Abstract
Background and Objectives
Linezolid, a oxazolidinone, was the first in class to be approved for the treatment of bacterial infections arising from both susceptible and resistant strains of Gram-positive bacteria. Since overt exposure of linezolid may precipitate serious toxicity issues, therapeutic drug monitoring (TDM) may be required in certain situations, especially in patients who are prescribed other co-medications.
Methods
Using appropriate oral pharmacokinetic data (single dose and steady state) for linezolid, both maximum plasma drug concentration (Cmax) versus area under the plasma concentration–time curve (AUC) and minimum plasma drug concentration (Cmin) versus AUC relationship was established by linear regression models. The predictions of the AUC values were performed using published mean/median Cmax or Cmin data and appropriate regression lines. The quotient of observed and predicted values rendered fold difference calculation. The mean absolute error (MAE), root mean square error (RMSE), correlation coefficient (r), and the goodness of the AUC fold prediction were used to evaluate the two models.
Results
The Cmax versus AUC and trough plasma concentration (Ctrough) versus AUC models displayed excellent correlation, with r values of >0.9760. However, linezolid AUC values were predicted to be within the narrower boundary of 0.76 to 1.5-fold by a higher percentage by the Ctrough (78.3 %) versus Cmax model (48.2 %). The Ctrough model showed superior correlation of predicted versus observed values and RMSE (r = 0.9031; 28.54 %, respectively) compared with the Cmax model (r = 0.5824; 61.34 %, respectively).
Conclusions
A single time point strategy of using Ctrough level is possible as a prospective tool to measure the AUC of linezolid in the patient population.
Key Points
| The linear regression model of maximum plasma drug concentration (C max) versus area under the plasma concentration–time curve (AUC) C max and trough plasma concentration (C trough) versus AUC showed excellent correlation. |
| Linezolid AUC values were accurately predicted with the Ctrough model compared with the C max model, with better error predictions. |
| The single time point C trough model can be utilized in a prospective fashion to measure the AUC of linezolid in patients. |
Introduction
Linezolid, belonging to the oxazolidinone class of antibacterials, was the first in the class to be granted global approval for treating a variety of infections related to Gram-positive pathogens [1, 2]. Both oral and intravenous drug formulations are available to provide convenient therapy for patients [2]. Linezolid’s mechanism of action is unique and suggested to occur via significant inhibition of the bacterial protein synthesis complex initiation in the bacterial system via the direct action of linezolid on the binding site for initiator transfer RNA (t-RNA) [3, 4]. Linezolid significantly inhibits the growth of a variety of Gram-positive bacterial strains, including staphylococci, streptococci, and enterococci. Furthermore, it shows antimicrobial activity against both methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) [5–7]. The hallmark of linezolid’s antibacterial activity is its persistent and long-acting post-antibiotic effect, which may render it useful in strains that are difficult to treat. In addition, this effect may also curb the development of bacterial resistance to linezolid. Linezolid has been found to be an important option in the treatment of multiple drug-resistant tuberculosis [MDR-TB] [8]. Linezolid has an excellent minimum inhibitory concentration (MIC) against Mycobacterium tuberculosis and several first-line drug-resistant isolates [9–11]. The same dosing regimen (every 12 h) used to treat patients with Gram-positive infections has been used to treat patients with MDR-TB [11–13].
The safety, tolerability, and pharmacokinetics of linezolid in humans has been investigated for both intravenous and oral use [14–16]. It has been shown to be well tolerated in doses up to 625 mg given intravenously twice daily for up to 7 days in the clinic and in doses of either 400 mg or 600 mg given orally twice daily for up to 28 days [14–16]. Pharmacokinetic investigation has confirmed complete bioavailability of oral linezolid; this suggests it can be used interchangeably permitting oral and intravenous drug switches during therapy, if necessary. After oral administration, linezolid reached peak levels within 1–1.5 h, suggesting relatively rapid absorption of the drug. After intravenous administration, the peak levels were reached at the end of the 30-min drug infusion [14, 16]. Both maximum plasma drug concentration (Cmax) and area under the plasma concentration–time curve (AUC) values appeared to increase in a dose-proportional manner after oral or intravenous routes of administration. Almost two-thirds of linezolid total clearance was renal; the remaining one-third was via non-renal routes [14–16]. Regardless of the administration route, the half-life of linezolid ranged from 5 to 7 h, supporting twice daily dosing of the drug. Drug accumulation occurred at steady state, albeit numerically small. A mass balance study showed that approximately 50 % of administered linezolid was recovered in the urine, and comprised two inactive metabolites; another 35 % of the dose was represented by the intact parent compound [14–16].
We were interested in predicting the AUC of linezolid using a simple and straightforward approach for universal application. To be rigorous, we assembled published pharmacokinetic data of linezolid from various studies with different subject populations to make the dataset very heterogeneous in nature. However, for the model development we used data from a single pharmacokinetic study that provided a wide spread of the pharmacokinetic parameters, such as Cmax, trough plasma concentration (Ctrough), and AUC for modelling purposes.
Scope
To develop relationship using linear regression correlations of Ctrough versus AUC and Cmax versus AUC of linezolid from a published oral pharmacokinetic study.
To perform an internal validation to predict the AUC of linezolid following intravenous dosing from the same study using both the developed models.
To perform an external validation for the prediction of the linezolid AUC following oral and intravenous administration from scores of other published studies using the relevant Ctrough and Cmax data.
Methods
We searched the National Center for Biotechnology Information PubMed® database for relevant abstracts and full-length texts pertaining to the pharmacokinetics of linezolid. The keywords used in the search included linezolid, pharmacokinetics, humans, and clinical. The aim of the present analysis was to seek a relationship between Ctrough versus AUC and Cmax versus AUC for linezolid using unweighted linear regression analysis. Once established, we then used the appropriate regression lines in the prediction of AUC values for linezolid.
