Abstract
Purpose
This study investigated changes in selected tear cytokine concentrations (IL-1β, IL-1Ra, IL-6, IL-10, IL-12(p70) and TNF-α) following a 1-week washout from soft contact lens wear (CLW), and the repeatability of cytokine measurements using custom Multiplex assays.
Methods
A total of 10 subjects completed this 6-visit (immediately following contact lens removal, and after 1, 2, 3, 4 and 7 (±1) days without CLW) pilot study. Approximately 20–30μl of pooled basal tears were collected from both eyes at each visit. Two custom Multiplex assays were used by 2 operators to quantify the concentration of tear cytokines. Tear samples from subjects 1–6 were analysed using the first kit by operator 1. Tear samples from subject 7–10 plus additional tear samples from subjects 1–5, which were used to determine between-kit/operator repeatability, were analysed using the second kit by operator 2. Linear mixed models were used to determine changes in tear cytokine concentrations over time. Between-kit/operator and within-kit/operator repeatability was assessed using a Bland and Altman analysis.
Results
There were no significant changes in tear cytokine concentrations over a 1-week washout of CLW. More than 99% of the tear samples had detectable levels of cytokines using custom Multiplex assays. Within-kit/operator repeatability was good, but between-kit/operator repeatability was poor; likely due to protein degradation, differences in operator experience and operating procedures.
Conclusion
A washout period may not be necessary when evaluating changes in tear cytokines with new contact lenses or lens care products. A well-trained operator using standardized operating procedures can produce repeatable measurements using custom Multiplex assays.
Keywords: Washout period, Contact lens, Tear cytokines, Multiplex, Repeatability
Introduction
A range of pro-inflammatory cytokines can be detected in human tears and the relationship with ocular surface inflammation is beginning to be identified. Tear pro-inflammatory cytokines, including interleukin (IL)-1β, 6, 12(p70) and tumor necrosis factor-α (TNF-α) promote the inflammatory response on the ocular surface, and are detected in dry eye1–4, ocular allergy,5 bacterial keratitis,6 graft versus host disease7 and contact lens wear.8–11 Specifically, mature IL-1β is involved in upregulating inflammatory cellular activity and defending the ocular surface against infection.12–16 IL-6 and TNF-α also regulate inflammatory cells on the ocular surface to defend against pathogens and promote epithelial wound healing.17–21 IL-6 stimulates activation of cytotoxic T cells and the expression of intercellular adhesion molecule.16 TNF-α stimulates the acute phase reaction, reacts with other cytokines (IL-1 and 17a), and leads to pathogen apoptosis.6,16,22 IL- 12 is a potent regulator of T-helper type 1 (Th1) cells which help to eliminate bacteria on the ocular surface.23,24 Other cytokines in tears include inhibitory factors, including IL-1Ra and IL-10. IL-1Ra inhibits the activity of IL-1β by binding to the Type 1 IL-1 receptor.25 IL-10 downregulates IL-12, TNF- α, and IL-8, but not IL-6.23
Many inflammatory proteins on the ocular surface are upregulated with soft contact lens wear.26–29 For example, higher tear concentrations of TNF-α were found in rigid contact lens wearers compared to non-lens wearers.30 A few studies show that IL-6 and 8 increased significantly after daily disposable or reusable soft contact lens wear.8,9,31 A period of washout from contact lens wear or ocular drop use has been suggested to return the ocular surface to a “baseline” state prior to further study.27–29 A washout of between one day and one month has been used in studies of the impact of contact lenses and lens care products on the inflammatory state of the eye, but there is no evidence to support the need for, or appropriate duration of, a washout period.27–29
Repeatability and reproducibility of Multiplex assays using tears have been previously reported, including intra-assay with different dilutions,32 and intra- and inter-day variation.33 In healthy non-contact lens wearers and dry eye subjects, the day to day and diurnal differences in tear IL-1Ra,33,34 IL-633 and IL-834 were stable, whereas diurnal variation was significantly higher in IL-10 and IL-1β.33
This study aimed to investigate the time course of changes in tear cytokine concentration following contact lens removal, and the reproducibility of cytokine measurements using Multiplex assays.
Methods
A prospective pilot study was carried out between December 2014 and February 2015. A convenience sample of 10 subjects was enrolled in this pilot study. The study followed the tenets of the Declaration of Helsinki. The Institutional Review Board at the State University of New York, College of Optometry (SUNY Optometry) approved the research before data collection began. Written informed consent was obtained from all subjects prior to their participation in this study.
Adult subjects were recruited from the faculty, staff and students of SUNY Optometry. Only full time soft contact lens wearers (who wore contact lenses for at least one year, more than 5 days per week, 6 hours per day) were recruited. Exclusion criteria included a prior history of refractive surgery, self or practitioner diagnosis of dry eye, ocular or systemic disease likely to affect the ocular surface [e.g. thyroid disease, diabetes], ocular treatment with anti-inflammatory medications, and pregnancy during the study period. No eye-drop or eye-wash use was allowed during the study period.
