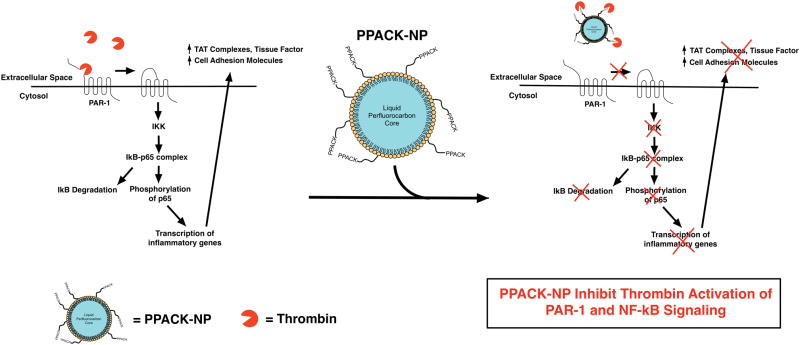
The principal new observation in this work is that focal inhibition of plaque thrombin, and possibly other trypsin-like proteases, in fat-fed ApoE-null mice results in rapid recovery of damaged endothelial barriers and attenuated vascular procoagulant activity in spite of a continued Western diet. These beneficial outcomes were achieved with the use of anti-thrombin nanoparticles that passively permeated plaque intimal regions after i.v. injection and were focally retained to exert sustained pleiotropic anti-inflammatory effects. Additionally, the progression of atherosclerotic plaque in lesion-prone areas of the ascending aorta was forestalled during the 1 month treatment period. Potential mechanisms for promoting quiescence in activated endothelium related to downregulation of inflammatory NFkB signaling activity through inhibition of the thrombin-PAR1 signaling are illustrated in Fig. 7.
Figure 7.
Schematic depiction of the effect of PPACK-NP on reducing the inflammatory effects of thrombin. Thrombin promotes the release of inflammatory molecules (cell adhesion molecules) and procoagulant molecules (TAT complexes, tissue factor) as demonstrated in this work, through the activation of the NF-κB pathway as measured through IκB degradation and phosphorylation of p65.
The direct relationship between thrombotic risk and endothelial barrier disruption (Suppl. Fig. I and Fig. 1D in 11) according to the metrics employed in these models confirms a primary role for intact endothelium in maintaining vascular homeostasis in atherosclerosis. Recent reports of the relationship between endothelial damage/sloughing and acute coronary syndromes in patients20 recalls original descriptions of hypercoagulable vascular erosions by the Virmani group21 and focuses attention on ways to measure and preserve endothelial integrity as a strategic path to the detection and reduction of thrombotic risk. Prior work in our lab indicates that the effects of cholesterol feeding elicit both procoagulant effects and barrier disruption only after some time on a sustained high fat diet: >3 months in ApoE-null mice11 and >6 months in NZW rabbits10). In ApoE-null mice, barrier disruption worsens progressively over time on a high fat diet, but can resolve rapidly within 2 months after switching to normal chow.11 Although leaky vasculature and reduced vasodilatory capacity associated with endothelial dysfunction may occur within weeks of inception of an atherogenic diet in the ApoE-null model22, the barrier disruption and procoagulant activity identified by our nanoparticle permeability metrics emerge later and may serve as more direct harbingers of thrombotic risk.