Data Source for Model Development
We obtained the mean pharmacokinetic data that provided Cmax and AUC values for linezolid from published pharmacokinetic data in healthy subjects [15–49]. The oral pharmacokinetic data to create the reference model for linezolid were from a double-blind, placebo-controlled study with 3:1 randomization of subjects to active relative or placebo at all dose levels [16]. The goal of the clinical study was to obtain clinical safety, tolerability, and pharmacokinetics data for linezolid after single and multiple oral administration to healthy subjects. In total, three doses (375, 500, and 625 mg) of linezolid were administered orally on day 1 (single dose) and from day 2 onwards (multiple doses). The same oral doses were administered for another 14.5 days every 12 h. The second study examined the safety, tolerability, and pharmacokinetics of linezolid in healthy subjects following intravenous drug administration. Two doses (500 and 625 mg) of linezolid were administered via a 30-min infusion on day 1 (single dose) and from day 2 (multiple doses) onwards for another 7.5 days; the same intravenous doses were administered via a 30-min infusion every 12 h [16].
The pharmacokinetic data were gathered after single and multiple doses following both oral and intravenous administration of linezolid. The frequency of the blood samples was adequate to assess linezolid pharmacokinetics with single and multiple doses regardless of the drug administration route. The AUC values used for linezolid in the Cmax regression model represented both AUCinf (single-dose study) and AUCtau (multiple-dose study) values. However, for the Ctrough regression model, AUCtau values (multiple-dose study) were used. The AUC data for linezolid obtained from the intravenous study were used for internal validation of the two regression models. In addition, for each pair of observed Cmax versus AUC and Ctrough versus AUC, four additional data points were generated via the addition or subtraction of either one or two standard deviations from the corresponding mean values of each parameter (i.e., Cmax, Ctrough, and AUC). This provided a basis for a larger spread of the Cmax, Ctrough, and AUC data to facilitate the model development. The incorporation of standard deviation assisted spread of the parameter values has been documented in the linear regression analysis of cyclosporine [50].
For the Cmax model, 30 pairs of Cmax and AUC values for linezolid were used as raw reference data in establishing the regression model (Table 1). For the Ctrough model, 14 pairs of Ctrough and AUC values for linezolid were used as raw reference data in establishing the regression model (Table 1). The data spread of Cmax, Ctrough, and AUC for linezolid were approximately 7.67-fold (4.07–31.23 µg/ml), approximately 54.57-fold (0.28–15.28 ng/ml), and 16.74-fold (15.7–262.8 µg × h/ml), respectively (Table 1).
Table 1.
Pharmacokinetic data used for developing linear regression models for linezolid
| Model type | Route, dose, type | Data tabulation | Single dose | Multiple dose | Reference | ||
|---|---|---|---|---|---|---|---|
| C max (µg/ml) | AUCinf (µg × h/ml) | C max (µg/ml) | AUCtau (µg × h/ml) | ||||
| C max | Oral, 375 mg, single dose | Mean | 8.21 | 65.5 | 13.1 | 82.8 | Stalker et al. [16] |
| Mean (−1 SD) | 6.14 | 40.6 | 10.2 | 60.2 | |||
| Mean (+1 SD) | 10.28 | 90.4 | 16 | 105.4 | |||
| Mean (−2 SD) | 4.07 | 15.7 | 7.3 | 37.6 | |||
| Mean (+2 SD) | 12.35 | 115.3 | 18.9 | 128 | |||
| Oral, 500 mg, single dose | Mean | 10.4 | 74.3 | 15.3 | 99.2 | ||
| Mean (−1 SD) | 7.87 | 46.4 | 11.58 | 62.5 | |||
| Mean (+1 SD) | 12.93 | 102.2 | 19.02 | 135.9 | |||
| Mean (−2 SD) | 5.34 | 19.3 | 7.86 | 25.8 | |||
| Mean (+2 SD) | 15.46 | 130.1 | 22.74 | 172.6 | |||
| Oral, 625 mg, single dose | Mean | 12.7 | 102 | 18.75 | 147 | ||
| Mean (−1 SD) | 9.34 | 72.3 | 12.51 | 89.1 | |||
| Mean (+1 SD) | 16.06 | 131.7 | 24.99 | 204.9 | |||
| Mean (−2 SD) | 5.98 | 42.6 | 6.27 | 31.2 | |||
| Mean (+2 SD) | 19.42 | 161.4 | 31.23 | 262.8 | |||
| C trough | Oral, 375 mg, single dose | Mean | NA | NA | 3.9a | 82.8 | |
| Mean (−1 SD) | 2.05 | 60.2 | |||||
| Mean (+1 SD) | 5.75 | 105.4 | |||||
| Mean (−2 SD) | 0.18b | 37.6b | |||||
| Mean (+2 SD) | 7.6 | 128 | |||||
| Oral, 500 mg, single dose | Mean | NA | NA | 5.04 | 99.2 | ||
| Mean (−1 SD) | 2.66 | 62.5 | |||||
| Mean (+1 SD) | 7.42 | 135.9 | |||||
| Mean (−2 SD) | 0.28 | 25.8 | |||||
| Mean (+2 SD) | 9.8 | 172.6 | |||||
| Oral, 625 mg, single dose | Mean | NA | NA | 8.02 | 147 | ||
| Mean (−1 SD) | 4.39 | 89.1 | |||||
| Mean (+1 SD) | 11.65 | 204.9 | |||||
| Mean (−2 SD) | 0.76 | 31.2 | |||||
| Mean (+2 SD) | 15.28 | 262.8 | |||||
AUC area under the plasma concentration–time curve, C max maximum plasma drug concentration, C trough trough plasma concentration, NA not available
a C trough reported
bValue excluded from the regression analyses
Linear Regression Model
Separate linezolid models representing Cmax versus AUC and Ctrough versus AUC were established by performing an un-weighted linear regression of the respective paired datasets to obtain the regression lines:
where m is the slope of the line and C is the intercept value. For each regression model of the paired datasets, a correlation coefficient was established. The developed Cmax versus AUC model was utilized in the prediction of the AUC for the linezolid. The in-built statistical package in Microsoft® Excel 2010 (Microsoft Company, Redmond, WA, USA) was used to perform linear regressions and establish correlation coefficients.
Prediction Using Published Cmax and Ctrough Data
Internal Dataset Validation
The intravenous data obtained from the same study that supplied the raw reference data for establishing the regression models using both Cmax and Ctrough were used for the internal validation [16].
