SUMMARY
Reward learning gives rise to strong attentional biases. Stimuli previously associated with reward automatically capture visual attention regardless of intention [1–12]. Dopamine signaling within the ventral striatum plays an important role in reward learning, representing the expected reward initiated by a cue [13,14]. How dopamine and the striatum may be involved in maintaining behaviors that have been shaped by reward learning, even after reward expectancies have changed, is less well understood [15]. Nonspecific measures of brain activity have implicated the striatum in value-based attention [11,12,16–18]. However, the neurochemical mechanisms underlying the attentional priority of learned reward cues remain unexplored. Here, we investigated the contribution of dopamine to value-based attention using positron emission tomography (PET) with [11C]raclopride. We show that, in the explicit absence of rewards, the magnitude of attentional capture by previously reward-associated but currently task-irrelevant distractors is correlated across individuals with changes in available D2/D3 dopamine receptors (presumably due to intrasynaptic dopamine) linked to distractor processing within the right caudate and posterior putamen. Our findings provide direct evidence linking dopamine signaling within the striatum to the involuntary orienting of attention, and specifically to the attention-grabbing quality of learned reward cues. These findings also shed light on the neurochemical basis of individual susceptibility to value-driven attentional capture, which is known to play a role in addiction [19–21]. More broadly, the present study highlights the value and feasibility of using PET to relate changes in the release of a neurotransmitter to learning-dependent changes in healthy adults.
RESULTS
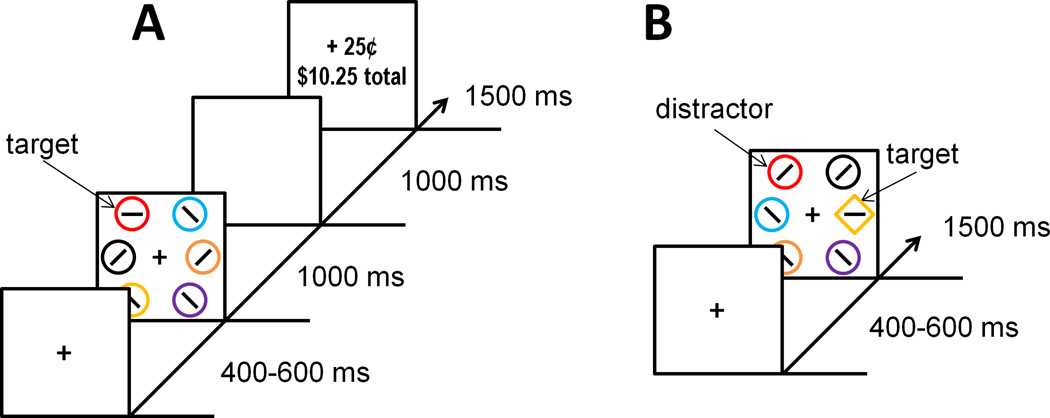
Healthy human participants (n = 20) performed an experiment comprising a training phase and a test phase. The training phase involved a visual search task in which locating and reporting each of two color-defined targets was associated with a monetary reward outcome (Fig. 1a). The following day, two PET scans were conducted while participants completed an unrewarded shape-search task in which the color of the shapes was task-irrelevant (Fig. 1b). During the distractor absent scan, no stimuli were ever rendered in the color of a formerly reward-predictive target shown during training, whereas during the distractor present scan, half of the trials contained a distractor rendered in a previously reward-associated color (the scans were otherwise identical).
Figure 1.
Time course and trial events for the training phase (A) and test phase (B) of the experimental task.