There were six study visits (baseline, and after 1, 2, 3, 4 and 7(±1) days without contact lens wear. All study visits were conducted between 12pm and 7pm, within a 3 hour visit window for each subject in order to minimize diurnal variation in tear cytokine levels.29,33 At the baseline (first) visit, general health and contact lens history information was queried. Subjects were asked to wear their contact lenses to the first study visit and remove them just prior to the slit lamp examination. An anterior segment slit lamp examination was conducted at visits 1 and 6 to assess the presence or absence of lid flakes, lid margin notching, matted lashes, discharge, bulbar and limbal redness, papillae and follicles, corneal and conjunctival edema, corneal vascularization or scaring. Tear collection was conducted at each visit as described below. After tear collection at visits 1 and 6, sodium fluorescein dye was instilled to assess corneal surface staining using the Cornea and Contact Lens Research Unit (CCLRU) scale.35 The total staining score was the sum of all five corneal regions.
At each visit, approximately 20–30 microliters (μl) of pooled basal tears from both eyes were collected from the lateral canthus using disposable glass capillaries (Blaubrand intraMARK, Wertheim, Germany) taking care to avoid touching the conjunctiva. To ensure the basal tears were collected, the tears with the flow rate of <4μl/minute were only collected. The collected tears were expelled from the glass capillary into an extended capacity centrifuge tube using a tear pump and centrifuged at 4000 rpm for 5 minutes at 4°C. Collected tears were transferred to a new extended capacity centrifuge tube, leaving any cells or lipid debris at the bottom of the tube. In order to optimise the analysis of tear cytokine levels, a 1:10 dilution was utilized.32,36 A described previously,32 twelve μl of tears were required to allow duplicates for the analysis (6μl per well).The 12μl of tears were diluted with 108 μl of 0.55% Bovine Serum Albumin (BSA) in the provided sample buffer and stored at −80°C until analysis. Each diluted sample was incubated at −80°C for at least 24 hours before Multiplex assay analysis since pre-dilution of samples has been demonstrated to provide better repeatability.32,36 A total of 50μl of the diluted samples were allocated to each well with the additional 10μl prepared for potential loss during pipetting. A multi-channel pipette was used to distribute the samples and assay buffers into the wells. A Bio-Rad Multiplex assay (Bio-rad Laboratories Inc., CA, USA) was selected because of the low coefficients of variation and good sensitivity for low concentration cytokines in tears.32,37 Custom Multiplex assays (X plex format, Human cytokine group 1, 6 factors: IL-1β, IL-1Ra, IL-6, IL-10. IL-12p70 and TNF-α) were analyzed the tear samples following the manufacturers’ protocols. According to the manufacturer, modified limits of detection (pg/ml) were: IL-1β 0.03, IL-1Ra 4.17, IL-6 0.44, IL-10 0.13, IL-12p70 0.63, TNF-α 0.22. The beads were counted and analyzed in Bio-Plex® Magplex™ Multiplex reader (Bio-Rad, CA, USA) and standard curves of known concentrations of each cytokine were used to calculate the concentration in the tear samples. The percentage of cytokine recovery was also calculated as the expected concentration of the cytokines divided by the observed concentration of the cytokines times 100 using the Multiplex software.
The tear samples from subjects 1 to 6 were analysed using one custom Multiplex kit (kit 1) by operator 1 and a month later when tear collection of subject 7–10 was completed, samples from subjects 7 to 10 were analysed using a second kit (kit 2) by operator 2. To examine the between kit/operator repeatability, extra tear samples from subjects 1–5 (17 samples in total) were also allocated in the second kit. The same protocol was followed for both kits but the procedures were conducted by different operators (kit 1-operator 1; kit 2-operator 2).
Statistical analysis
Data were analyzed using SPSS version 22 (SPSS for Mac, Chicago, IL), and significance was considered when p values were less than 0.05. A convenient sample size of 10 subjects was included in this study. Linear mixed models were used to assess changes in tear cytokine concentrations over time. Where significant overall mean change was found, post-hoc comparisons were conducted to examine mean changes between visits. Fisher’s Exact Test was carried out to assess the presence or absence of clinical signs between visit 1 and visit 6. Wilcoxon Signed Rank test was carried out to examine the difference in corneal staining between visit 1 and 6.