External Dataset Validation
Scores of publications that described the pharmacokinetics of linezolid after oral and intravenous dosing in a variety of patient populations and heathy subjects were gathered [15–49], and the respective observed individual, mean/median Cmax or Ctrough values were used to predict AUC for linezolid using the regression lines as applicable. The predicted AUC values obtained from the two models were then subjected for additional statistical tests.
Statistical Tests and Fold-Difference Computation
The fold difference of the linezolid AUC prediction was separately calculated for the two regression models and was defined as the quotient of observed AUC and predicted AUC value. Various categories of fold difference ranging from <0.5-fold, 0.51- to 0.75-fold, 0.76- to 1.25-fold, 1.26 to 1.5-fold, 1.51 to 2-fold, and >2-fold were created to understand the spread and goodness of the prediction.
For the purpose of the current analysis, a prediction within 0.5 to 2-fold difference was considered satisfactory for the external dataset validation and a narrower prediction of within 1.5-fold difference was considered appropriate for the internal dataset validation. Fold difference-based statistical comparison has previously been employed and validated for several drugs [50–56].
We used a double-sided paired t-test to evaluate the observed (literature data) versus predicted AUC for the linezolid. The mean absolute error (MAE) was defined as the mean of the observed AUC values minus the predicted AUC values of linezolid; 95 % confidence interval limits were generated and an appropriate p-value was assigned for the statistical significance using the T-test calculator (Graphpad, San Diego, CA, USA).
In addition, we calculated mean square error and root means square error (RMSE) for linezolid (shown below) using Microsoft® Excel 2010.
Data Utility and Conversions
All data points from the reference data, with the exception of a single pair for the Ctrough model were used in the model development for linezolid. For consistency for the data assessment, Cmax values were reported in µg/ml units; AUC values were reported in µg × h/ml. Data unit conversions, if necessary, were made as applicable during compilation and tabulation of the pharmacokinetic data using the same uniform unit format.
Results
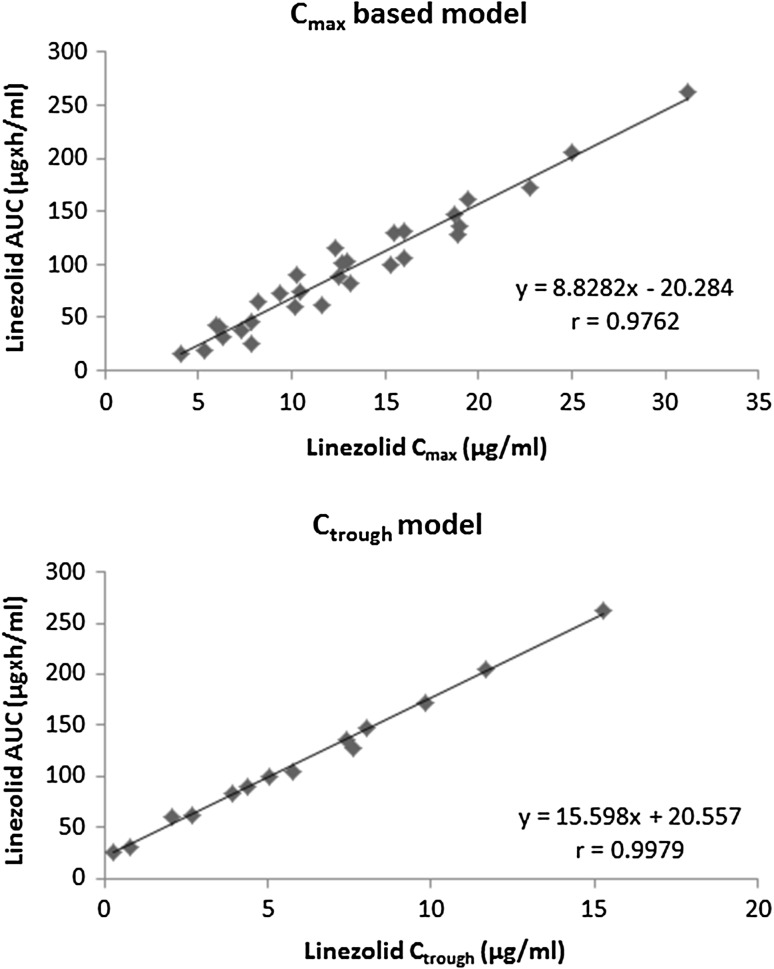
As illustrated in Fig. 1, the Cmax versus AUC and Ctrough versus AUC linear regression models were established for linezolid using the reference data presented in Table 1. An excellent correlation coefficient (r) value of 0.9762 (p < 0.001) and 0.9979 (p < 0.001) were obtained for the Cmax and Ctrough models, respectively.
Fig. 1.
Linear regression models developed by linezolid C max vs. linezolid AUC and linezolid C trough vs. linezolid AUC. AUC area under the plasma concentration–time curve, C max maximum plasma drug concentration, C trough trough plasma concentration
The prediction of AUC values for linezolid using the two models was performed using the regression equations described below:
Internal Dataset Prediction
As shown in Table 2, the use of either Cmax or Ctrough regression models developed using oral linezolid data adequately predicted the AUC values obtained after intravenous administration at steady state. The fold difference in the predicted AUC for linezolid was 0.84 and 1.15, for Cmax and Ctrough models, respectively.
Table 2.
