Based on their relative distance from sites of CNS injury or disease and the severity of insult, astrocytes and other glial cells characteristically become “reactive”. Reactive astrocytes are phenotypically different from mature protoplasmic or fibrous astrocytes that reside in normal, healthy tissue. During the process of reactive astrogliosis, mature astrocytes undergo dramatic changes in gene and protein expression that beget changes in morphology (hypertrophy) and cell cycle status (Fig 1). Proliferating reactive astrocytes in close proximity to damaged/dying cells carry out key functions that include neuroprotection, preservation/re-establishment of the blood-brain barrier (BBB), regulation of immune cell responses, and glial scar formation [1]. Importantly, mechanistic understanding and mapping of the signaling network that controls reactive astrogliosis has great potential to elucidate therapeutic targets and inform the development of new treatments to promote recovery and improve outcomes in patients with CNS injury or disease.
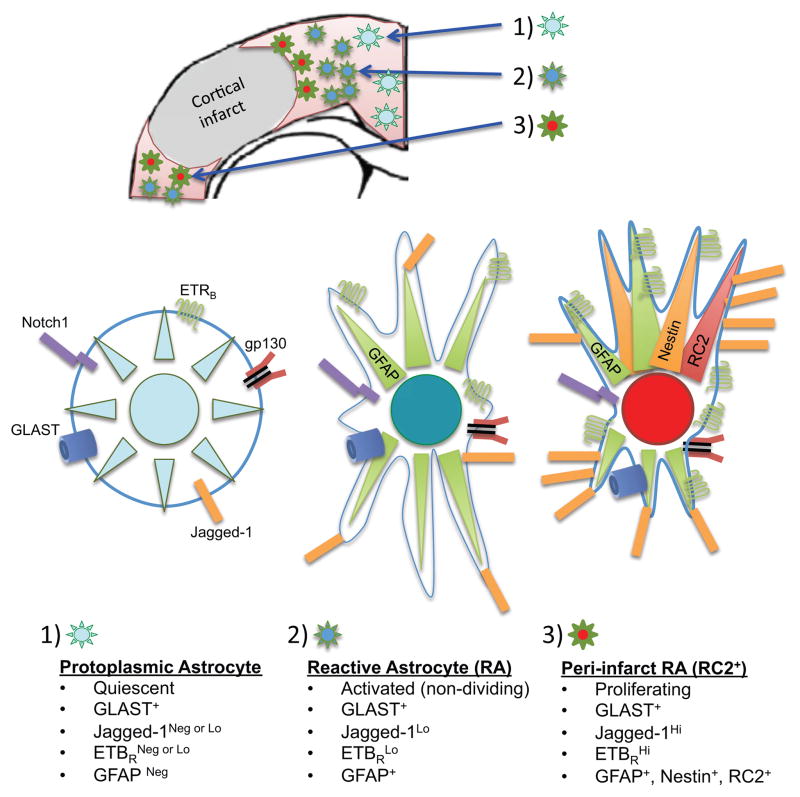
Fig 1. Sub-types of reactive astrocytes in the cerebral cortex 3 days after stroke.
(A) Distal to the stroke infarct, GLAST+ protoplasmic astrocytes within uninjured parenchymal tissue express undetectable or low levels of Jagged-1 and ETBR and are negative for GFAP. (B) Towards the outer edge of the peri-infarct area, GLAST+ reactive astrocytes express low levels of Jagged-1, ETBR and GFAP, but do not undergo cell division. (C) Located immediately adjacent to the infarct core, proliferating GLAST+ reactive astrocytes express GFAP, Nestin, and the RC2 antigen. Notably, sub-type (C) is significantly depleted after conditional knockout of Notch1 or ETBR in GFAP-positive reactive astrocytes in transgenic mice with stroke. Note that all three astrocyte subtypes express cytokine receptors such as gp130, and are thus sensitive to inflammatory mediators such as IL-6.
Multiple proteins/peptides commonly released during CNS injury have been shown to stimulate reactive astrocyte proliferation such as epidermal growth factor (EGF), fibroblast growth factor (FGF), and endothelin (ET-1). Other cytokines/growth factors, molecules, and conditions known to trigger reactive astrogliosis include (but are not limited to): IL-1, IL-6, IL-10, LIF, CNTF, BMP, TGF-β, TNF-α, INF-γ, lipopolysaccharide, glutamate, adenosine triphosphate, nitric oxide, reactive oxygen species, and hypoxia and glucose deprivation during tissue ischemia [1]. Although numerous signals released or produced during CNS injury have been shown to stimulate reactive astrocytes in culture, the complex signaling network that regulates reactive astrocyte proliferation and function(s) in vivo remains poorly understood.