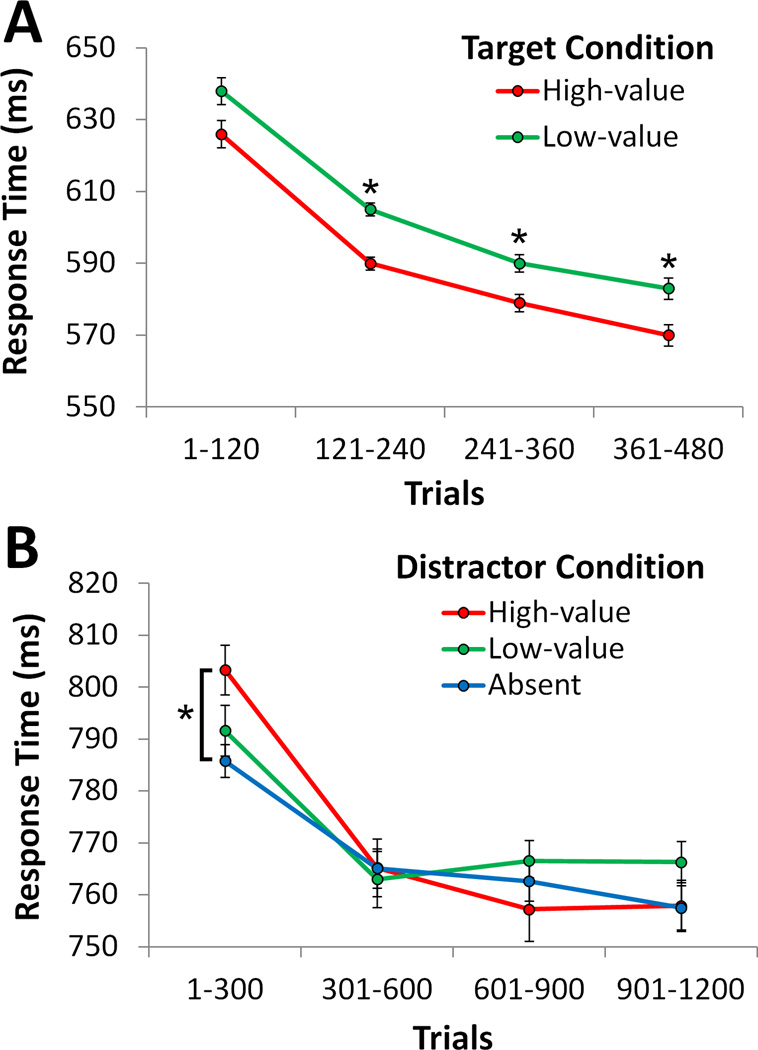
During the training phase, participants were faster to report the target that was associated with higher reward, indicating learning of the stimulus-reward associations. This value-driven bias was reliable from the second epoch (trials 121–240) of the training phase onward [ts > 2.12, ps < .048; first epoch: t = 1.57, p = .135] (Fig. 2a). An attentional bias for these previously reward-associated stimuli was evident in the first epoch of trials during the test phase (trials 1–300), as indicated by a slowing of response time (RT) on high-value distractor trials compared to distractor absent trials [t = 2.74, p = .014] (Fig. 2b), replicating previous behavioral results [1–5,10,11]. This learned bias then extinguished in the continued absence of reward and by the second epoch was no longer statistically reliable. Consistent with prior reports [1,3,4,20,22], the low-value distractor did not significantly impair performance [t = 1.03, p = .316]. There were no differences in accuracy across the experimental conditions in either phase of the experiment (see Table S1 and S2).
Figure 2.
Response time by value condition during the training phase (A) and test phase (B) of the experiment. Error bars reflect the within-subjects SEM. *p < .05. See also Table S1 (for panel A) and S2 (for panel B).
As in prior studies [1,3,7,19,20], there were substantial individual differences in the extent to which the previously reward-associated distractors influenced performance. For some participants, the previously-high-value distractors greatly impaired target detection, while for other participants these same distractors had no measurable cost associated with their presence. To better understand the nature of these individual differences, we examined the relationship between the attentional bias observed in behavior and the availability of D2/D3 dopamine receptors in the striatum as measured using PET. Regional concentrations of [11C]raclopride, a radiolabelled D2/D3 receptor antagonist, provide a measure of available dopamine receptors. By comparing the binding potential of [11C]raclopride across scans, relative increases or decreases in the release of endogenous dopamine over a broad timescale can be determined. The relatively weak affinity of [11C]raclopride makes it well-suited for detecting changes in dopamine release specifically in the striatum.