Internal dataset validation: prediction of intravenous area under the plasma concentration–time curve data for linezolid using regression models from oral data
| Model type | Route, dose, type | Observed | Predicted | Fold difference | Reference |
|---|---|---|---|---|---|
| AUCtau (µg × h/ml) | AUCtau (µg × h/ml) | ||||
| C max | Intravenous, 500 mg, multiple dose | 81.2 | 106.84 | 0.76 | Stalker et al. [16] |
| 61.6 | 79.65 | 0.77 | |||
| 100.8 | 134.03 | 0.75 | |||
| 42 | 52.46 | 0.80 | |||
| 120.4 | 161.22 | 0.75 | |||
| Intravenous, 625 mg, multiple dose | 93.4 | 118.32 | 0.79 | ||
| 61.1 | 95.19 | 0.64 | |||
| 125.7 | 141.45 | 0.89 | |||
| 158 | 164.58 | 0.96 | |||
| C trough | Intravenous, 500 mg, multiple dose | 81.2 | 75.31 | 1.08 | |
| 61.6 | 54.09 | 1.14 | |||
| 100.8 | 96.52 | 1.04 | |||
| 42 | 32.88 | 1.28 | |||
| 120.4 | 117.73 | 1.02 | |||
| Intravenous, 625 mg, multiple dose | 93.4 | 80.45 | 1.16 | ||
| 61.1 | 42.08 | 1.45 | |||
| 125.7 | 118.82 | 1.06 | |||
| 158 | 157.20 | 1.01 |
AUC area under the plasma concentration–time curve, C max maximum plasma drug concentration, C trough trough plasma concentration
External Dataset Prediction
Cmax Model
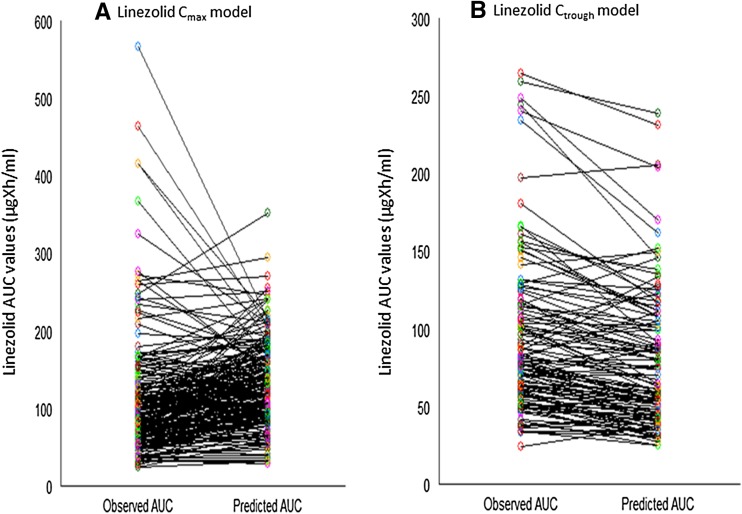
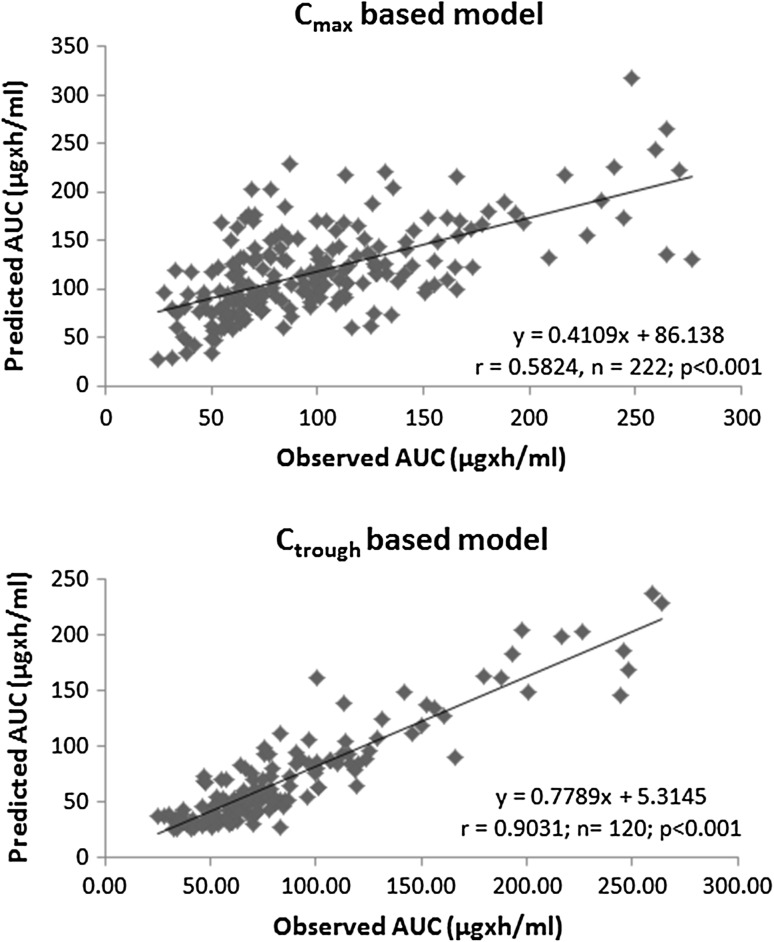
Figure 2 displays the comparison of the observed AUC values versus predicted AUC values for linezolid. Less than 50 % of the predicted AUC values were within the 0.76- to1.5-fold limit of the original values (Table 3). Furthermore, AUC fold difference was distributed across the various segments, suggesting a greater variability in the prediction of AUC (Table 3). For instance, 16.6 % of the AUC predictions were <0.5-fold difference, and 1.4 % of the AUC predictions were >2.0-fold difference. The plot of observed AUC versus predicted AUC values for linezolid is shown in Fig. 3 and had a correlation of 0.5824, n = 222 (p < 0.001). The MAE and RMSE (expressed as %) were 21.34 and 61.34 %, respectively (Table 3).
Fig. 2.
Spread of the observed AUC vs. predicted AUC for either linezolid C max model (a) or linezolid C trough model. AUC area under the plasma concentration–time curve, C max maximum plasma drug concentration, C trough trough plasma concentration
Table 3.
Statistical comparisons and fold difference summary between observed vs. predicted area under the plasma concentration–time curve values for linezolid
| Model type | N size | Prediction criteriaa | Mean AUC (µg × h/ml) | Mean absolute error (difference %) | Mean square error | Root mean square error (%) | Correlation coefficient (r value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.5-fold | >0.5 to 0.75-fold | 0.76 to 1.25-fold | 1.26 to 1.5-fold | 1.51 to <2-fold | >2-fold | Observed | Predicted | ||||||
| C max | 222 | 37 (16.7) | 66 (29.7) | 96 (43.2) | 11 (5.0) | 9 (4.0) | 3 (1.4) | 107.26 | 130.22 | −22.90 (21.34)b | 4331.1 | 65.81 (61.34)b | 0.5824 |
| C trough | 120 | – | 4 (3.4) | 67 (55.8) | 27 (22.5) | 22 (18.3) | – | 93.05 | 77.79 | 15.26 (16.40)c | 705.37 | 26.56 (28.54)c | 0.9031 |
AUC area under the plasma concentration–time curve, C max maximum plasma drug concentration, C trough trough plasma concentration
aData presented as N (%)
bDerived by the equation:
cDerived by the equation:
Fig. 3.