With respect to the underlying mechanisms responsible for endothelial damage, inflammatory signaling and immunomodulatory events orchestrated by various plaque cell types interacting with activated endothelium have been described in detail.6 Here we have focused on thrombin as a key instigator of plaque growth and instability contributing to endothelial activation, vessel inflammation, and hypercoagulability, as summarized in Figure 7. Surprisingly, after an aggressive one-month treatment period with PPACK-NP following 3 months of initial cholesterol feeding, marked benefits were observed even in the face of persistently elevated serum cholesterol. The role of thrombin not only as a principal prothrombotic agent, but also as an atherogenic molecule is not unexpected given that it drives many of the inflammatory molecules that participate in plaque growth such as NFkB17, NADPH oxidase23, VCAM-124, PDGF25, among many others.6 Thrombin’s role as a proinflammatory molecule through the activation of the NF-kB pathway results in numerous downstream effects that accelerate plaque development, cell infiltration, expression of inflammatory molecules, and promotion of hypercoagulability through stimulation and secretion of procoagulant enzymes.26
Of particular interest is the role of endothelial-specific NFkB activation in atherogenesis as demonstrated by Gareus et al. with the use of genetically engineered conditional knockouts of endothelial NFkB that markedly suppressed plaque formation in fat-fed ApoE−/− mice.27 Sun et al achieved similar results by delivering MiR-181b to fat-fed ApoE null mice for 12 weeks to inhibit nuclear translocation of NFkB in endothelium via importin, even in the face of high cholesterol levels, which is consistent with our present observations.28 The seminal observations of very early upregulation of NFkB in lesion prone aortic arch regions by Cybulsky’s group29 raises the interesting speculation of a role for thrombin even at these incipient time points, particularly in the context of previously documented clusters of intense, albeit small, clusters of endothelial apoptosis and replication in these aortic arch regions even in normal subjects.30,31 The ability to achieve focal suppression of NFkB with nanoparticle delivery systems that might abrogate endothelial PAR-1 activation could help to maintain a more quiescent endothelial phenotype (e.g., reduced sVCAM: Fig 5B), preserve barrier integrity, and simultaneously reduce paracrine crosstalk with other inflammatory plaque components.
A pleiotropic response to the suppression of thrombin signaling in diverse cell types that participate in atherogenesis is evidenced by modulation of NFkB in THP-1 as well as HAEC cells (Fig. 4A–J, and Suppl. Fig. II), and our previous reports of reduced platelet content in clots that are produced by vessel injury.12,13 Regarding platelet activation, synergistic benefits also could accrue by local inhibition of thrombin-PAR-1 signaling through the NFkB axis.32 However, as a potential caveat, it is interesting to note that selective inhibition of NFkB in macrophage populations has been associated with increased atherosclerosis as contrasted with more specific inhibition of endothelial NFkB.33 Although we show that NFkB may be downregulated in representative human monocyte cell lines in vitro by interrupting thrombin/PAR-1 activation (Fig. 3A) and in vivo through quantification of pp65 staining in PPACK-NP treated mice, the exact relationships between macrophages and endothelial signaling and responses to this intervention remain to be defined.
Recent experimental reports have explored the role of thrombin and related coagulation enzymes in promoting atherosclerosis with the use of orally administered antithrombotic agents or genetically modified mice for primary prevention of atherosclerosis.34–36 All such studies report significant decreases in overall plaque extent and reduced expression of inflammatory mediators. Secondary prevention clinical trials in patients with acute coronary syndromes using oral anti-thrombotic agents have shown very modest effects on subsequent clinical events related to atherosclerosis progression, but at the risk of significantly increased bleeding.9 Interestingly, nanoparticle-based thrombin inhibition exhibits similar therapeutic effects to that of the experimental studies mentioned above, but with significantly fewer treatments (12 doses over 4 weeks), no requirement for cholesterol reduction (Fig. 6F), and a more promising safety profile as coagulation parameters and bleeding times have been shown to normalize within 30–60 minutes after i.v. injection.12
We observed no significant change in plaque macrophage content between treatment groups (Fig. 6A–D) in contrast to the 50% decrease in plaque macrophages reported by Hara et al. after 5 months of Xa inhibition.37 However, despite our shorter 1-month time window of therapeutic intervention that may not have allowed for reduced plaque macrophage content, our observations of rapid downregulation of NF-kB in macrophage-rich regions and downstream inflammatory markers (TF, TAT complexes, sVCAM) (Fig. 4E–H), is consistent with the similar observations of Kadoglou et al in dabigatran-treated ApoE-null mice.35 These results also accord with previously published data38 demonstrating the effect of thrombin and PPACK-thrombin in modulating the expression of TF in human saphenous vein endothelial cells, which is thought to be due to activation of NF-kB. Future work will aim to elucidate the effects of PPACK-NP on plaque healing, with respect to local vascular smooth muscle cell populations and fibrosis in response to therapy over longer follow-up intervals.