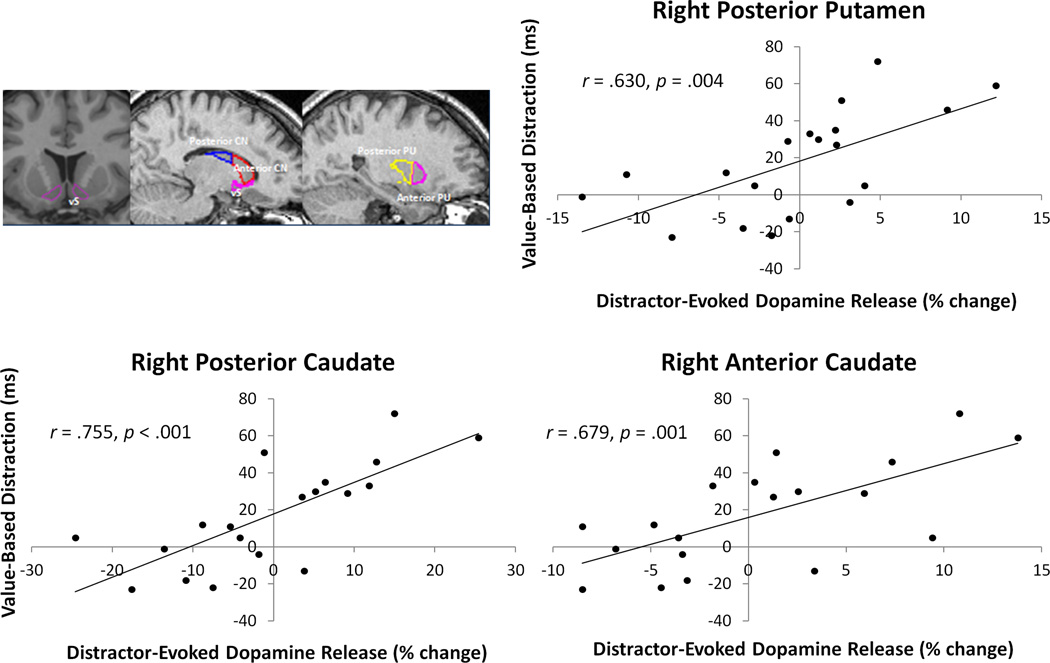
Within each of the striatal volumes of interest (VOIs) (see Experimental Procedures), we tested for a correlation across subjects of the magnitude of an individual's value-based attentional bias during the first epoch of the test phase to the magnitude of change in that participant's dopamine release attributable to the presence of the previous reward-associated distractors (see Experimental Procedures: Brain-behavior correlations). Significant correlations, Bonferroni corrected for multiple comparisons (α = .005), were observed in the right anterior and posterior caudate, along with the right posterior putamen (see Fig. 3). A complete list of the observed correlations across the ten VOIs tested, along with mean binding potential across the ten VOIs for each scan, is provided in Table S3. The findings remain significant using a non-parametric randomization test (ps < .005, see Experimental Procedures). As expected, no significant correlations were observed using RT differences computed from any of the latter three epochs of the test phase, by which point the value-driven attentional bias had extinguished.
Figure 3.
Visual depiction of VOIs and observed correlations between value-based distraction and distractor-evoked dopamine release (see Methods for calculations) across participants. vS = ventral striatum, CN = caudate nucleus, PU = putamen. See also Table S3.
Baseline RT (mean RT on distractor absent trials) did not correlate with the distractor-related dopamine release in any of the 10 VOIs (ps > .27), and the three reported correlations (Fig. 3) remain significant when baseline RT is included as a covariate (ps ≤ .005). Thus, our findings are not confounded by individual differences in overall processing speed. The correlations reported in Fig. 3 either remain significant (caudate: ps < .04) or marginally significant (putamen: p = .09) if value-driven attentional bias is measured as the difference in RT on high-value compared to low-value distractor trials. The difference in RT between low-value distractor trials and distractor absent trials was unrelated to dopamine release in these regions (ps > .76), as were measures of learning rate during the training phase and the extinction rate of value-based distraction during the first epoch of the test phase (ps > .49; see Experimental Procedures: Brain-behavior correlations).