Seventeen tear samples were used to assess between-kit/operator repeatability while 31 for kit 1/operator 1 and 21 samples for kit 2/operator 2 were used to assess within-kit/operator repeatability. Bland and Altman analysis was used to assess between- and within-kit/operator repeatability. For between-kit/operator repeatability, the results were plotted as per Bland and Altman38,39 and the coefficient of repeatability (CoR = 1.96*SD) was calculated from the bias (mean of the difference between kit 1/operator 1 and kit 2/operator 2) and the limits of agreement (LoA = bias ± 1.96*SD of the differences between 2 kits/operators). The CoRs of the cytokine levels indicate that 95% of the differences between 2 kits/operators can be expected to lie between the limits of agreement. For within-kit/operator repeatability, bias, CoR and LoA were calculated between repeat measurements. The Wilcoxon signed rank test was used to examine the differences in tear cytokine concentrations between two repeats within- and between-kits/operators.
Results
Five males and five females, aged 21 to 56 years (Mean [±SD], 25.6 [±6] years) completed the study. Four reported use of daily disposables contact lenses and the other six were wearers of monthly reusable contact lenses. Fifty-nine tear samples were available for analysis as the tear volume of one sample (subject 1, visit 3) was less than 6μl. All samples were detectable in the Multiplex assays except for 1 (subject 10, visit 2), for which the concentrations of IL-10 and IL-12(p70) were beyond the linear range of detection. All detectable measured tear cytokines were within the middle range of the standard curves, which demonstrates that they were well within the detection range of the kits. The recoveries of the cytokine concentrations in both kits were between 95 and 103%, indicating that the kits were sensitive and reliable.
Between-kit/operator repeatability
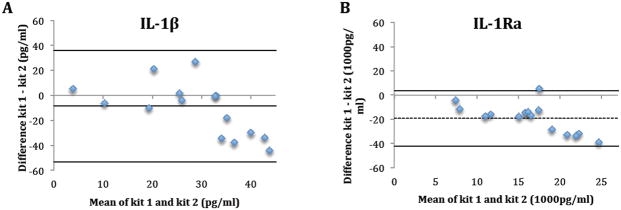
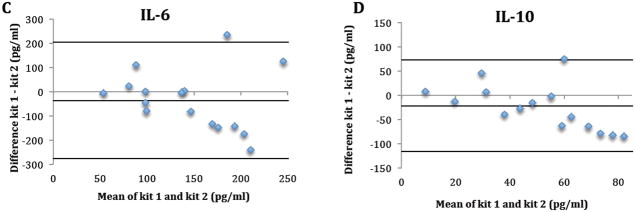
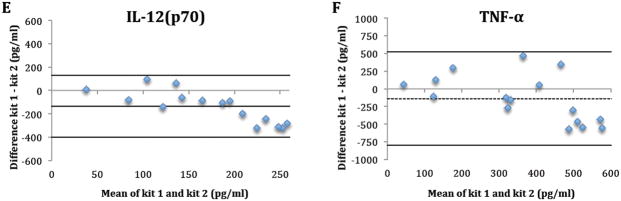
Seventeen tear samples from 5 subjects were available for between-kit repeatability. One sample (subject 4, visit 3) was later excluded due to low bead counts (<25), which indicates less than 25 readings for tear analysts in a sample. Table 1 and Figure 1 summarize the between-kit/operators repeatability of tear cytokine concentrations. For all cytokines, concentrations determined using kit 1 by operator 1 were higher than concentrations using kit 2 by operator 2 as indicted by the negative bias (Figure 1). The only statistically significant difference between kits was for IL-1Ra (p<0.01) (Table 1). The between-kit/operator CoRs were larger than the mean measurements of the cytokines in both kits/operators, indicating poor between-kit/operators repeatability. (Table 1 and Figure 1).
Table 1.
Between custom Multiplex assays repeatability of the selected tear cytokine concentrations.
| Tear cytokine concentration | IL-1β | IL-1Ra | IL-6 | IL-10 | IL-12(p70) | TNF-α |
|---|---|---|---|---|---|---|
| Kit 1 by operator 1 (pg/ml) | 33.6±20.1 | 25608.4±10854.5 | 156.4±91.9 | 61.5±39.7 | 239.0±131.3 | 416.9±301.0 |
| Kit 2 by operator 2 (pg/ml) | 25.2±15.9 | 7034.5±5118.9 | 120.8±77.4 | 41.1±27.2 | 115.6±59.1 | 282.0±164.7 |
| p value | 0.11 | <0.01 | 0.14 | 0.09 | 0.15 | 0.80 |
| Bias (pg/ml) | −8.41 | −18573.9 | −35.7 | −20.3 | −133.7 | −134.9 |
| CoR (pg/ml) | ±43.2 | ±22666.6 | ±233.8 | ±92.3 | ±264.6 | ±640.1 |
| LoA (pg/ml) | 34.8; −51.7 | 4092.7; −41240.6 | 198.2; −269.5 | 71.9; −112.7 | 130.9; −398.3 | 505.2; −775.1 |
Data presented as mean± standard deviation (n=16).
CoR: coefficient of repeatability; LoA: Limits of agreement
Figure 1.