Reactive astrocytes commonly exhibit increased expression of several intermediate filament proteins such as Glial Fibrillary Acidic Protein (GFAP), Nestin, and RC2, a specialized isoform of Nestin with post-translational modifications (Fig 1). Previously, our group demonstrated Notch1 signaling was required for the proliferation of reactive astrocytes after stroke. Using an inducible transgenic mouse model with Cre-loxP-mediated gene excision (GFAP-CreER-Notch1-cKO), we reported that conditional knockout (cKO) of Notch1 in reactive astrocytes significantly reduced the number of RC2-positive cells immediately adjacent to the infarct core and increased immune cell invasion after stroke [2].
Recently, we demonstrated that the RC2 antigen marks specifically the subpopulation of proliferating reactive astrocytes after stroke and traumatic brain injury and reported a method to prospectively isolate the subpopulation of proliferating reactive astrocytes from injured CNS tissue based on expression of the glutamate-aspartate transporter (Glast) and Jagged1, a Notch1 ligand [3]. In the peri-infarct area after stroke, RC2-positive reactive astrocytes expressed high levels of ETBR, prompting us to investigate whether Notch1 signaling interacted with ETBR. We found that Jagged1/Notch1 signaling increased ETBR levels in an indirect manner, by stabilizing phosphorylated STAT3 (p-STAT3), a transcriptional activator for EDNRB (ETBR gene). Subsequent experiments with GFAP-CreER-ETBR-cKO mice demonstrated that ETBR was necessary for reactive astrocyte proliferation after stroke. Notably, the deficit observed in GFAP-CreER-ETBR-cKO mice closely resembled that of GFAP-CreER-Notch1-cKO mice. Notch1-STAT3-ETBR signaling may affect numerous cell types that proliferate during development, tissue repair, and cancer. In addition to primary adult astrocytes, we observed that Notch1-STAT3-ETBR signaling regulated proliferation and/or survival of primary neonatal astrocytes as well as human tumor cell lines derived from kidney (HEK 293) or brain (U87; glioma, astrocytoma)[3].
Whereas we found that Jagged1/Notch1 signaling promoted increased ETBR levels in postnatal astrocytes and adult reactive astrocytes, ET-1 was recently shown to induce Jagged1 expression in cultured astrocytes and to alter Notch signaling in vivo during the first week after lysolecithin-induced focal demyelination [4]. In the same injury model, reactive astrogliosis was previously shown to result in ET-1 signaling through ETBR, ERK and JNK pathways, and activation of the transcription factor c-jun [5]. Importantly, c-jun can bind to cis-acting AP-1 DNA elements in the Jag1 promoter to drive Jagged1 expression [6]. In light of these observations, our recent data suggest a feed-forward loop whereby ET-1 signaling through ETBR increases Jagged1 levels, which then can activate Notch1 signaling in adjacent astrocytes. Notch1 signaling increases the expression of ETBR by stabilizing p-STAT3, and in turn enhances expression of Jagged1 by amplifying ET-1 signaling through ETBR. Accordingly, we expect that Jagged1/Notch1, STAT3, and ET-1/ETBR signaling all contribute to driving reactive astrocyte proliferation after CNS injury through this feed-forward loop. Similar feed-forward signaling loops promoted by STAT3 may be a common driver for rapid cell proliferation after tissue injury and during cancer. Feed-forward signaling through STAT3 is reported to regulate cell proliferation in tumors and transformed cells from several tissue types as well as those of the blood [7–9].
Acknowledgments
This work was supported by National Institutes of Health (NIH) R01 NS073815 (to J.L.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimada IS, Borders A, Aronshtam A, Spees JL. Proliferating reactive astrocytes are regulated by Notch-1 in the peri-infarct area after stroke. Stroke. 2011;42:3231–3237. doi: 10.1161/STROKEAHA.111.623280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeComte MD, Shimada IS, Sherwin C, Spees JL. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proc Natl Acad Sci USA. 2015;112:8726–87. doi: 10.1073/pnas.1501029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond TR, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre AA, et al. Astrocyte-Derived Endothelin-1 Inhibits Remyelination through Notch Activation. Neuron. 2014;81:588–602. doi: 10.1016/j.neuron.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal S, Lakoma A, Chen Z, Hicks J, Metelitsa LS, Kim ES, et al. G-CSF Promotes Neuroblastoma Tumorigenicity and Metastasis via STAT3-Dependent Cancer Stem Cell Activation. Cancer Res. 2015;75:2566–2579. doi: 10.1158/0008-5472.CAN-14-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong AH, Wei P, Zhang S, Yao J, Yuan Y, Zhou AD, et al. FoxM1 Drives a Feed-Forward STAT3-Activation Signaling Loop That Promotes the Self-Renewal and Tumorigenicity of Glioblastoma Stem-like Cells. Cancer Res. 2015;75:2337–2348. doi: 10.1158/0008-5472.CAN-14-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann AA, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]