Interestingly, the observed correlations seemed to be driven to some extent by dopamine release that was greater on distractor absent scans in some participants. To explore this further, we performed a median split over value-driven attentional bias (y-axis in Fig. 3) and examined dopamine release separately in the resulting two groups. Collapsing across the three regions identified in Fig. 3, significantly elevated dopamine release [t = 3.47, p = .008] was observed in the participants exhibiting robust value-driven attentional bias [t = 8.15, p < .001]. Importantly, for the low-bias group, which showed no evidence of attentional capture [t = −1.14, p = .285], dopamine release was suppressed on distractor present scans [t = −3.15, p = .012]. This suggests a bivalent relationship between dorsal striatal dopamine and value-driven attention: robust value-driven attentional capture is associated with significantly elevated levels of endogenous dopamine, whereas the ability to ignore previously reward-associated stimuli is associated with the suppression of dopamine release in these same regions.
DISCUSSION
In the context of learning, striatal dopamine is known to play a role in representing the expected reward signaled by a predictive cue [13,14,23]. Striatal dopamine also plays a role in voluntary motor behavior [24], the learning and execution of habits [15], and cue-elicited motivation [25]. When a cue is consistently paired with a reward outcome, an automatic bias to orient attention to this cue develops that is evident even when reward is not expected [1–5,9–12]. In the present study, we demonstrate a link between the release of dopamine within the dorsal striatum and involuntary perseveration of attentional bias after a change in the reward-related priority given to a cue.
Here we show that the orienting of attention to previously reward-associated stimuli is positively correlated with the release of dopamine within the caudate and posterior putamen. These findings are anatomically consistent with recent fMRI and single-unit recording studies of value-based attention [11,16–18], and further reveal the neurochemical basis for these attentional priority signals. Importantly, we demonstrate such automatic attentional orienting in a context in which reward is no longer available and where participants are informed that the previously reward-associated features (color) are completely irrelevant to the task. The results are thus isolated to involuntary, cue-triggered processes not tied to currently expected reward.
As would be expected from the correlation, individuals exhibiting robust value-based distraction also exhibited significantly elevated dopamine release on the scan in which the distractors were present. Interestingly, the ability to resist attentional capture by the distractors was associated with a corresponding suppression of dopamine release. Suppression of attentional capture by reward cues has been documented previously, particularly in the context of attention to drug cues in successful abstainers [20,21,26]. The observed suppression provides converging evidence for striatal dopamine signals underlying value-based attention, and offers a neural mechanism by which the suppression of distraction due to previously-learned value associations might occur.
Dopamine release within the caudate and putamen is known to underlie habit learning and the expression of habitual behaviors [15]. Relatedly, dopamine release within these same two structures is associated with craving elicited by drug cues [27,28]. Our findings suggest that value-based attention may be governed by similar neural mechanisms. The caudate in particular has been implicated in the shifting of covert attention [29]. More specifically, neural responses in the tail of the caudate have been shown to be sensitive to learned value [17,18,30] and play an important role in value-based distraction [11,17,18]. Given the involvement of this structure in the representation of value and its close connections to the visual system through the visual corticostriatal loop [31], the caudate tail is well situated to integrate these two sources of information into a value-modulated attentional priority signal. It should be noted, however, that in the present dataset, the standardized segmentation procedures we employed divide the caudate in the middle of the body and thus preclude direct comparison to prior fMRI [11] and single-unit [17] findings specific to the tail of the caudate.
Our findings contribute to a growing literature relating dopamine levels as measured via PET to individual differences in measures such as drug craving [27,28], impulsiveness [32], romantic excitement [33], willingness to work for reward [34], eating behavior [35], and harm avoidance [36]. In each of these cases, such individual differences reflect preexisting variation across participants. To our knowledge, the ability to measure learning-related changes in the dopamine system using PET has not been established. Our study highlights the feasibility of using PET to examine the role of dopamine in attentional bias and experimentally-induced learning. Multimodal imaging, combining PET with the temporal and spatial resolution of fMRI [11,12,16], holds promise in uncovering the temporal dynamics of dopamine signaling as it relates to attention and the expression of learned value in humans.