Correlation of the observed vs. predicted values for either the linezolid C max model or the linezolid C trough model. AUC area under the plasma concentration–time curve, C max maximum plasma drug concentration, C trough trough plasma concentration
Ctrough Model
Figure 2 displays the comparison of the observed AUC values versus predicted AUC values for linezolid. More than 75 % of the predicted AUC values (i.e., 78.3 %) were within the 0.76- to 1.5-fold limit of the original values (Table 3). Unlike the Cmax model, no AUC predictions of linezolid were either <0.5- or >2.0-fold difference, suggesting the containment of the AUC values within 0.5- to 2-fold difference (Table 3). The plot of observed AUC versus predicted AUC values for linezolid is shown in Fig. 3 and had a correlation of 0.9031, n = 120 (p < 0.001). The MAE and RMSE (expressed as percentages) were 16.40 and 28.54 %, respectively (Table 3).
Discussion
The increased risk posed by resistant Gram-positive pathogens causing frequent fatalities can be circumvented with the prudent use of linezolid to treat a variety of infections. Linezolid is one of the few antibiotics that possess excellent pharmacokinetic properties, such as almost 100 % [14–16] bioavailability and rapid Cmax after oral administration (almost matching the Cmax obtained after standard intravenous infusion of the drug), meaning it is easily possible to switch from intravenous to oral drug administration regimens. Therefore, transitioning patients from a hospital/institutional setting to a home setting is made easy with the possibility of changing an intravenous prescription of linezolid to an oral regimen with a dose alteration. This prompted us to establish simple regression models using oral pharmacokinetic data that would enable the prediction of AUC data for linezolid using a single time point strategy regardless of the administration route.
The AUC of linezolid is a vital parameter, and the ratio of AUC/MIC has been used as a surrogate for both bacteriological and clinical outcomes [14]. Note also that the linezolid AUC has also been linked to the occurrence of thrombocytopenia [14].
The reference data for linezolid AUC used for building either Cmax or Ctrough models represented either AUCtau (every 12 h dosing schedule) or AUCinf (single-dose) values. Because linezolid exhibits linear pharmacokinetics, steady state exposure was expected to be comparable to the single-dose AUCinf data. The calculated AUC values from either of the two models are representative of the exposure of linezolid in a dosing interval since the majority of the examples used in the dataset were from multiple-dose pharmacokinetic studies of linezolid.
Although we were limited by not having individual datasets to build the Cmax versus AUC and Ctrough versus AUC linear regression models, the mean ± standard deviation approach enabled us to generate additional data points. While this strategy enabled a wider spread of the Cmax, Ctrough, and AUC values for linezolid, it did not compromise the scientific integrity of the analysis. For instance, the Cmax versus AUC analysis would have yielded a slope value of 7.3458 using as is data, which was in close proximity to the value of 8.8282 with additional data points. Similarly, for the Ctrough versus AUC analysis, the slope value of 15.6750 (as is data) was almost overlapping with the slope value of 15.5980 (with additional data points). The internal validation unequivocally supported the ability of models developed with oral data to predict the intravenous exposure data of linezolid, irrespective of Cmax or Ctrough models.
Based on statistical comparisons, the superiority of Ctrough over that of Cmax in predicting the AUC of linezolid was established with >2-fold better error prediction rendered by the Ctrough model (RMSE: 28.54 %) as compared with the Cmax model (RMSE: 61.34 %). The distribution of AUC fold-differences in the prediction suggested that the Ctrough model predicted the AUC values to a large extent within the narrow band of 0.75- to 1.5-fold differences. This ability of the Ctrough model to consistently predict linezolid AUC values within a narrower boundary may be useful in determining the potential for any drug–drug interaction with other drugs co-administered with linezolid. For instance, in the drug–drug interaction study of clarithromycin with linezolid [35], the mean observed AUC for linezolid was 61 (34.6–63.9) ng × h/ml and the Ctrough model predicted AUC values were 53.1 (34.6–54.9) ng × h/ml, which confirmed its utility.
A clinical pharmacokinetic study was performed previously to explore a limited sampling strategy for the therapeutic drug monitoring (TDM) of linezolid in patients with MDR-TB [34]. Interestingly, the strategy comprised Ctrough (alone) and Ctrough combined with two to three additional time points within the 0- to 12-h dosing interval of linezolid. The use of Ctrough alone was identified as useful for the TDM of linezolid. This was a well planned and executed study with a homogenous patient population, and it yielded an r value of 0.91 and an RMSE of 15 % [34]. To put things into perspective, the present analysis of linezolid was heterogeneous in terms of the nature of studies carried out in different geographies with applicable clinical protocols and collated data for over a decade, covering different patient populations being treated with linezolid for various resistant Gram-positive pathogens, and it also included oral and intravenous administration routes. Despite the enormous heterogeneity, we were able to establish an r value of 0.90 and an RMSE of 29 % using the CtroughCtrough-based model. Furthermore, we also examined two individual patient studies of linezolid that had a sample size of at least n = 10 and performed the regression analysis of Ctrough versus AUC values to further validate our developed model, which was based on mean data in healthy subjects.
The first study involved critically ill patients with ventilator-associated pneumonia, where plasma and intrapulmonary linezolid concentrations were determined [25]—the Ctrough versus AUC regression analysis yielded:
The second study involved critically ill neurological patients where both cerebrospinal fluid and serum concentrations were measured [44]—the Ctrough versus AUC regression analysis yielded:
Using the examples of the individual patient studies, our present analysis when put into context with previously reported limited sampling strategy work on linezolid [34] strongly suggests that a Ctrough model could be used prospectively in patients. A single sample collection at Ctrough has the distinct advantage of minimizing the risk of other opportunistic infections in a community setting. Also, the Ctrough model would be beneficial when other concomitant drugs are administered, since the sample time is distant from absorption and metabolism processes that may affect the pharmacokinetics of the drug. Perhaps the same sample collected for linezolid may also be useful for measuring other concomitant drugs.