The optimal dosing interval for this therapy and the duration of the local effect on barrier integrity and procoagulant activity remain to be defined. Fortunately, the pharmacokinetics (nanoparticle clearance half life: ~ 2 hours, Suppl. Fig. III) is dominated primarily by the nanoparticle itself, with only modest alterations induced by conjugation of the active pharmacological ingredient (Suppl. Fig. IV) or other surface constituents. This minor difference in PK may allow for convenient swapping of alternative anticoagulants such as bivalirudin as we have shown previously13, while allowing for adequate circulation time to allow for sufficient plaque uptake of nanoparticles, demonstrated in mouse, rabbit and human samples through prior work in our lab.10,11 These PK parameters are advantageous for potential clinical applications, as we have shown in mice that nanoparticle clearance through the RES (Suppl. Fig. V) results in reduction of residual circulating (i.e., nontrapped) bioactive conjugated PPACK or bivalirudin moieties within 30–60 minutes to a level below that required to alter systemic clotting and bleeding parameters.12,13 The potential disadvantage of intravenous nanotherapy also is notable, but there are a number of clinical scenarios that might benefit from early and aggressive treatment for a period of time before effective cholesterol control could be established. It is also important to note that the particular thrombin inhibitor PPACK may not be entirely specific to thrombin as a serine protease inhibitor, with PPACK exerting inhibitory effects on other trypsin-like proteases such as Factor Xa. Despite its effects on other proteases, however, PPACK remains a strong inhibitor of thrombin, where the inhibitory effect of PPACK on Xa has been previously shown to be 3 orders of magnitude less than on thrombin.39 Nevertheless, local inhibition of other coagulation proteases may in fact exert a synergistic but still locally constrained effect in preventing the activation of thrombin.
Supplementary Material
Significance.
This study illustrates a principal mechanistic role for thrombin in vascular inflammation and atherosclerosis progression that depends in part on its deleterious consequences for vascular barrier function. The direct and progressive relationship between endothelial barrier disruption and vessel thrombotic risk provides a clear rationale for designing therapeutic approaches that seek to preserve or restore endothelial integrity as a strategy to ameliorate atherosclerosis. To this end, the application of thrombin-inhibiting nanoparticles for focal control of procoagulant plaque activity without the need for systemic anticoagulation is demonstrated for the first time.
Acknowledgments
The authors thank Mike Scott, Xiaoxia Yang, and Stacy Allen for animal experiment and nanoparticle formulation assistance and Huiying Zhang and Noriko Yanaba for cryostat sectioning and histology assistance.
Sources of Funding
This work was supported in part by NIH grants HL073646, HL112303, DK095555, AR056223, The Foundation for Barnes-Jewish Hospital, Foundation Fund Number B5076-40, Award Number 3570 and the James R. Hornsby Family Dream Garden Investment Partnership to S.A.W.
Non-standard Abbreviations and Acronyms
- NP
Nanoparticle
- PFC
Perfluorocarbon
- 19F
Fluorine
- PFOB
Perfluorooctylbromide
- CE
crown ether
- MRS
Magnetic Resonance Spectroscopy
Footnotes
Disclosures
SAW reports financial disclosures for Acuplaq, LLC (founder and equity).