Individuals differ in both the degree to which reward history biases attention and the amount of striatal dopamine released in response to reward information. Abnormally high susceptibility to value-driven attentional capture is associated with addiction [19–21], and dopamine release within the dorsal striatum has been linked to cue-evoked drug craving [27,28]. Here, we demonstrate a neurochemical link that underlies these two sources of individual variation: dopamine release within the caudate and posterior putamen predicts value-driven attentional capture, and the suppression of value-driven attentional capture is associated with the suppression of dopamine in these same regions. The present study thereby identifies a potential target for pharmacological manipulation of value-based attention, which could have benefits for a variety of problematic behaviors to which such attentional biases contribute.
EXPERIMENTAL PROCEDURES
Participants
Twenty (10 female) healthy adult volunteers (18–31 years of age, mean = 23.4 y) who were free of medical or neuropsychiatric disorders participated in the experiment. Screening criteria included a negative drug test and the exclusion of major medical or neuropsychiatric disorders past or present. All subjects received a detailed physical exam including vital signs, 12 lead ECG, blood for complete blood count with differential, complete metabolic panel, blood clotting parameters, creatinine (CPK) for muscle toxicity, urine for urinalysis, and toxicology for drugs of abuse and alcohol breathalyzer before the PET scans. Informed consent was obtained from all participants, and all procedures were approved by the Institutional Review Board of the Johns Hopkins University School of Medicine and conformed to the principles outlined in the Declaration of Helsinki.
Experimental Task
The experiment consisted of a training phase and a test phase. Both phases of the experiment were performed while the participant lay within the PET scanner, although only during the test phase was PET data acquired -- this was done to match the context within which the two phases were completed as much as possible, as value-based attentional biases can be sensitive to contextual information [5]. Participants viewed the stimuli on a LCD monitor using prism mirrors that allow horizontal viewing in the supine position while retaining the same right left orientation. The experiment was run on a Dell Latitude E6400 computer running Matlab software with Psychophysics Toolbox extensions [37], and behavioral responses were made using a modified keyboard with all keys except "z" and "m" removed. The training phase was performed the evening before the test phase.
Training Phase
During the training phase (see Fig. 1a), each trial consisted of a fixation display, a search array, and a feedback display. The fixation display was presented for 400, 500, or 600 ms (randomly determined on each trial), the search array for 1000 ms, and the reward feedback display for 1500 ms. A 1000 ms blank screen was inserted between the search and feedback displays and between trials. Participants were instructed to search for a target circle that was unpredictably red or green and report the orientation of a bar within the target as either vertical or horizontal via a button press ("z" and "m", respectively). Half of the trials contained a red target and half contained a green target; each target color appeared in each of the six possible stimulus positions equally-often. The order of trials was randomized. Each circle in the search array was approximately 3.4° × 3.4° visual angle in size. The middle of the three circles on each side of the screen was presented 10° center-to-center from fixation, and the two other circles were presented 8° from the vertical meridian, 6° above and below the horizontal meridian. The six stimuli in the search array were all distinct, salient colors.
Following a correct response that fell within a 1000 ms response deadline, money was added to a bank total in the reward-feedback display. If participants responded incorrectly or too slowly, the reward feedback display indicated that 0¢ had been earned for that trial. Additionally, if a response was not made before the 1000 ms deadline, participants were presented with a 250 ms 1000 Hz tone. One of the two target colors (counterbalanced across participants) was followed by a high reward of $1.50 on 80% of the trials on which it was correctly reported, and by a low reward of 25¢ on the remaining 20% of correct trials (high-value color); for the other (low-value) color, these mappings were reversed. Participants were provided with a brief rest period every 120 trials.
Test Phase
For the test phase (see Fig. 1b), each trial consisted of a fixation display (400–600 ms), a search array (1500 ms), and an inter-trial-interval during which the fixation cross was visible for 400 ms and then removed for 100 ms. Targets were now defined as the unique shape, either a diamond among circles or a circle among diamonds (equally-often), and participants made the same identity judgment concerning the orientation of the bar contained within the target. The colors of the shapes were now irrelevant to the task, and participants were instructed to ignore color and focus on identifying the unique shape. No trial-by-trial feedback about performance was provided.