Although we understood that the Cmax versus AUC model may not be ideal, we attempted to build the model and validate it further. We believe that since Cmax is largely influenced by the sampling times to define the pharmacokinetic profile of the drug, it may exhibit more intra- and inter-subject variability. From a practicality viewpoint, it may be difficult to sample for a precise Cmax estimation because it would involve intensive pharmacokinetic sampling. In the present analysis, Cmax may also have been influenced by differences in the duration of intravenous infusion of linezolid (30 min vs. 1 h infusion). Therefore, institution of a Cmax-based model as a strategy should be considered after carefully weighing the number of limitations it imposes.
As published pharmacokinetic data were lacking, we were unable to examine the predictability of linezolid AUC in obese subjects using either the Cmax or the Ctrough models. However, we used the recently published data by Bhalodi et al. [57] to examine the predictability of the AUCtau of linezolid using the Cmax model. Using the mean Cmax (20.9 µg/ml) of linezolid in moderately obese patients [57], the predicted AUCtau value was 182.4 µg × h/ml as compared with the observed AUCtau of 130.3 µg × h/ml. Similarly, using the mean Cmax (18.8 µg/ml) in morbidly obese patients [57], the predicted AUCtau was 161.9 µg × h/ml as compared with the observed AUCtau of 109.2 µg × h/ml. Although Ctrough data were not available in this study [57], using the Cmax model suggested that the developed models were applicable for the prediction of linezolid AUCtau in obese patients.
Our work has additional limitations: first, the linear regression models, either Cmax or Ctrough, developed for linezolid were based on mean data but not on individual subject datasets; second, the AUC predictions for either of the models were based on mean data, while the prediction errors may not truly reflect the errors of the population at large. Third, although the Ctrough model appeared to provide the best accuracy and bias for predicting AUC values, the clinical pharmacokinetic data in patients should be interpreted with utmost caution, keeping in mind polypharmacy and/or attenuated pathophysiological considerations because of the disease state. Fourth, the Ctrough model can only be used to render the AUC prediction of linezolid in a dosing interval (τ = 12 h), but it may be less than ideal for the prediction of AUCinf following single-dose administration of linezolid.
Conclusions
The Cmax versus AUC and Ctrough versus AUC models were unambiguously established for linezolid using published data. The predictions of AUC values using the Ctrough model were found to be superior to those of the Cmax model as judged by fold-difference calculations and error predictions such as MAE and RMSE values and correlation coefficients. Since excellent predictions of the AUC values of linezolid were obtained by the Ctrough model, a single time point strategy of measuring Ctrough level is possible as a prospective tool in the patient population.
Compliance with Ethical Standards
Funding
There was no funding that supported the current work.
Conflicts of interest
NRS and MS have no conflicts of interest to declare.
References
- 1.Bounthavong M, Hsu DI. Cost-effectiveness of linezolid in methicillin-resistant Staphylococcus aureus skin and skin structure infections. Expert Rev Pharmacoecon Outcomes Res. 2012;12(6):683–98. [DOI] [PubMed]
- 2.ZYVOX® [(linezolid) injection; (linezolid) tablets; (linezolid) for oral suspension]—package insert. Pfizer Inc, NY, Last revised November 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021132s027lbl.pdf. Accessed 15 Sept 2015.
- 3.Lin AH, Murray RW, Vidmar TJ, Marotti KR. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with the binding of chloramphenicol and lincomycin. Antimicrob Agents Chemother. 1997;41:2127–2131. doi: 10.1128/aac.41.10.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinabarger DL, Marotti KR, Murray RW, Lin AH, Melchior EP. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother. 1997;41:2132–2136. doi: 10.1128/aac.41.10.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noskin GA, Siddiqui F, Stosor V, Hacek D, Peterson LR. In vitro activities of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1999;43:2059–2062. doi: 10.1128/aac.43.8.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel R, Rouse MS, Piper KE, Steckelberg JM. In vitro activity of linezolid against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 1999;34:119–122. doi: 10.1016/S0732-8893(99)00016-4. [DOI] [PubMed] [Google Scholar]
- 7.Rybak MJ, Cappelletty DM, Moldovan T, Aeschlimann JR, Kaatz GW. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob Agents Chemother. 1998;42:721–724. doi: 10.1093/jac/42.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Falagas ME, Vardakas KZ, Wang R, Qin R, Wang J, et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis. 2015;7:603–615. doi: 10.3978/j.issn.2072-1439.2015.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez JC, Ruiz M, Lopez M, et al. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2002;20:464–467. doi: 10.1016/S0924-8579(02)00239-X. [DOI] [PubMed] [Google Scholar]
- 10.Bostic GD, Perri MB, Thal LA, Zervos MJ. Comparative in vitro and bactericidal activity of oxazolidinone antibiotics against multidrug-resistant enterococci. Diagn Microbiol Infect Dis. 1998;30:109–112. doi: 10.1016/S0732-8893(97)00210-1. [DOI] [PubMed] [Google Scholar]
- 11.Alcalá L, Ruiz-Serrano MJ, Pérez-Fernández Turégano C, García De Viedma D, Díaz-Infantes M, et al. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother. 2003;47:416–417. doi: 10.1128/AAC.47.1.416-417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortun J, Martin-Davila P, Navas E, Pérez-Elías MJ, Cobo J, Tato M, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005;56:180–185. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]