The difference in tear cytokine concentrations (IL-1β (A), 1Ra (B), 6 (C), 10 (D), 12(p70) (E) and TNF-α (F)) between kits/operators plotted against their mean. Dashed line: bias; Solid line: Limits of agreement.
Within-kit/operator Repeatability
Thirty-one samples in kit 1 conducted by operator 1 and 21 samples in kit 2 conducted by operator 2 were assessed for within-kit/operator repeatability. Table 2 shows the within-kit/operator repeatability for both kits. Mean cytokine concentrations between repeats within each kit were not significantly different and the within-kit/operator bias was also low for each kit (Table 2). The within-kit/operator CoRs in kit 2 conducted by operator 2 was smaller than kit 1, indicating that kit 2 conducted by operator 2 was more repeatable than kit 1.
Table 2.
Within custom Multiplex assay repeatability of the selected tear cytokine concentration.
| IL-1β | IL-1Ra | IL-6 | IL-10 | IL-12(p70) | TNF-α | ||
|---|---|---|---|---|---|---|---|
| Kit1 by operator1 (n= 31 samples) | Duplicate 1 (pg/ml) | 32.6±22.6 | 25737.0±15214.4 | 147.9±103.1 | 56.1±42.2 | 237.9±158.0 | 390.8±391.9 |
| Duplicate 2(pg/ml) | 31.1±19.1 | 29013.3±13770.1 | 157.2±110.1 | 59.0±43.4 | 247.7±168.6 | 430.3±401.3 | |
| p-value | 0.61 | 0.12 | 0.47 | 0.62 | 0.48 | 0.33 | |
| Bias (pg/ml) | 0.3 | −2814.0 | 3.1 | −4.1 | −14.8 | −29.2 | |
| CoR (pg/ml) | ±31.1 | ±22725.5 | ±75.5 | ±20.9 | ±149.3 | ±305.0 | |
| LoA (pg/ml) | 31.4, −30.8 | 19911.5, −25539.5 | 78.6, −72.4 | 16,8, −25.0 | 134.5, −164.1 | 275.8, −334.2 | |
| Kit2 by operator2 (n=21 samples) | Duplicate 1 (pg/ml) | 31.0±8.5 | 9737. 9±4475.5 | 138.4±38.8 | 46.3±10.9 | 145.0±32.0 | 326.9±110.9 |
| Duplicate 2 (pg/ml) | 33.5±8.3 | 10118.9±4009.6 | 146.3±38.2 | 49.8±9.1 | 153.9±29.7 | 367.9±112.8 | |
| p-value | 0.11 | 0.61 | 0.38 | 0.22 | 0.39 | 0.11 | |
| Bias (pg/ml) | −0.7 | −151.4 | −0.4 | −0.9 | 1.8 | −12.6 | |
| CoR (pg/ml) | ±12.9 | ±3905.7 | ±62.4 | ±21.8 | ±67.1 | ±208.5 | |
| LoA (pg/ml) | 12.2 −13.6 |
3754.3 −4057.1 |
62.8 −62.0 |
20.9 −22.7 |
68.8 −65.3 |
195.9 −221.1 |
|
Data presented as mean± standard deviation.
CoR: coefficient of repeatability; LoA: Limits of agreement.
Contact Lens Washout
Slit lamp findings at baseline and 7 days after contact lens removal were not significantly different (p>0.05). Several epithelial microcysts were observed in one subject (subject 3) at baseline but had recovered by day 7. The presence of mild bulbar conjunctival redness was observed in all subjects at both visits. Corneal neovascularization and limbal redness were observed in one subject in both visits. Two subjects showed significant lid flakes at baseline and one at day 7. Average corneal staining was mild (4.9±4.3 at baseline and 2.9±2.5 at day 7 [p>0.05]).
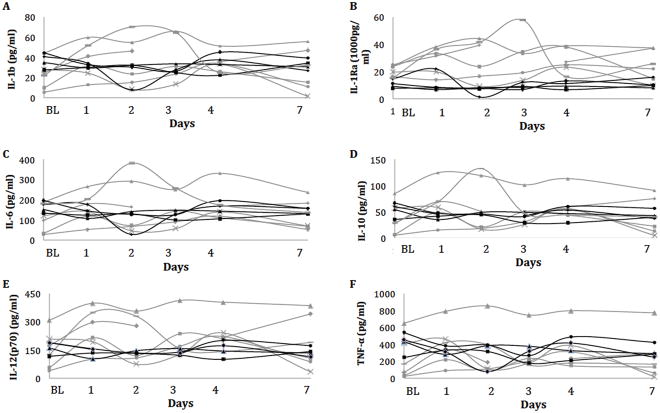
Tear samples of subject 1–6 in kit1 and tear samples of subject 7–10 in kit2 ware used for the washout analysis. Overall, there was no significant change in tear cytokine concentrations up to 7 days after contact lens removal (Figure 2) (all p>0.05). The overall mean change in tear cytokine concentrations from baseline over 7 days is presented in Table 3.