The test phase consisted of two 1200-trial scans. On the distractor present scan, one of the nontarget shapes was rendered in the color of the formerly high-value target (high-value distractor) on 25% of trials, and likewise in the color of the formerly low-value target (low-value distractor) on another 25% of trials. On the remaining 50% of the trials, none of the shapes were rendered in the color of a formerly reward-predictive target (distractor absent trials). During the distractor absent scan, none of the trials contained a previously reward-associated color (the same as distractor absent trials from the distractor present scan). The order of distractor present and distractor absent scans was counterbalanced across participants. Targets and distractors appeared equally-often in each of the six possible stimulus positions. Participants were provided with a brief rest period every 60 trials.
Acquisition of Neuroimaging Data
MRI
Anatomical MRI scans were obtained for each participant on a day prior to PET scanning. A 3T Siemens Trio MRI was used to acquire a T1 sagittal (TR = 500 ms TE = 8 ms), T1 SPGR (spoiled grass sequence; TR = 35 ms, TE = 6 ms), and T2 (TR = 5900 ms, TE = 6 ms) image.
PET
Participants performed the test phase task over the course of two 60 min PET scans. PET was performed on a high resolution research tomograph (HRRT) in three dimensional mode with a 2.5 mm resolution [38]. For each scan, 20 mCi of [11C]raclopride was administered intravenously as a bolus injection (mean ± SD injected radioactivity: 19.0 ± 1.6 vs. 19.8 ± 0.8 mCi; mean ± SD injected non-radioactive mass of raclopride: 1.2 ± 0.4 vs. 1.1 ± 0.3 µg, for distractor absent and distractor present scans, respectively; no statistical differences). The two scans were separated by 75 min. The head is stabilized for both PET and MRI by an individualized thermoplastic mask and Velcro straps. A laser light in the PET scanner is used to line up an axial line on the mask and the scanner bed and subject head tilt are monitored by the PET technologist for the entire scan.
Definition of VOIs
Volumes of interest (VOIs) were defined from the MRI data using the 3-D interactive-segmentation mode of a locally developed VOI defining tool (VOILand), as previously reported [39], and using published segmentation guidelines [39–41]. Then, striatal VOIs were subdivided according to the model advanced by Mawlawi et al. [42] to the ventral striatum, and anterior/posterior putamen and caudate nucleus (5 subdivisions per side) using a semi-automated method that incorporated anatomical guidance based on post-mortem human materials [39,43]. VOIs were transferred from MRI to PET space according to MRI-to-PET coregistration parameters obtained with the coregistration module [44] in SPM5 (The Statistical Parametric Mapping 5; The Wellcome Trust Centre for Neuroimaging; available at www.fil.ion.ac.uk/spm), and applied to PET frames to obtain regional time (radio-)activity curves (TACs).
Reconstruction of PET Data
Emission PET scans were reconstructed using the iterative ordered-subset expectation-maximization algorithm correcting for attenuation, scatter, randoms and dead-time [45] and including inter-frame head motion correction including transmission-emission alignment for the individual frames [46]. The radioactivity was corrected for physical decay to the injection time. Reconstructions included dynamic PET frames of 256 (left-to-right) by 256 (nasion-to-inion) by 207 (neck-to-cranium) voxels with 1.22mm isotropic dimensions. The frame schedules were four 15-s, four 30-s, three 1-min, two 2-min, five 4-min, and twelve 5-min frames.
Data Analysis
Behavior
Mean RT and accuracy were computed for each experimental condition. Only correct RTs were included in the mean, and RTs faster than 200 ms or exceeding 3 SD of the mean were trimmed as in prior studies [1–5,19,20]. Data from each phase of the experiment were further broken down into four equally-sized trial bins, as in [2].
PET
Nondisplaceable binding potential (BPND; ref. 47) of [11C]raclopride was obtained by the reference tissue graphical analysis (RTGA; ref. 48) for striatum subdivisions. Then, dopamine release (DARel in %; ref. 49) was obtained using the following formula: (BPND[A] − BPND[P])/BPND[A] × 100, where [A] and [P] stands for BPND of the distractor absent and distractor present scans, respectively. Data for one participant was unusable due to a technical error that resulted in an asynchrony between PET data acquisition a nd the administration of the experimental task.