References
- 1.Libby PP. Inflammation in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borissoff JIJ, Spronk HMHH, Cate ten HH. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 3.Kalz J, Cate ten H, Spronk HMH. Thrombin generation and atherosclerosis. J Thromb Thrombolysis. 2014;37:45–55. doi: 10.1007/s11239-013-1026-5. [DOI] [PubMed] [Google Scholar]
- 4.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci US A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borissoff JI, Spronk HMH, Heeneman S, Cate ten H. Is thrombin a key player in the “coagulation-atherogenesis” maze? Cardiovascular Research. 2009;82:392–403. doi: 10.1093/cvr/cvp066. [DOI] [PubMed] [Google Scholar]
- 7.Olson ES, Whitney MA, Friedman B, Aguilera TA, Crisp JL, Baik FM, Jiang T, Baird SM, Tsimikas S, Tsien RY, Nguyen QT. In vivo fluorescence imaging of atherosclerotic plaques with activatable cell-penetrating peptides targeting thrombin activity. Integr Biol (Camb) 2012;4:595–605. doi: 10.1039/c2ib00161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borissoff JI, Heeneman S, Kilinç E, Kassák P, van Oerle R, Winckers K, Govers-Riemslag JWP, Hamulyák K, Hackeng TM, Daemen MJAP, Cate ten H, Spronk HMH. Early atherosclerosis exhibits an enhanced procoagulant state. Circulation. 2010;122:821–830. doi: 10.1161/CIRCULATIONAHA.109.907121. [DOI] [PubMed] [Google Scholar]
- 9.Costopoulos C, Niespialowska-Steuden M, Kukreja N, Gorog DA. Novel oral anticoagulants in acute coronary syndrome. Int J Cardiol. 2013;167:2449–2455. doi: 10.1016/j.ijcard.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Zhang L, Myerson J, Bibee K, Scott M, Allen J, Sicard G, Lanza G, Wickline S. Quantifying the evolution of vascular barrier disruption in advanced atherosclerosis with semipermeant nanoparticle contrast agents. PLoS ONE. 2011;6:e26385–e26385. doi: 10.1371/journal.pone.0026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palekar RU, Jallouk AP, Goette MJ, Chen J, Myerson JW, Allen JS, Akk A, Yang L, Tu Y, Miller MJ, Pham CTN, Wickline SA, Pan H. Quantifying progression and regression of thrombotic risk in experimental atherosclerosis. FASEB J. 2015;29:3100–3109. doi: 10.1096/fj.14-269084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myerson J, He L, Lanza G, Tollefsen D, Wickline S. Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. J Thromb Haemost. 2011;9:1292–1300. doi: 10.1111/j.1538-7836.2011.04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myerson JW, He L, Allen JS, Williams T, Lanza G, Tollefsen D, Caruthers S, Wickline S. Thrombin-inhibiting nanoparticles rapidly constitute versatile and detectable anticlotting surfaces. Nanotechnology. 2014;25:395101–395101. doi: 10.1088/0957-4484/25/39/395101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1831–1834. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]
- 15.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:2079–2093. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- 16.Maganto-Garcia E, Tarrio M, Lichtman AH. Mouse models of atherosclerosis. Curr Protoc Immunol. 2012;15:1–23. doi: 10.1002/0471142735.im1524s96. [DOI] [PubMed] [Google Scholar]
- 17.Rahman A, Fazal F. Blocking NF-κB: an inflammatory issue. Proceedings of the American Thoracic Society. 2011;8:497. doi: 10.1513/pats.201101-009MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nylaende M, Kroese A, Stranden E, Morken B, Sandbaek G, Lindahl AK, Arnesen H, Seljeflot I. Prothrombotic activity is associated with the anatomical as well as the functional severity of peripheral arterial occlusive disease. Thromb Haemost. 2006;95:702–707. [PubMed] [Google Scholar]
- 19.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 20.Lampka M, Grąbczewska Z, Jendryczka-Maćkiewicz E, Hołyńska-Iwan I, Sukiennik A, Kubica J, Halota W, Tyrakowski T. Circulating endothelial cells in coronary artery disease. Kardiol Pol. 2010;68:1100–1105. [PubMed] [Google Scholar]