Figure 2.
Tear concentration of IL-1β (A), 1Ra (B), 6 (C), 10 (D), 12(p70) (E) and TNF-α (F) over time after contact lens removal measured using the custom Multiplex assays. Y-axis: Tear cytokine concentrations in pg/ml; X-axis: Days after contact lens removal. BL: Baseline, days following contact lenses removal. Grey lines shows subjects analysed with kit 1; black lines shows subjects analysed with kit 2.
Table 3.
Changes in tear cytokine concentration from baseline over six study visits (n=59; * n=58)
| IL-1β | IL-1Ra | IL-6 | IL-10* | IL-12(p70)* | TNF-α | ||
|---|---|---|---|---|---|---|---|
| Overall mean change from baseline | pg/ml | 4.6±2.8 | 4143.7±1550.9 | 14.6±11.2 | 7.9±6.0 | 39.8±16.8 | 24.9±46.3 |
| % | 16% | 25% | 11% | 18% | 25% | 8% |
Discussion
This study showed that the tear concentrations of selected cytokine did not significantly change over a 7-day period after contact lens removal. The repeatability of custom Multiplex assays was poor between different kits/operators while the within-kit/operator repeatability was good in kit 2.
No changes in tear cytokine concentrations were found in the 7 days following contact lens removal. This finding suggests that a washout period in 1 week may not be necessary. It has previously been shown that IL-6, IL-8 and TNF-α concentrations increased in new wearers,8,9,30,31 However the tear cytokine concentrations were similar between established contact lens wearers and non-wearers across studies as shown in Table 4. If it is not possible to observe changes in tear cytokine concentration in the 7 days following contact lens removal, a longer washout may not be feasible in many clinical trials. This study indicates that the cytokine levels are relatively stable over one week of discontinuation for >1 year contact lens wearers and thus any changes can be attributed to new interventions (ie, refitting in another contact lens or introducing a new lens care product).
Table 4.
Summaries of the selected cytokine concentration measured using Multiplex assays in normal subjects.
| n / age (years) ELISA and tear volume | IL-1β | IL-1Ra | IL-6 | IL-10 | IL-12 | TNF-α | |
|---|---|---|---|---|---|---|---|
| Current study# | 10 (6 visits) / 26 Bio-Rad, basal tears (6 μl) | 31±14 | 19264±11421 | 141±66 | 50±27 | 190±99 | 327±217 |
| Kalsow, 2013 * # | 26 (BL) / 26 Bio-Rad, basal tear (5.5μl) | 8 | 18000 | 85 | 78 | 170 | 400 |
| Wilcox 2015 # | 44 $ / 26 Bio-Rad, basal tear (3 μl) | 22 | 2697 | 54 | NA | 134 | 74 |
| Malvitte, 2007 | 12 / 69 Bio-Rad, basal tear (2 μl) | 80 | NA | 1000 | 95 | 90 | 71 |
| Liu, 2010 | 15 / 59 Bio-Rad, basal tear (10 μl) | 20 | 3989 | 65 | 32 | 143 | NA |
| Lam, 2009 | 14 / 45 Milliplex, basal tear (1 μl) | 3 | NA | 27 | 1 | 118 | 127 |
| Enriquez-de-Salamanca, 2010 * | 9 / 58 Milliplex, basal tear (1 μl) | NA | 6500 | 200 | ^ | NA | NA |
| Carreno, 2010 | 9 / 33 Milliplex, basal tear (4 μl) | 101 | 3883 | 130 | 37 | 47 | 48 |
| Benito 2014 * + | 24 / 22 Milliplex, basal tear (4 μl) | 24 | 12000 | 30 | 10 | NA | NA |
| Leonardi,2006 * | 14 / 31 Milliplex, basal tear (50–100 μl) | 10 | NA | 8 | 2 | 10 | 10 |
| Massingale, 2009 | 14 / 50 Invitrogen, basal tear (10 μl) | 436 | NA | 632 | 256 | NA | 250 |
| Huang, 2012 | 22 / 28 Luminex, basal tear (10 μl) | NA | NA | NA | NA | NA | |
| Chong, 2010 | 29 / 67 Bio-Rad, NA, from strip | ||||||
| VanDerMeid, 2011* | 5/ 40 Luminex, NA, from strip | 0 | |||||
| Yamahuchi, 2014* | 14 / 39 Luminex, Flush tear (20 μl) | 11 | 83 | 667 | 17 | NA | NA |
All data presented in mean in pg/ml.
extrapolation from the published figure;
average of 3 evening visit;
contact lens wear;
BL: baseline visit only;
out of range.
10 days pooled tear.
Bold and italic: values that comparable with our results.