- 13.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN, et al. Case series report of a linezolid containing regimen for extensively drug-resistant tuberculosis. Chest. 2008;134:187–192. doi: 10.1378/chest.07-1988. [DOI] [PubMed] [Google Scholar]
- 14.MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother. 2003;51(Suppl 2):ii17–25. [DOI] [PubMed]
- 15.Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB, Sams JP, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [(14)C]linezolid to healthy human subjects. Drug Metab Dispos. 2001;29:1136–1145. [PubMed] [Google Scholar]
- 16.Stalker DJ, Jungbluth GL, Hopkins NK, Batts DH. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother. 2003;51:1239–1246. doi: 10.1093/jac/dkg180. [DOI] [PubMed] [Google Scholar]
- 17.Gee T, Ellis R, Marshall G, Andrews J, Ashby J, Wise R. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob Agents Chemother. 2001;45:1843–1846. doi: 10.1128/AAC.45.6.1843-1846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, et al. Linezolid dosage in pediatric patients based on pharmacokinetics and pharmacodynamics. J Infect Chemother. 2015;21:70–73. doi: 10.1016/j.jiac.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Eslam RB, Burian A, Vila G, Sauermann R, Hammer A, Frenzel D, et al. Target site pharmacokinetics of linezolid after single and multiple doses in diabetic patients with soft tissue infection. J Clin Pharmacol. 2014;54:1058–1062. doi: 10.1002/jcph.296. [DOI] [PubMed] [Google Scholar]
- 20.Flanagan SD, Bien PA, Muñoz KA, Minassian SL, Prokocimer PG. Pharmacokinetics of tedizolid following oral administration: single and multiple dose, effect of food, and comparison of two solid forms of the prodrug. Pharmacotherapy. 2014;34:240–250. doi: 10.1002/phar.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beer R, Engelhardt KW, Pfausler B, Broessner G, Helbok R, Lackner P, et al. Pharmacokinetics of intravenous linezolid in cerebrospinal fluid and plasma in neurointensive care patients with staphylococcal ventriculitis associated with external ventricular drains. Antimicrob Agents Chemother. 2007;51:379–382. doi: 10.1128/AAC.00515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunder G, Zysset-Aschmann Y, Vollenweider F, Maier T, Krähenbühl S, Drewe J. Lack of pharmacokinetic interaction between linezolid and antacid in healthy volunteers. Antimicrob Agents Chemother. 2006;50:68–72. doi: 10.1128/AAC.50.1.68-72.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beringer P, Nguyen M, Hoem N, Louie S, Gill M, Gurevitch M, et al. Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings. Antimicrob Agents Chemother. 2005;49:3676–3681. doi: 10.1128/AAC.49.9.3676-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boselli E, Breilh D, Rimmelé T, Djabarouti S, Toutain J, Chassard D, et al. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit Care Med. 2005;33:1529–1533. doi: 10.1097/01.CCM.0000168206.59873.80. [DOI] [PubMed] [Google Scholar]
- 25.Dehghanyar P, Bürger C, Zeitlinger M, Islinger F, Kovar F, Müller M, et al. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob Agents Chemother. 2005;49:2367–2371. doi: 10.1128/AAC.49.6.2367-2371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer B, Kornek GV, Nikfardjam M, Karth GD, Heinz G, Locker GJ, et al. Multiple-dose pharmacokinetics of linezolid during continuous venovenous haemofiltration. J Antimicrob Chemother. 2005;56:172–179. doi: 10.1093/jac/dki133. [DOI] [PubMed] [Google Scholar]
- 27.Bosso JA, Flume PA, Gray SL. Linezolid pharmacokinetics in adult patients with cystic fibrosis. Antimicrob Agents Chemother. 2004;48:281–284. doi: 10.1128/AAC.48.1.281-284.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brier ME, Stalker DJ, Aronoff GR, Batts DH, Ryan KK, O’Grady M, et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 2003;47:2775–2780. doi: 10.1128/AAC.47.9.2775-2780.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkhardt O, Borner K, von der Höh N, Köppe P, Pletz MW, et al. Single- and multiple-dose pharmacokinetics of linezolid and co-amoxiclav in healthy human volunteers. J Antimicrob Chemother. 2002;50:707–712. doi: 10.1093/jac/dkf163. [DOI] [PubMed] [Google Scholar]
- 30.Gordi T, Tan LH, Hong C, Hopkins NJ, Francom SF, Slatter JG, et al. The pharmacokinetics of linezolid are not affected by concomitant intake of the antioxidant vitamins C and E. J Clin Pharmacol. 2003;43:1161–1167. doi: 10.1177/0091270003257455. [DOI] [PubMed] [Google Scholar]
- 31.Hendershot PE, Antal EJ, Welshman IR, Batts DH, Hopkins NK. Linezolid: pharmacokinetic and pharmacodynamic evaluation of coadministration with pseudoephedrine HCl, phenylpropanolamine HCl, and dextromethorpan HBr. J Clin Pharmacol. 2001;41:563–572. doi: 10.1177/00912700122010302. [DOI] [PubMed] [Google Scholar]
- 32.Sisson TL, Jungbluth GL, Hopkins NK. A pharmacokinetic evaluation of concomitant administration of linezolid and aztreonam. J Clin Pharmacol. 1999;39:1277–1282. doi: 10.1177/00912709922011962. [DOI] [PubMed] [Google Scholar]
- 33.Adembri C, Fallani S, Cassetta MI, Arrigucci S, Ottaviano A, Pecile P, et al. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versuscontinuous infusion. Int J Antimicrob Agents. 2008;31:122–129. doi: 10.1016/j.ijantimicag.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Alffenaar JW, Kosterink JG, van Altena R, van der Werf TS, Uges DR, et al. Limited sampling strategies for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Ther Drug Monit. 2010;32:97–101. doi: 10.1097/FTD.0b013e3181cc6d6f. [DOI] [PubMed] [Google Scholar]
- 35.Bolhuis MS, van Altena R, van Soolingen D, de Lange WC, Uges DR, van der Werf TS, et al. Clarithromycin increases linezolid exposure in multidrug-resistant tuberculosis patients. Eur Respir J. 2013;42:1614–1621. doi: 10.1183/09031936.00001913. [DOI] [PubMed] [Google Scholar]