Brain-Behavior Correlations
Within each of the striatal VOIs, we tested for a correlation (Pearson's r) across participants of the magnitude of an individual's value-based attentional bias (slowing of RT on high-value distractor trials compared to distractor absent trials during the first epoch, see Fig. 2b) to the magnitude of dopamine release attributable to distractor processing using the calculation described above. Bonferroni correction was used to set the overall type-I error rate at .05 (α = .005 for each of ten correlations). Significant correlations obtained using Pearson's r were further scrutinized via a randomization test in which the probability of each correlation was estimated non-parametrically by randomly shuffling the xy pairings (n interations = 10000). To examine the potential influence of learning rate during the training phase on our measure of striatal dopamine release, for each participant we defined learning rate both as the difference in the RT facilitation by the high-value target (low-value minus high-value target RT) between the first and last epoch, as well as a linear fit to the change in this measure over all four epochs. To examine the potential influence of extinction rate during the test phase, for each participant we computed the difference in value-based attentional bias (slowing of RT on high-value distractor trials compared to distractor absent trials) during the first and second half of the first epoch (i.e., trials 1–150 vs trials 151–300).
Supplementary Material
Acknowledgments
The reported research was supported by NIH grants R01-DA013165 to S.Y. & S.M.C., S10-RR017219 to D.F.W., S10-RR023623 to D.F.W., and F31-DA033754 to B.A.A. The funding sources played no role in the study beyond financial support. Special thanks to Andrew Crabb, MS, Ayon Nandi, MS and Joshua Roberts, PhD for technical and/or editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
B.A.A. & S.Y. developed the study concept; B.A.A., H.K., D.F.W., & S.Y. designed the experiment; D.F.W., E.G.G., J.R.B., N.G., & B.F. conducted the experiment; B.A.A., H.K., D.F.W., & A.R. analyzed and chose the analytic tools for the data; A.R. contributed custom software used in data analysis; all authors contributed to the interpretation of the data and the writing and editing of the manuscript.
REFERENCES
- 1.Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proc Natl Acad Sci, USA. 2011;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson BA, Laurent PA, Yantis S. Learned value magnifies salience-based attentional capture. PLoS ONE. 2011;6(11):e27926. doi: 10.1371/journal.pone.0027926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BA, Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Atten Percept Psychophys. 2012;74:1644–1653. doi: 10.3758/s13414-012-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson BA, Yantis S. Persistence of value-driven attentional capture. J Exp Psychol Hum Percept Perform. 2013;39:6–9. doi: 10.1037/a0030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson BA. Value-driven attentional priority is context specific. Psychon Bull Rev. 2015;22:750–756. doi: 10.3758/s13423-014-0724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Libera C, Chelazzi L. Learning to attend and to ignore is a matter of gains and losses. Psychol Sci. 2009;20:778–784. doi: 10.1111/j.1467-9280.2009.02360.x. [DOI] [PubMed] [Google Scholar]
- 7.Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. J Neurosci. 2010;30:11096–11103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond JE, O'Brien JL. Selective visual attention and motivation: The consequences of value learning in an attentional blink task. Psychol Sci. 2009;20:981–988. doi: 10.1111/j.1467-9280.2009.02391.x. [DOI] [PubMed] [Google Scholar]
- 9.Le Pelley ME, Pearson D, Griffiths O, Beesley T. When goals conflict with values: Counterproductive attentional and oculomotor capture by reward-related stimuli. J Exp Psychol Gen. 2015;144:158–171. doi: 10.1037/xge0000037. [DOI] [PubMed] [Google Scholar]