The large concentration range for different cytokines between studies could be due to the variations in the assays, tear dilution and preparation methods, or the methods of tear collection (Table 4). The differences are less likely to be due to variations in subject age (Table 4). The tear IL-1β, IL-6, IL-10 and TNF-α concentration in our study were comparable with Kalsow et al,27 which also examined contact lens wearers and used the same assays and similar dilution of the tears (Table 4). IL-12 is the least variable cytokine across studies (Table 4). Five previous studies have assessed IL-12 in healthy non-contact lens wearers,3,5,40–42 and 2 studies have assessed IL-12 in healthy contact lens wearers.27,36 We present comparable findings for healthy contact lens wearers during a washout period. IL-1β is the most variable cytokines, with only 2 out of 11 studies being comparable with our findings. Tears collected from a strip were unlikely to comparable to tears collected from micro-capillary tubes because of the use of dry strips, where the collected area on the ocular surface is larger than a capillary tube and is more likely to stimulate reflex tearing. Therefore, micro-capillary method is more likely to be representative of true cytokine levels in basal tear than the strip.43,44 The collection of flush tears rather than basal tears, or use of dilution that are too high or low may also lead to increased variation.32 Also, it may not be possible to compare across brands of assays, but studies using Bio-Rad Multiplex assays were more comparable (Table 4). In summary, the results presented here align well with studies in habitual soft contact lens wearers 27,36 using similar tear collection and storage methods, Biorad assays and sample dilutions41.
This is the first study to report on the use of custom Multiplex assays to assess tear cytokine concentrations. Our findings show ≥99% of the selected cytokines could be detected in the tear samples; this compares favorably with earlier studies using pre-prepared assays (Table 5). This study showed that the six selected cytokines could be detected in tears using a custom Multiplex assay. Other study groups have reported that many cytokines cannot be detected in tears using pre-prepared Multiplex assays in normal tears.2,4,5,32–34,36,40 This may be because other Multiplex assay include multiple cytokines which could introduce noise through non-specific binding and interactions.34,36,40
Table 5.
The available percentage of the basal tear samples collected from micro-capillary tubes which the selected cytokine was detected.
| n/visits ELISA and tear volume | IL-1B | IL-1Ra | IL-6 | IL-10 | IL12(p70) | TNF-α | |
|---|---|---|---|---|---|---|---|
| Current study | 10/6 Bio-Rad, basal tears (6μl) | 100% | 100% | 100% | 100% | 99% | 99% |
| Leonardi, 2006 | 14/1 Milliplex, basal tear (50–100μl) | 7% | NA | 100% | 7% | 7% | 7% |
| LaFrance, 2008 | Unk/1 Milliplex, basal tear (5μl) | 61% | 94% | 94% | 79% | 73% | 12% |
| Enriquez-deSalamanca, 2010 | 9/1 Milliplex, basal tear (1μl) | 30% | 52% | 65% | 48% | NA | 2% |
| Carreno, 2010 | 9/1 Milliplex, basal tear (4μl) | 100% | 100% | 100% | 100% | 5% | 50% |
| Benito 2014 | 24/3 Milliplex, basal tear (4μl) | 50–79% | 97–99% | 64–89% | 18–69% | NA | 1–13% |
| Willcox 2014 | 44/1 Bio-Rad, basal tear (3ul) | 50–60% | |||||
Unk: Unknown.
The repeatability of tear cytokine measurements was poor when two separate custom assay kits were used. For each of the cytokines, the coefficient of repeatability (CoR) between the two kits was similar in magnitude to the mean concentration (Table 1). In addition, a size effect was apparent for all tested cytokines, which is possible to affect the repeatability by increasing the tear cytokine concentrations. While this levels of repeatability may be acceptable for studies of corneal infiltration or bacterial keratitis and glaucoma, where the cytokine concentrations differ more than 2–5 times between cases and controls,6,42,44,45 it can introduce an unacceptably high level of noise in studies of contact lens wearers where small differences in cytokines are examined. Poor between-kit repeatability suggests that it may not be feasible to make absolute comparisons across studies using Multiplex.
Tear cytokine concentrations were measured to be higher in the first kit than the second kit, particularly for IL-1Ra. The higher concentrations could be due to protein degradation which occurred in the 30 days during which the tears were stored; however, a previous study,36 also suggests that IL-1Ra may not be a highly reproducible cytokine in tears. Even though higher concentration of other tested tear cytokines was found in the first kit, the differences did not reach to the significance levels. Therefore, this suggests that tear IL-1β, IL-6, IL-10, IL(p70) and TNF-α degrade in 30 days in a −80°C freezer but custom Multiplex still provides reproducible readings in these cytokines.
The within kit repeatability, especially as conducted by the second operator, were repeatable and reliable. As indicted in Table 2, the bias and the CoRs in the second kit were generally smaller than the first kit. The more reliable results were likely because the second operator had more experience with tear analysis.