- 36.Cai Y, Chai D, Falagas ME, Karageorgopoulos DE, Wang R, Bai N, et al. Weight-adjusted versus fixed dose of linezolid for Chinese healthy volunteers of higher and lower body weight: a phase I pharmacokinetic and pharmacodynamic study. Expert Opin Investig Drugs. 2013;22:309–315. doi: 10.1517/13543784.2013.766716. [DOI] [PubMed] [Google Scholar]
- 37.Dong H, Wang X, Dong Y, Lei J, Li H, You H, et al. Clinical pharmacokinetic/pharmacodynamic profile of linezolid in severely ill intensive care unit patients. Int J Antimicrob Agents. 2011;38:296–300. doi: 10.1016/j.ijantimicag.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Helmy SA. Pharmacokinetics and relative bioavailability evaluation of linezolid suspension and tablet formulations. Drug Res (Stuttg). 2013;63:489–494. doi: 10.1055/s-0033-1347189. [DOI] [PubMed] [Google Scholar]
- 39.Islinger F, Dehghanyar P, Sauermann R, Bürger C, Kloft C, Müller M, et al. The effect of food on plasma and tissue concentrations of linezolid after multiple doses. Int J Antimicrob Agents. 2006;27:108–112. doi: 10.1016/j.ijantimicag.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Kosaka T, Kokufu T, Shime N, Sugioka N, Kato R, Hamaoka K, et al. Pharmacokinetics and tolerance of linezolid for meticillin-resistant Staphylococcus aureus mediastinitis in paediatric patients. Int J Antimicrob Agents. 2009;33:368–370. doi: 10.1016/j.ijantimicag.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Lovering AM, Le Floch R, Hovsepian L, Stephanazzi J, Bret P, Birraux G, et al. Pharmacokinetic evaluation of linezolid in patients with major thermal injuries. J Antimicrob Chemother. 2009;63:553–559. doi: 10.1093/jac/dkn541. [DOI] [PubMed] [Google Scholar]
- 42.Majcher-Peszynska J, Haase G, Sass M, Mundkowski R, Pietsch A, Klammt S, et al. Pharmacokinetics and penetration of linezolid into inflamed soft tissue in diabetic foot infections. Eur J Clin Pharmacol. 2008;64:1093–1100. doi: 10.1007/s00228-008-0531-5. [DOI] [PubMed] [Google Scholar]
- 43.Myrianthefs P, Markantonis SL, Vlachos K, Anagnostaki M, Boutzouka E, Panidis D, Baltopoulos G. Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob Agents Chemother. 2006;50:3971–3976. doi: 10.1128/AAC.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Rosa FG, Corcione S, Baietto L, Ariaudo A, Di Perri G, Ranieri VM, et al. Pharmacokinetics of linezolid during extracorporeal membrane oxygenation. Int J Antimicrob Agents. 2013;41:590–591. doi: 10.1016/j.ijantimicag.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Santos RP, Prestidge CB, Brown ME, Urbancyzk B, Murphey DK, Salvatore CM, et al. Pharmacokinetics and pharmacodynamics of linezolid in children with cystic fibrosis. Pediatr Pulmonol. 2009;44:148–154. doi: 10.1002/ppul.20966. [DOI] [PubMed] [Google Scholar]
- 46.Viaggi B, Paolo AD, Danesi R, Polillo M, Ciofi L, Del Tacca M, et al. Linezolid in the central nervous system: comparison between cerebrospinal fluid and plasma pharmacokinetics. Scand J Infect Dis. 2011;43:721–727. doi: 10.3109/00365548.2011.582140. [DOI] [PubMed] [Google Scholar]
- 47.Welshman IR, Sisson TA, Jungbluth GL, Stalker DJ, Hopkins NK. Linezolid absolute bioavailability and the effect of food on oral bioavailability. Biopharm Drug Dispos. 2001;22:91–97. doi: 10.1002/bdd.255. [DOI] [PubMed] [Google Scholar]
- 48.Wiskirchen DE, Shepard A, Kuti JL, Nicolau DP. Determination of tissue penetration and pharmacokinetics of linezolid in patients with diabetic foot infections using in vivo microdialysis. Antimicrob Agents Chemother. 2011;55:4170–4175. doi: 10.1128/AAC.00445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vu DH, Bolhuis MS, Koster RA, Greijdanus B, de Lange WC, van Altena R, et al. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2012;56:5758–5763. doi: 10.1128/AAC.01054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srinivas NR. Therapeutic drug monitoring of cyclosporine and area under the curve prediction using a single time point strategy: appraisal using peak concentration data. Biopharm Drug Dispos. 2015 [Epub ahead of print]. [DOI] [PubMed]
- 51.Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data. I: impact of inter-individual variability. Xenobiotica. 2006;36:473–497. doi: 10.1080/00498250600683197. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Mao J, Hop CE. Physiologically based pharmacokinetic modeling to predict drug-drug interactions involving inhibitory metabolite: a case study of amiodarone. Drug Metab Dispos. 2015;43:182–189. doi: 10.1124/dmd.114.059311. [DOI] [PubMed] [Google Scholar]
- 53.Benjamin B, Sahu M, Bhatnagar U, Abhyankar D, Srinivas NR. The observed correlation between in vivo clinical pharmacokinetic parameters and in vitro potency of VEGFR-2 inhibitors. Can this be used as a prospective guide for the development of novel compounds? Arzneimittelforschung. 2012;62:194–201. doi: 10.1055/s-0031-1299772. [DOI] [PubMed] [Google Scholar]
- 54.Srinivas NR. Limited sampling strategy for the prediction of area under the curve (AUC) of statins: reliability of a single time point for AUC prediction for pravastatin and simvastatin. Drug Res (stuttg). 2015 [Epub ahead of print]. [DOI] [PubMed]
- 55.Srinivas NR . Differences in the prediction of area under the curve for a protease inhibitor using trough versus peak concentration: feasibility assessment using published pharmacokinetic data for indinavir. Am J Ther. 2015 [Epub ahead of print]. [DOI] [PubMed]
- 56.Srinivas NR. Prediction of area under the curve for a p-glycoprotein, a CYP3A4 and a CYP2C9 substrate using a single time point strategy: assessment using fexofenadine, itraconazole and losartan and metabolites. Drug Dev Ind Phar. 2015 [Epub ahead of print]. [DOI] [PubMed]
- 57.Bhalodi AA, Papasavas PK, Tishler DS, Nicolau DP, Kuti JL. Pharmacokinetics of intravenous linezolid in moderately to morbidly obese adults. Antimicrob Agents Chemother. 2013;57:1144–1149. doi: 10.1128/AAC.01453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]