- 21.Farb A, Burke AP, Tang AL, Liang Y, Mannan P, Smialek J, Virmani R. Coronary Plaque Erosion Without Rupture Into a Lipid Core : A Frequent Cause of Coronary Thrombosis in Sudden Coronary Death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 22.Meyrelles SS, Peotta VA, Pereira TM, Vasquez EC. Endothelial Dysfunction in the Apolipoprotein E-deficient Mouse: insights into the influence of diet, gender and aging. Lipids Health Dis. 2011;10(1):211. doi: 10.1186/1476-511X-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagadeesha DK, Takapoo M, Banfi B, Bhalla RC, Miller FJ. Nox1 transactivation of epidermal growth factor receptor promotes N-cadherin shedding and smooth muscle cell migration. Cardiovascular Research. 2012;93:406–413. doi: 10.1093/cvr/cvr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. Journal of Clinical Investigation. 2001;107:1209–1210. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowen-Pope DF, Raines EW. History of discovery: platelet-derived growth factor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:2397–2401. doi: 10.1161/ATVBAHA.108.179556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffel J, Lüscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 27.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJJ, Kardakaris R, Polykratis A, Kollias G, de Winther MPJ, Pasparakis M. Endothelial Cell-Specific NF-κB Inhibition Protects Mice from Atherosclerosis. Cell Metabolism. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, He S, Wara AKM, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, Feinberg MW. Systemic delivery of microRNA-181b inhibits nuclear factor-κB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2014;114:32–40. doi: 10.1161/CIRCRESAHA.113.302089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci US A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson G, Chao S, Schwartz SM, Reidy MA. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. The American Journal of Pathology. 1985;121:123–127. [PMC free article] [PubMed] [Google Scholar]
- 31.Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- 32.Malaver E, Romaniuk MA, D’Atri LP, Pozner RG, Negrotto S, Benzadon R, Schattner M. NF-kappaB inhibitors impair platelet activation responses. J Thromb Haemost. 2009;7:1333–1343. doi: 10.1111/j.1538-7836.2009.03492.x. [DOI] [PubMed] [Google Scholar]
- 33.Kanters E, Pasparakis M, Gijbels MJJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJA, Clausen BE, Förster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MPJ. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. Journal of Clinical Investigation. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pingel S, Tiyerili V, Mueller J, Werner N, Nickenig G, Mueller C. Thrombin inhibition by dabigatran attenuates atherosclerosis in ApoE deficient mice. Arch Med Sci. 2014;10:154–160. doi: 10.5114/aoms.2014.40742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadoglou NPE, Moustardas P, Katsimpoulas M, Kapelouzou A, Kostomitsopoulos N, Schafer K, Kostakis A, Liapis CD. The beneficial effects of a direct thrombin inhibitor, dabigatran etexilate, on the development and stability of atherosclerotic lesions in apolipoprotein E-deficient mice : dabigatran etexilate and atherosclerosis. Cardiovasc Drugs Ther. 2012;26:367–374. doi: 10.1007/s10557-012-6411-3. [DOI] [PubMed] [Google Scholar]
- 36.Lee I-O, Kratz MT, Schirmer SH, Baumhäkel M, Böhm M. The effects of direct thrombin inhibition with dabigatran on plaque formation and endothelial function in apolipoprotein E-deficient mice. Journal of Pharmacology and Experimental Therapeutics. 2012;343:253–257. doi: 10.1124/jpet.112.194837. [DOI] [PubMed] [Google Scholar]
- 37.Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, Yagi S, Yamada H, Soeki T, Wakatsuki T, Shimabukuro M, Sata M. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis. 2015;242:639–646. doi: 10.1016/j.atherosclerosis.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Bartha K, Brisson C, Archipoff G, la Salle de C, Lanza F, Cazenave JP, Beretz A. Thrombin regulates tissue factor and thrombomodulin mRNA levels and activities in human saphenous vein endothelial cells by distinct mechanisms. Journal of Biological Chemistry. 1993;268:421–429. [PubMed] [Google Scholar]
- 39.Kettner C, Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14:969–973. doi: 10.1016/0049-3848(79)90014-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.