There are two major limitations of this study. First, only ten subjects were enrolled in this pilot study. This small sample size may not be adequate to detect small but significant differences in cytokine levels. Therefore, a larger sample size and longer duration of study is needed to fully understand cytokine changes over time after contact lens removal. Another limitation of this study is the long time period gap between kit/operator testing. Our results suggest there may be some protein degradation over time or with repeated thawing and re-freezing of samples. Future studies should attempt to collect and analyze samples as quickly as possible and limit freeze-thaw cycles to minimize protein degradation.
In conclusion, this study confirmed that the selected tear cytokine concentrations did not change significantly within 1 week after discontinuation of contact lens wear. This indicates that a 7-day washout period may not significantly affect the ocular surface inflammatory state with contact lens, although a longer period may be required to the return to the levels to pre-contact lens wear. Also, a customized Multiplex assay was able to detect all the selected cytokines. While the between kit repeatability was poor, this study also showed that a well-trained operator can provide repeatable and reliable findings using custom Multiplex.
Acknowledgments
Funding: NIH NEI K23-EY019097
We would like to acknowledge the Magplex support from Mr Bob Laffer and Dr Tara Ellison from Bio-Rad Laboratories. We would like to thank Prof. Mark Willcox, School of Optometry and Vision Science, UNSW for his kind assistant with interpretation of the data.
Footnotes
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Massingale ML, Li X, Vallabhajosyula M, et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 2.Boehm N, Riechardt AI, Wiegand M, et al. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52:7725–7730. doi: 10.1167/iovs.11-7266. [DOI] [PubMed] [Google Scholar]
- 3.Lam H, Bleiden L, de Paiva CS, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205. e191. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 5.Leonardi A, Curnow SJ, Zhan H, et al. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36:777–784. doi: 10.1111/j.1365-2222.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Calvacanti BM, Cruzat A, et al. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55:7457–7466. doi: 10.1167/iovs.14-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riemens A, Stoyanova E, Rothova A, et al. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012;18:797–802. [PMC free article] [PubMed] [Google Scholar]
- 8.Thakur A, Willcox MD. Contact lens wear alters the production of certain inflammatory mediators in tears. Exp Eye Res. 2000;70:255–259. doi: 10.1006/exer.1999.0767. [DOI] [PubMed] [Google Scholar]
- 9.Poyraz C, Irkec M, Mocan MC. Elevated tear interleukin-6 and interleukin-8 levels associated with silicone hydrogel and conventional hydrogel contact lens wear. Eye Contact Lens. 2012;38:146–149. doi: 10.1097/ICL.0b013e3182482910. [DOI] [PubMed] [Google Scholar]
- 10.Thakur A, Willcox MD. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp Eye Res. 1998;67:9–19. doi: 10.1006/exer.1998.0480. [DOI] [PubMed] [Google Scholar]
- 11.Carnt NA, Willcox MD, Hau S, et al. Association of single nucleotide polymorphisms of interleukins-1beta, -6, and -12B with contact lens keratitis susceptibility and severity. Ophthalmology. 2012;119:1320–1327. doi: 10.1016/j.ophtha.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 13.Black RA, Kronheim SR, Sleath PR. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989;247:386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- 14.van de Veerdonk FL, Netea MG, Dinarello CA, et al. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan S, Glasser A, Hu YS, et al. The effect of interleukin-1 on cytokine gene expression by human corneal epithelial cells. Exp Eye Res. 2005;80:175–183. doi: 10.1016/j.exer.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Benitez del Castillo JM, Lemp MA. Ocular surface disorder. London, UK: JP Medical Ltd; 2002. [Google Scholar]
- 17.Arranz-Valsero I, Schulze U, Contreras-Ruiz L, et al. Involvement of corneal epithelial cells in the Th17 response in an in vitro bacterial inflammation model. Mol Vis. 2013;19:85–99. [PMC free article] [PubMed] [Google Scholar]
- 18.Duan F, Liao J, Huang Q, et al. HSV-1 miR-H6 inhibits HSV-1 replication and IL-6 expression in human corneal epithelial cells in vitro. Clin Dev Immunol. 2012;2012:192791. doi: 10.1155/2012/192791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebihara N, Matsuda A, Nakamura S, et al. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Invest Ophthalmol Vis Sci. 2011;52:8549–8557. doi: 10.1167/iovs.11-7956. [DOI] [PubMed] [Google Scholar]