- 10.Anderson BA. A value-driven mechanism of attentional selection. J Vis. 2013;13(3):7, 1–16. doi: 10.1167/13.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson BA, Laurent PA, Yantis S. Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Res. 2014;1587:88–96. doi: 10.1016/j.brainres.2014.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickey C, Peelen MV. Neural mechanisms of incentive salience in naturalistic human vision. Neuron. 2014;85:512–518. doi: 10.1016/j.neuron.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 14.Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- 15.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 16.Krebs RM, Boehler CN, Roberts KC, Song AW, Woldorff MG. The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cereb Cortex. 2012;22:607–615. doi: 10.1093/cercor/bhr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto S, Kim HF, Hikosaka O. Reward value-contingent changes in visual responses in the primate caudate tail associated with a visuomotor skill. J Neurosci. 2013;33:11227–11238. doi: 10.1523/JNEUROSCI.0318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hikosaka O, Yamamoto S, Yasuda M, Kim HF. Why skill matters. Trends Cogn Sci. 2013;17:434–441. doi: 10.1016/j.tics.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson BA, Faulkner ML, Rilee JJ, Yantis S, Marvel CL. Attentional bias for non-drug reward is magnified in addiction. Exp Clin Psychopharmacol. 2013;21:499–506. doi: 10.1037/a0034575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson BA, Kronemer SI, Rilee JJ, Sacktor N, Marvel CL. Reward, attention, and HIV-related risk in HIV+ individuals. Neurobiol Dis. doi: 10.1016/j.nbd.2015.10.018. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Anderson BA. Value-driven attentional capture in the auditory domain. Atten Percept Psychophys. doi: 10.3758/s13414-015-1001-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A. Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci. 2004;24:4105–4112. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 25.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 26.Stromark KM, Field NP, Hugdahl K, Horowitz M. Selective processing of visual alcohol cues in abstinent alcoholics: an approach-avoidance conflict? Addict Behav. 1997;22:509–519. doi: 10.1016/s0306-4603(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brašić JR, Kimes AS, Maris MA, Kumar A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 29.Fairhall SL, Indovina I, Driver J, Macaluso E. The brain network underlying serial visual search: comparing overt and covert spatial orienting, for activations and for effective connectivity. Cereb Cortex. 2009;19:2946–2958. doi: 10.1093/cercor/bhp064. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S, Monosov IE, Yasuda M, Hikosaka O. What and where information in the caudate tail guides saccades to visual objects. J Neurosci. 2012;32:11005–11016. doi: 10.1523/JNEUROSCI.0828-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seger CA. The visual corticostriatal loop through the tail of the caudate: circuitry and function. Front Syst Neurosci. 2013;7(104) doi: 10.3389/fnsys.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi K, Mizuno K, Sasaki AT, Wada Y, Tanaka M, Ishii A, Tajima K, Tsuyuguchi N, Watanabe K, Zeki S, et al. Imaging the passionate stage of romantic love by dopamine dynamics. Front Hum Neurosci. 2015;9:191:1–6. doi: 10.3389/fnhum.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Logan J, Gatley SJ, Ding YS, Wong C, et al. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: a high resolution PET study. Biol Psychol. 2011;87:164–167. doi: 10.1016/j.biopsycho.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 38.Sossi V, de Jong HWAM, Barker WC, Bloomfield P, Burbar Z, Camborde M-L, Comtat C, Eriksson LA, Houle S, Keator D, et al. The second generation HRRT: a multi-centre scanner performance investigation. IEEE Nucl Sci Symp Conf Rec (2015) 2005:2195–2199. [Google Scholar]
- 39.Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brašić JR, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- 40.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 42.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci. 1999;11:71–78. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- 44.Ashburner J, Friston KJ. Rigid body re gistration. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human Brain Function. 2nd. Academic Press; 2003. [Google Scholar]
- 45.Rahmim A, Cheng JC, Blinder S, Camborde ML, Sossi V. Statistical dynamic image reconstruction in state-of-the-art high-resolution PET. Phys Med Biol. 2005;50:4887–4912. doi: 10.1088/0031-9155/50/20/010. [DOI] [PubMed] [Google Scholar]
- 46.Keller SH, Sibomana M, Olesen OV, Svarer C, Holm S, Andersen FL, Højgaard L. Methods for motion correction evaluation using 18F-FDG human brain scans on a high-resolution PET scanner. J Nucl Med. 2012;53:495–504. doi: 10.2967/jnumed.111.095240. [DOI] [PubMed] [Google Scholar]
- 47.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 48.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Innis RB, Malison RT, al-Tikriti M, Hoffer PB, Sybirska EH, Seibyl JP, Zoghbi SS, Baldwin RM, Laruellel M, Smith EO, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.