- 20.Cope AP, Londei M, Chu NR, et al. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest. 1994;94:749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 22.Taylor PR, Roy S, Leal SM, Jr, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu TF, Jones BM. Impaired production of IL-12 in systemic lupus erythematosus. I. Excessive production of IL-10 suppresses production of IL-12 by monocytes. Cytokine. 1998;10:140–147. doi: 10.1006/cyto.1997.0268. [DOI] [PubMed] [Google Scholar]
- 24.Hazlett LD, McClellan SM, Hume EB, et al. Extended wear contact lens usage induces Langerhans cell migration into cornea. Exp Eye Res. 1999;69:575–577. doi: 10.1006/exer.1999.0728. [DOI] [PubMed] [Google Scholar]
- 25.Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 26.Korb DR, Greiner JV, Glonek T. Tear film lipid layer formation: implications for contact lens wear. Optom Vis Sci. 1996;73:189–192. doi: 10.1097/00006324-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Kalsow CM, Reindel WT, Merchea MM, et al. Tear cytokine response to multipurpose solutions for contact lenses. Clin Ophthalmol. 2013;7:1291–1302. doi: 10.2147/OPTH.S44642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markoulli M, Papas E, Cole N, et al. Effect of contact lens wear on the diurnal profile of matrix metalloproteinase 9 in tears. Optom Vis Sci. 2013;90:419–429. doi: 10.1097/OPX.0b013e31828d7d3b. [DOI] [PubMed] [Google Scholar]
- 29.Markoulli M, Papas E, Cole N, et al. The diurnal variation of matrix metalloproteinase-9 and its associated factors in human tears. Invest Ophthalmol Vis Sci. 2012;53:1479–1484. doi: 10.1167/iovs.11-8365. [DOI] [PubMed] [Google Scholar]
- 30.Lema I, Duran JA, Ruiz C, et al. Inflammatory response to contact lenses in patients with keratoconus compared with myopic subjects. Cornea. 2008;27:758–763. doi: 10.1097/ICO.0b013e31816a3591. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Perez J, Villa-Collar C, Sobrino Moreiras T, et al. Tear film inflammatory mediators during continuous wear of contact lenses and corneal refractive therapy. Br J Ophthalmol. 2012;96:1092–1098. doi: 10.1136/bjophthalmol-2012-301527. [DOI] [PubMed] [Google Scholar]
- 32.LaFrance MW, Kehinde LE, Fullard RJ. Multiple cytokine analysis in human tears: an optimized procedure for cytometric bead-based assay. Curr Eye Res. 2008;33:525–544. doi: 10.1080/02713680802190085. [DOI] [PubMed] [Google Scholar]
- 33.Benito MJ, Gonzalez-Garcia MJ, Teson M, et al. Intra- and inter-day variation of cytokines and chemokines in tears of healthy subjects. Exp Eye Res. 2014;120:43–49. doi: 10.1016/j.exer.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Huang JF, Zhang Y, Rittenhouse KD, et al. Evaluations of tear protein markers in dry eye disease: repeatability of measurement and correlation with disease. Invest Ophthalmol Vis Sci. 2012;53:4556–4564. doi: 10.1167/iovs.11-9054. [DOI] [PubMed] [Google Scholar]
- 35.Brautaset RL, Nilsson M, Leach N, et al. Corneal and conjunctival epithelial staining in hydrogel contact lens wearers. Eye Contact Lens. 2008;34:312–316. doi: 10.1097/ICL.0b013e3181891439. [DOI] [PubMed] [Google Scholar]
- 36.Willcox MD, Zhao Z, Naduvilath T, et al. Cytokine changes in tears and relationship to contact lens discomfort. Mol Vis. 2015;21:293–305. [PMC free article] [PubMed] [Google Scholar]
- 37.Khan SS, Smith MS, Reda D, et al. Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 38.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 39.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 40.Carreno E, Enriquez-de-Salamanca A, Teson M, et al. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol. 2010;88:e250–258. doi: 10.1111/j.1755-3768.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Shi B, He S, et al. Changes to tear cytokines of type 2 diabetic patients with or without retinopathy. Mol Vis. 2010;16:2931–2938. [PMC free article] [PubMed] [Google Scholar]
- 42.Malvitte L, Montange T, Vejux A, et al. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br J Ophthalmol. 2007;91:29–32. doi: 10.1136/bjo.2006.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VanDerMeid KR, Su SP, Krenzer KL, et al. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011;17:1056–1063. [PMC free article] [PubMed] [Google Scholar]
- 44.Chong RS, Jiang YZ, Boey PY, et al. Tear cytokine profile in medicated glaucoma patients: effect of monocyte chemoattractant protein 1 on early posttrabeculectomy outcome. Ophthalmology. 2010;117:2353–2358. doi: 10.1016/j.ophtha.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 45.Santacruz C, Linares M, Garfias Y, et al. Expression of IL-8, IL-6 and IL-1beta in tears as a main characteristic of the immune response in human microbial keratitis. Int J Mol Sci. 2015;16:4850–4864. doi: 10.3390/ijms16034850. [DOI] [PMC free article] [PubMed] [Google Scholar]